Translate this page into:
Simultaneous Bilateral Carotid Stenting in a Series of 9 Patients: A Single-Center Experience with Review of Literature
Address for correspondence: Dr. Anand Alurkar, Department of Neurointervetion, King Edward Memorial Hospital, Pune - 411 011, Maharashtra, India. E-mail: anandalurkar@gmail.com
-
Received: ,
Accepted: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Objectives:
Simultaneous bilateral carotid artery stenting (SBCAS) is a challenging procedure, and selection criteria play an important role in determining the final outcome. The aim of the present study was to determine the efficacy and safety of the SBCAS in a series of 9 patients with significant bilateral carotid artery disease (>50% on the symptomatic side and >60% on the asymptomatic side).
Materials and Methods:
The present study is a retrospective study of 9 patients from January 2005 to December 2012 in a tertiary care center. There were 8 males and 1 female in the age range 50 to 75 years and an average mean age of 63 years. Inclusion criteria of the present study were patients with bilateral internal carotid artery stenosis >50% (50 - 99%) in the symptomatic side and >60% in the asymptomatic side as seen on digital subtraction angiography (DSA). SBCAS with use of distal protection device (Spider device, ev3), to prevent intra-procedural embolic migration, was done in all the patients.
Results:
Technical success was achieved in all patients (100%). Post-procedural events in the form of hypotension and bradycardia occurred in 3 patients after the placement of stent on both the sides, in 2 patients after the placement of the first stent, and in 1 patient after the placement of the second stent. We did not encounter any cases of hyperperfusion, which was a concern in these patients. There were no deaths, major or minor strokes, or myocardial infarction either in the post-procedural period (up to 1 month) or on clinical follow-up 3 and 6 months post-treatment.
Conclusion:
SBCAS was an effective and safe alternative treatment method in a select group of patients with bilateral carotid artery disease. It can be considered as a feasible treatment option with acceptable risks.
Keywords
Carotid artery stenting
hyperperfusion
stroke
SBCAS (simultaneous bilateral carotid artery stenting)
INTRODUCTION

Carotid angioplasty with or without stenting is a minimally invasive alternative to endarterectomy. The percutaneous transluminal angioplasty (PTA) and carotid artery stenting (CAS) have largely emerged as the treatment option over the past decade. Bilateral simultaneous carotid artery stenting done in the same session had been reported in the literature. In our present study, we want to discuss the safety and efficacy of the simultaneous bilateral carotid artery stenting (SBCAS) in a series of 9 patients. There are limited number of series in the literature that have evaluated the details of the SBCAS.[1–3] We present the first case series of SBCAS from India in our study. We aim to highlight the selection criteria, clinical presentation, the treatment strategy, and the clinical outcome in these patients.
MATERIALS AND METHODS
A total of 308 patients underwent carotid artery stenting in our institution from January 2005 to December 2011. Among them, 32 (10.3%) patients had significant bilateral carotid artery disease. Inclusion criteria of the present study were patients who on digital subtraction angiography (DSA) showed bilateral carotid artery stenosis >50% in the symptomatic side and >60% in the asymptomatic side. The criteria were based on the measurement using NASCET (North American Society carotid endarterectomy trial) criteria, where smallest luminal diameter at the level of stenosis was compared to the normal arterial diameter distal to the stenosis on DSA. The inclusion criteria were similar to the eligibility criteria of the CREST trial. According to our inclusion criteria, simultaneous bilateral carotid artery stenting was performed on 9 patients (2.9% of total cases). The study included 8 males and 1 female (ages range: 50-75 years; average age: 63 years). Patient symptoms ranged from recurrent TIAs lasting for a few minutes to hemiparesis [Table 1]. All the patients in the series had a history of at least one significant medical illness (diabetes mellitus (DM), hypertension (HT), ischemic heart disease (IHD)). Magnetic resonance imaging (MRI) with magnetic resonance angiography (MRA) was done in all the patients. Since time of flight MRA overestimates the stenosis, the inclusion criteria were only based on the DSA findings. The exclusion criteria of our present study, in spite of presence of significant bilateral carotid artery disease, included deranged renal function (serum creatinine >2 mg%) and compromised cardiac reserve (ejection fraction of <40% on 2D-echocardiogram), and severely tortuous anatomy of the vessels. However, in 1 patient among the 9 cases selected, we went ahead with the procedure in spite of morbid obesity, orthopnea, and deranged cardiac function (ejection fraction of 30%) since the patient presented with severe triple vessel disease requiring early coronary artery bypass graft (CABG) and had an increasing frequency of recurrent bilateral transient ischemic attacks. High-risk consent forms with explanation of benefits versus risks were signed by the patient. This patient also underwent CABG at a later date. All the patients in the series underwent treatment within 1 week of the onset of the most recent episode of TIA or stroke.
