Translate this page into:
Acute Necrotizing Encephalopathy in an Adult
Address for correspondence: Dr. Ramakrishna Narra, Department of Neuroradiology, Katuri Medical College and Hospital, Chinnakondrupadu, Andhra Pradesh, India. E-mail: narra.ramki29@gmail.com
-
Received: ,
Accepted: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Acute necrotizing encephalopathy (ANE) is a rapidly progressing neurologic disorder that occurs in children after common viral infections of the respiratory or gastrointestinal systems. This disease is commonly seen in East Asia. Normal healthy infants and children can get affected. The condition carries a poor prognosis with high morbidity and mortality rates. We report here a case of a 23-year-old female with ANE and describe its neuroimaging findings. Magnetic resonance imaging examination performed showed symmetric lesions involving the thalami, brainstem, and cerebellum.
Keywords
Acute necrotizing encephalopathy
diffusion imaging
gradient imaging
hemorrhage
magnetic resonance imaging
necrosis
thalami
INTRODUCTION

Acute necrotizing encephalopathy (ANE) is a rare central nervous system (CNS) complication secondary to influenza or other viral infections which is characterized by altered mental status and seizures, and often this further leads to profound disability or death. It is generally considered to be a parainfectious disease secondary to immune response that is triggered mainly by these viral infections. Initially described as a neurological syndrome in pediatric patients of Asian descent, sporadic cases are now recognized worldwide. Many cases of ANE have been reported in the pediatric literature, but very few cases have been described in adults.[1] Though influenza A virus, mycoplasma, herpes simplex virus (HSV), and human herpes virus-6 have been reported as common causative agents, it is now believed that this disease is most likely due to immune-mediated reactions secondary to these infections. Cytokines such as tumor necrotizing factor-α and interleukin-1 and interleukin-6 can speed up the disease.[2] The initial recognizable symptoms of this syndrome are seizures, rapid neurologic decline, and vomiting. No specific treatment or preventive method has been identified for this disease. Only 10% of these patients recover completely.
CASE REPORT
A 23-year-old female was admitted to our hospital with a 3-day history of headache and 2-day history of moderate-grade fever which was not associated with chills and rigors. She had an episode of projectile vomiting a day earlier, following which she developed altered sensorium and had an episode of tonic posturing of limbs. Three days prior to the start of the present symptoms, the patient mentioned having cough and rhinorrhea. The patient had no diarrhea, pharyngitis, conjunctivitis, arthralgia, or rash. There was no history of dog bite, recent travel, herpes simplex virus (HSV) or varicella zoster infection, or hemorrhagic disease. Her pulse rate was 106/min, blood pressure 110/70 mm Hg, and respiratory rate 24 breaths/min. Petechial spots were present all over the body. Neurological examination revealed a Glasgow coma scale of E1M2V0 (Eye, Motor, Verbal). Pupillary reflex and corneal reflex were absent. Tone was normal with intermittent decerebration. Deep tendon reflexes were brisk bilaterally and plantars were not elicitable.
The patient had a platelet count of 90,000/mm3, WBC count of 7200 cells/mm3, with normal differential count. Cerebrospinal fluid (CSF) showed elevated protein and liver function tests were abnormal [aspartate transaminase 98 U/l (normal 8–48 U/l), alanine transaminase 110 U/l (normal 7–55 U/l), alkaline phosphatase 240 U/l (normal 45–115 U/l), total bilirubin 1.2 mg/dl (normal 0.1–1.0 mg/dl)].
Serology tests for dengue, influenza, mycoplasma, and HSV infections were negative. Other results of infectious (bacterial blood culture, serology for mycoplasma and influenza), metabolic, hematologic, and collagen vascular serologic evaluation were unremarkable.
Magnetic resonance imaging (MRI) done revealed areas of altered signal intensity (predominantly hyperintense) involving thalami, pons, midbrain, and cerebellum on fluid-attenuated inversion recovery (FLAIR) [Figure 1a and b]. The involved areas were predominantly hyperintense on T2W images with focal higher signal areas representing necrosis [Figure 2a and b]. On gradient sequence, blooming was noted in bilateral thalami representing hemorrhages [Figure 3a and b]. On diffusion-weighted MR imaging, focal areas of restriction were noted in bilateral thalami representing cytotoxic edema [apparent diffusion coefficient (ADC) 40 × 10-3 mm/s], surrounding necrosis [ADC 120 × 10-3 mm/s], and hemorrhage [Figure 4a and b]. Based on the temporal evolution of the clinical symptoms and MRI findings, a diagnosis of ANE was considered.