Clinical presentation
All the patients in the present series had symptomatic lesion at least on one side. Five patients among the 9 presented with bilateral recurrent anterior circulation transient ischemic attacks (TIAs) from a period ranging from 1 week to 6 months. Two of the patients (patient no. 1 and 4 in Table 1) had recurrent right-sided upper limb paresthesias and 2 others had recurrent left-side weakness (patient no. 2 and 8). MRI (T1-weighted sequence, T2-weighted sequence, diffusion-weighted imaging (DWI) with apparent diffusion coefficient (ADC) and gradient echo) and MRA with 3D time of flight was done in all the patients. Small foci of diffusion restriction suggesting acute infarcts in bilateral watershed territories were seen in 2 patients. Tiny acute infarcts were seen in left MCA territory in 2 patients, and similar foci were seen on the right side in 2 others. A chronic right MCA infarct (<1/3 of the territory) with no acute foci on DWI was seen in 1 patient (patient no. 7). MRI of the brain parenchyma was normal in 2 patients who presented with recurrent right-sided TIAs. High-risk informed consent was obtained from all patients.
Pre-treatment standard double anti-platelet regimen was given in all the cases. All the patients were on clopidogrel 75 mg/day and enteric-coated aspirin 150 mg/day for at least 10 days prior to the procedure. Anti-hypertensive medications, if any, were not stopped on the morning of the procedure.
Treatment
All the procedures were done under local anesthesia with careful hemodynamic monitoring except in 1 patient, in whom the procedure was done under general anesthesia. This patient had severe orthopnea due to morbid obesity and low ejection fraction (30%).
An 8 F right femoral access was secured, and guiding catheter (Vistabrite MPS) was placed in the common carotid artery (CCA). Systemic heparinization was given to maintain the activated clotting time >250 seconds. Baseline angiography was done, and stenoses were measured on both the sides by NASCET criteria, and intracranial circulation was evaluated. The stenosis was crossed with 0.014 wire. Emboli protection device (spider, ev3) was used in all patients. Predilation of the lesion prior to carotid stenting was based on the operator's discretion and was done in 4 out of 18 lesions. Subsequently, 8 × 6 × 40 mm self-expanding stent stent (Protege, ev3) was deployed across the lesion. Stenting was first done on the “dominant” side, which was the symptomatic side at the time of presentation. In case of bilateral TIAS, carotid stent was first placed on the side with more severe blood flow limitation. Post-dilation was strictly avoided after the placement of the first stent in all the cases, in order to minimize the baroreceptor response. Post-dilation after the placement of the second carotid stent was done in 3 of the 9 patients where the residual lesion was >30%. Post-procedure angiogram was done to evaluate the flow across the stented segment. Increased antegrade cerebral circulation was documented on the cerebral angiogram [Figures 1–3].
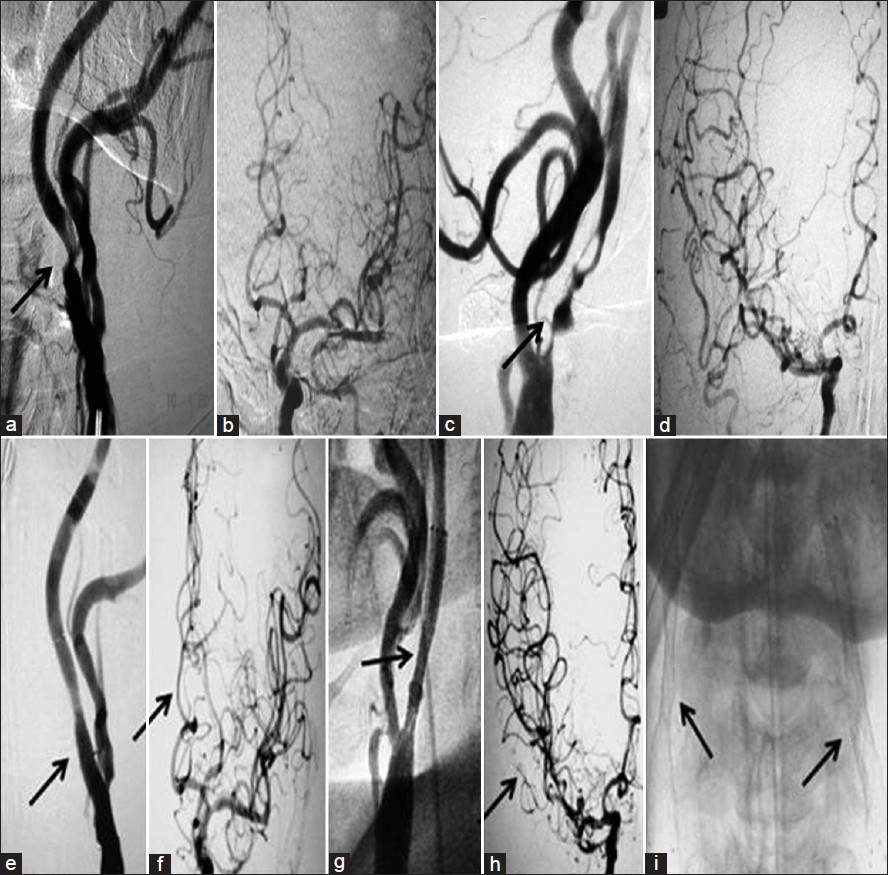
- 60-year-old male patient with recurrent-sided TIAs (Patient 4 in the table) (a) DSA shows 85% narrowing of left ICA with (b) decreased antegrade cerebral circulation. (c) DSA of right CCA shows critical stenosis of the right ICA with (d) reduced antegrade flow in the cerebral circulation. (e) Post-procedure right CCA angiogram shows good glow across the stented segment with (f) increased antegrade circulation. (g) Post-procedure right CCA angiogram shows good flow across the stented segment with (h) improved intracranial circulation, and (i) AP view shows stents on both the sides
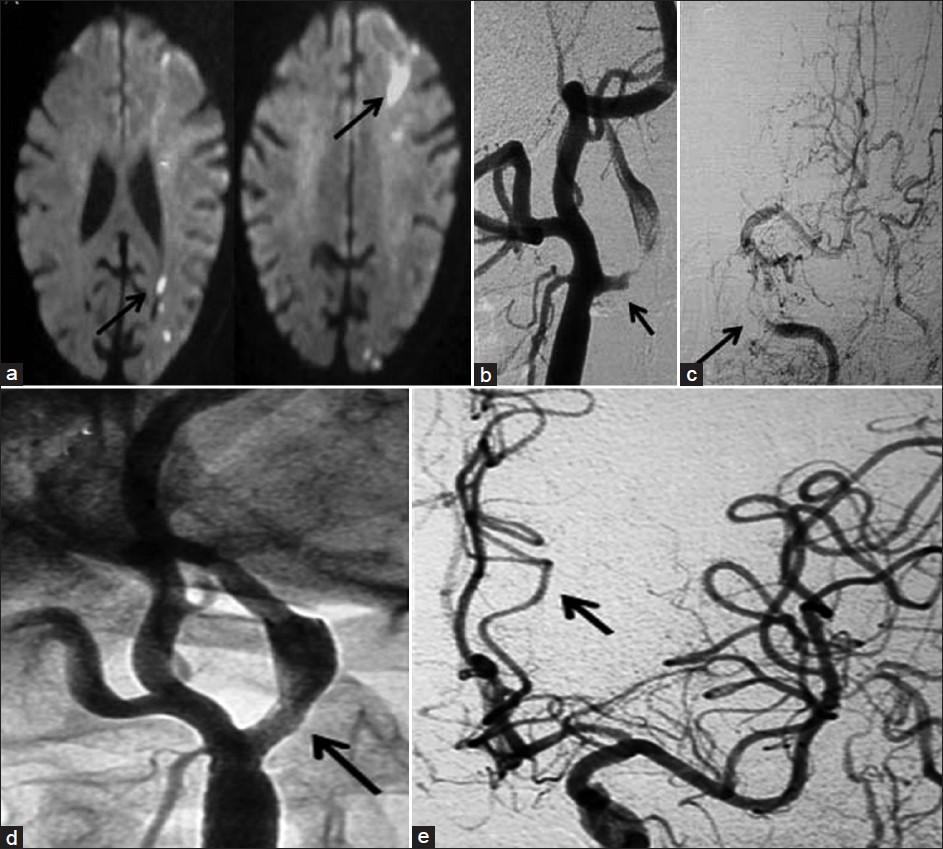
- 55-year-old man who presented with recurrent right sided TIAs (Patient 1 in the table) (a) MR DWI shows acute infarcts in the left MCA terrritory. (b) DSA of Left CCA shows critical stenosis of left ICA and (c) poor antegrade flow in the cerebral circulation (d) Post-procedure left CCA angiogram reveals the patent stented segment (e) with good antegrade flow in cerebral circulation.