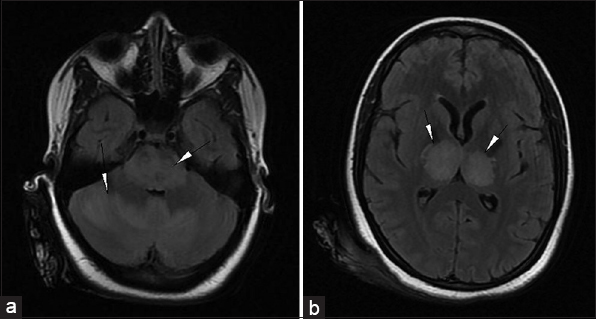
- 23-year-old female presented with headache and fever was diagnosed with acute necrotizing encephalopathy. Axial FLAIR MR images (a) at the level of pons and cerebellum show hyperintense signals (arrowheads) in the pons and cerebellum and (b) at the level of thalami shows hyperintense signals in bilateral thalami (arrowheads).
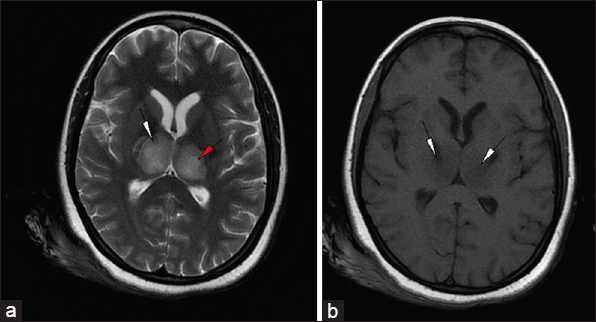
- 23-year-old female presented with headache and fever was diagnosed with acute necrotizing encephalopathy. Axial (a) T2-weighted (T2W) and (b) T1-weighted images at the level of thalami show hyperintense signal in bilateral thalami and the posterior limb of internal capsule on T2W (white arrowheads) and hypointense signal on T1-weighted images (arrowhead). T2W image shows increased signal (red arrowhead) in the center representing necrosis.
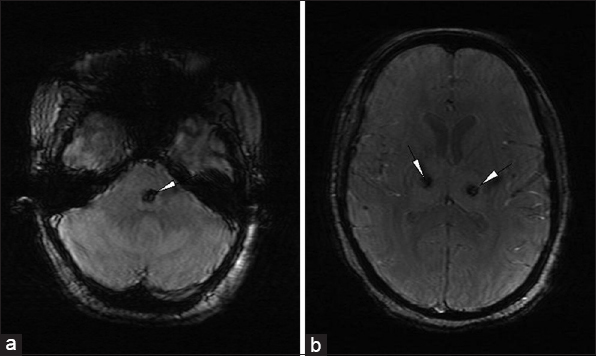
- 23-year-old female presented with headache and fever was diagnosed with acute necrotizing encephalopathy. Axial gradient images (a) at the level of pons and (b) thalami show blooming representing hemorrhages (white arrowheads) in bilateral thalami and the pons.
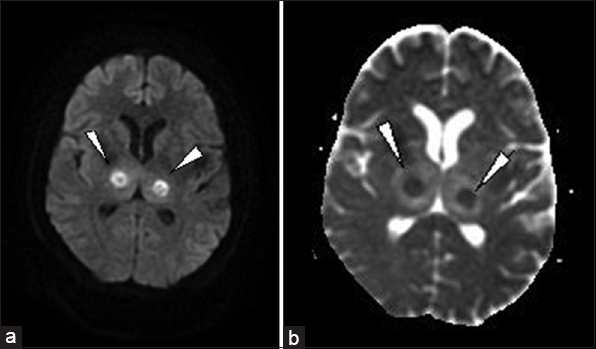
- 23-year-old female presented with headache and fever was diagnosed with acute necrotizing encephalopathy. (a) Axial diffusion and (b) apparent diffusion coefficient images show restrictions (arrowheads) representing necrosis and hemorrhages. Note the areas of hemorrhage are small compared to necrosis on diffusion images, suggesting coexisting hemorrhage and necrosis.
Intubation and mechanical ventilation was done in view of shallow breathing and profound alteration of sensorium. Pending the blood culture report, the patient was started empirically on IV ceftriaxone (rocephin). Signs and symptoms of raised intracranial pressure were present for which she received 3% NaCl (up to 1.5 ml/kg/h).
The patient was provided supportive care. Unfortunately 3 days later, the patient expired.
DISCUSSION
ANE is a disease entity seen in young, previously healthy children and starts with high fever, seizures, and rapid progressing neurologic disturbances. The etiology of ANE is unknown. Influenza A virus, mycoplasma, HSV, and human herpes virus-6 have been reported as common causative agents, directly or through an immune-mediated mechanism. It is believed that some virus or its variant causes rapid development of intracranial cytokine formation which causes blood–brain barrier damage in particular regions of brain resulting in localized edema, congestion, and hemorrhage, without any signs of direct viral invasion or post-infectious demyelination.[12] Most of the reported cases are related to influenza-associated infection.