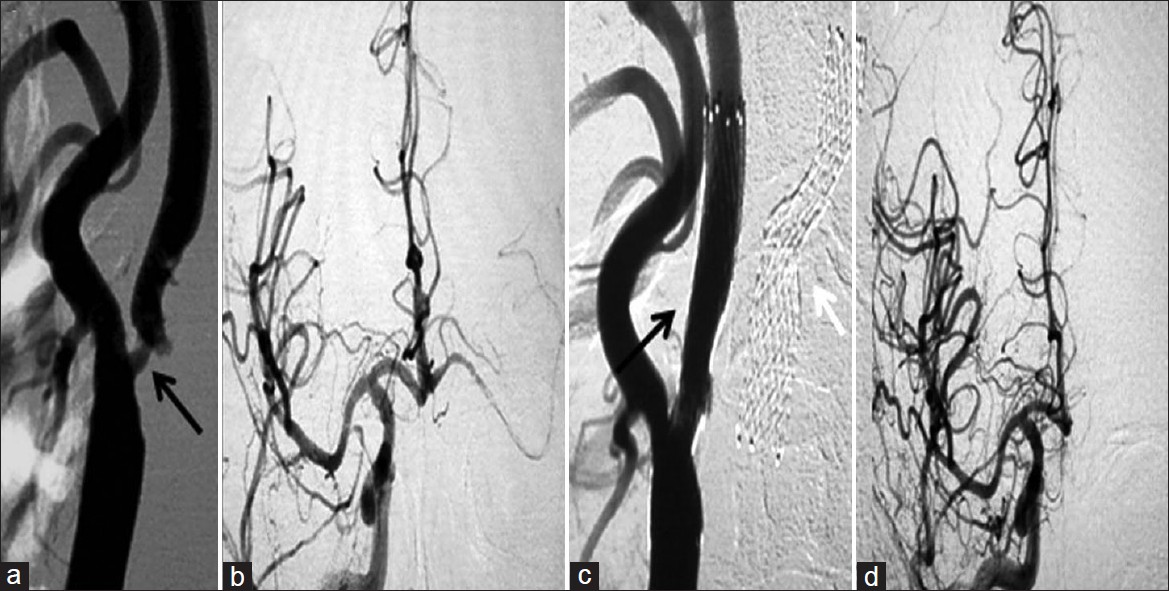
- (a) Pre-procedure right CCA angiogram of the same patient (patient 1 in the table) shows severe narrowing of the left ICA, and (b) right cerebral angiogram shows decreased flow. (c) Post-procedure left CCA angiogram shows good flow across the stented segment and (d) shows increased antegrade flow in the cerebral circulation. The stent on the left side is also seen (white arrow in C)
All patients were monitored in the neurointensive care unit after the procedure. Majority of the patients (6 out of the 9) required infusion of dopamine or noradrenaline to treat hypotension due to baroreceptor response during stent/balloon deployment. The mean arterial pressure (MAP) was maintained between 80 to 90 mm of Hg on the infusion. The patients were shifted out of neurointensive care unit after the infusion was stopped, which was 24 to 48 hours in all the cases.
Patients were also closely monitored for headache, vomiting, seizures, and any fresh neurological deficit suggesting cerebral hyper perfusion.