The disease usually affects young children of both sexes. It manifests as acute encephalopathy following 2–4 days of fever and minor symptoms of the respiratory and/or gastrointestinal systems.[345] The clinical diagnostic criteria of ANE include seizures, fever, vomiting, coma, and increased liver size with abnormal laboratory findings of liver dysfunction, renal disease (increased renal parameters) and hypoproteinemia, with increased CSF proteins and radiologically multifocal, symmetric brain lesions affecting the bilateral thalami, cerebral periventricular white matter, brainstem tegmentum, or cerebellum. Pathologically, these lesions are characterized by the presence of necrosis and hemorrhages.[34]
On MRI, the lesions are hypointense on T1-weighted images and hyperintense on T2-weighted and FLAIR images. When hemorrhage and necrosis are present, the lesions have mixed signal intensity. On gradient images, “blooming” may be present representing hemorrhage. Occasionally, on contrast, faint ring enhancement may be noted around necrosis. Lesions are typically multifocal and symmetric affecting the bilateral thalami, and/or cerebral periventricular white matter, brainstem tegmentum, or pons and cerebellum.[6] On diffusion imaging, concentric areas of high signal in the periphery representing cytotoxic edema with low ADC values (range of greater than 114 × 10−3 mm/s) and low signal in the center representing necrosis with high ADC values (range of less than 45 × 10−3 mm/s) are observed. Diffusion imaging also predicts the extent of neural damage in the disease.[7]
Wong et al., devised an MR prognostic scoring system based on the structures involved, presence or absence of cavitation/necrosis, and presence or absence of hemorrhage.[8] A point was given to each of the above findings and a comprehensive score was calculated. A score of 1 was assigned as good, 2 as moderate, 3 as poor, and 4 as worse than death. Based on the above scoring system, our patient was assigned a score of 4 on initial imaging.
Some diseases, such as Reye's syndrome, vascular occlusion, tumor, hemorrhage of the thalamus, metabolic disorders such as Sandhoff disease, Leigh and Wernicke encephalopathies, methyl l malonic acidemia, glutaric aciduria, carbon monoxide poisoning, acute disseminated encephalomyelitis (ADEM), and acute hemorrhagic leukoencephalitis may have similar clinical, radiological, or pathological findings and have to be considered in differential diagnosis.[349]
Of these, the metabolic disorders are differentiated based on clinical and biochemical investigations. However, it is difficult to differentiate ANE from Reye's syndrome and ADEM. Reye's syndrome is associated with metabolic alterations such as hypoglycemia and hyperammonemia, and ANE is usually associated with antecedent diarrhea and increased CSF protein compared to Reye's syndrome. Radiologically, the lesions in ADEM are asymmetric in distribution, and usually not associated with necrosis and hemorrhage compared to ANE. Acute hemorrhagic leukoencephalitis is asymmetric in distribution, has perivascular distribution, and is associated with meningeal inflammation compared to ANE.[349] In our patient, there was no suggestion of these diagnoses either clinically or radiologically.
The prognosis of ANE is usually poor. Focal neurologic deficits are common sequelae. The presence of hemorrhage and localized tissue loss on MRI usually suggests a poor prognosis. Okumura et al., concluded that administration of steroid within 24 h after the onset of symptoms was related to better outcome in patients with ANE without brainstem lesions.[10] So, early steroid treatment may be an important treatment option. In our patient, steroids were not given as the patient had brainstem lesions and diagnosis was made after 24 h.
CONCLUSION
Diagnosis of ANE is based on a combination of clinical and neuro-radiologic features. It is mostly a disease of children, but can occasionally occur in adults. MRI helps in diagnosis and a scoring system helps in predicting the prognosis of the disease.
Available FREE in open access from: http://www.clinicalimagingscience.org/text.asp?2015/5/1/20/156117
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- Fatal H1N1-Related Acute Necrotizing Encephalopathy in an Adult. Case Rep Crit Care 2011 2011 562516
- [Google Scholar]
- Detection of influenza virus RNA by reverse transcription-PCR and proinflammatory cytokines in influenza-virus-associated encephalopathy. J Med Virol. 1999;58:420-5.
- [Google Scholar]
- Acute necrotizing encephalopathy of childhood: A new syndrome presenting with multifocal, symmetric brain lesions. J Neurol Neurosurg Psychiatry. 1995;58:555-61.
- [Google Scholar]
- Acute necrotizing encephalopathy of childhood: A novel form of acute encephalopathy prevalent in Japan and Taiwan. Brain Dev. 1997;19:81-92.
- [Google Scholar]
- Concentric structure of thalamic lesion in acute necrotizing encephalopathy. Neuroradiology. 2002;44:489-93.
- [Google Scholar]
- Diffusion weighted imaging findings of acute necrotizing encephalopathy. AJNR Am J Neuroradiol. 2004;25:792-7.
- [Google Scholar]
- Acute necrotizing encephalopathy of childhood: Correlation of MR findings and clinical outcome. AJNR Am J Neuroradiol. 2006;27:1919-23.
- [Google Scholar]
- Toxic and metabolic brain disorders. In: Barkovich AJ, ed. Pediatric Neuroimaging (4th ed). Philadelphia: Lippincott Williams and Wilkins; 2005. p. :76-189.
- [Google Scholar]
- Outcome of acute necrotizing encephalopathy in relation to treatment with corticosteroids and gammaglobulin. Brain Dev. 2009;31:221-7.
- [Google Scholar]