RESULTS
Technical success was achieved in all patients (100%). Post-procedural transient events in the form of hypotension and bradycardia occurred in 3 patients after the placement of one stent on both the sides, in 2 patients after the placement of first stent, and in 1 patient after the placement of second stent. This was treated with intra-procedure atropine and post-procedure dopamine (5 - 15 μg/kg/min) or noradrenaline (2 - 4 μg/min) infusion for 6 - 36 hours to maintain the mean arterial pressure (MAP) at 80 to 90 mm Hg. However, none of our patients had any minor or major post-procedural complications, such as minor or major stroke, hyperperfusion, etc., patients were discharged on the fifth day on double anti-platelet regimen. We did not encounter any cases of hyperperfusion, which was a theoretical concern in these patients. There were no deaths, major or minor strokes, or myocardial infarction either in the peri-procedural period (up to 1 month), or on clinical follow-up after 3 and 6 months, in our series. Carotid Doppler imaging at follow-up after 6 months showed good antegrade flow across the patent stent in all 18 stented segments.
Out of the 9 patients, 1 patient was lost to follow-up. All the other 8 patients are being regularly monitored every 6 months, and none of these patients have had recurrent symptoms.
DISCUSSION
This is the first reported series of simultaneous bilateral carotid artery stenting in a select group of patients from India. We aim to discuss the importance of selection criteria, meticulous hemodynamic monitoring, and technical modification, such as strict avoidance of post-dilation after the placement of first stent and compulsory use of protection devices, and the technical modifications used to minimize the risk of occurrence of adverse events in these patients that were different from other published studies. Al-Mubarak et al., first reported a series of 5 patients who underwent simultaneous bilateral carotid stenting because of recurrent re-stenosis following carotid endarterectomy.[1] There are a few series reported in the literature about simultaneous bilateral carotid stenting.[23] Bilateral carotid artery stenosis is known to be treated by staged stent procedure since it is considered as high risk factor for carotid endarterectomy.[4] The advantage of simultaneous bilateral carotid artery stenting over staged carotid artery stenting had been described in the literature.[5–8]
The theoretical risks concerning SBCAS mainly include occurrence of hyper perfusion and excessive hemodynamic depression because of activation of bilateral carotid sinus reflex. We did not encounter any incidence of hyper perfusion syndrome (HPS) in our patients, which was mainly due to the meticulous hemodynamic monitoring and excellent post-procedure neurointensive care with careful blood pressure monitoring. Since cerebral auto regulation takes several days to normalize after revascularization, after discharge, patients were counseled regarding immediate reporting of onset of any new symptoms such as headache, vomiting, seizures, etc., (suggesting hyperperfusion). Careful monitoring of blood pressure is required for at least 1 month after SBCAS in order to avoid HPS.[9]
We believe that selection criteria of the patients for SBCAS among the patients with bilateral CAD play an important role in affecting the outcome of the procedure. The selection criteria were similar to the eligibility criteria, based on which patients were taken in the carotid revascularization endarterectomy vs. stenting trial (CREST), which was the recent randomized control trial, credentialing and training process of the same is the most rigorous reported to date and may serve as a model for future trials. Our selection criteria of the lesions were based on the same inclusion criteria as those of the CREST trial. The eligibility criteria in CREST trial included patients with >50% stenosis in symptomatic patients and >60% stenosis in asymptomatic lesions detected on angiography. In 1 of our patients (patient no. 3 in Table 1), who had triple vessel disease (EF =30%) and increasing frequency of recurrent transient ischemic attacks, decision was taken in favor of SBCAS in order to avoid delay of the CABG to a later date. Gabriella Visconti et al., described a case of simultaneous hybrid revascularization by CAS followed by immediate CABG in a patient with a severe coronary artery disease and bilateral carotid artery disease.[10] The major concern of staged CAS -CABG is double anti-platelet regimen (ecospirin 150 mg and clopidogrel 75 mg), which may increase the risk of peri-procedural bleeding. Chiarello et al., in their series[1112] suggested that CABG can be done soon after CAS is performed with single anti-platelet therapy (aspirin) and with loading of clopidogrel in the intensive care unit, when surgical bleeding had definitely stopped. In our present patient with severe co-existing coronary artery disease, CABG was done 1 week after SBCAS with a successful outcome.
Liu et al.,[13] in their series of 30 patients of SBCAS found higher incidence of HPS than in unilateral stent placement group (P = 0.036). They described the disadvantages of staged intervention, such as higher cost and delay of life-saving treatment procedures or occurrence of new cerebral infarction in unilateral CAS and concluded that outcome in their series after SBCAS revealed no significant difference compared with those of unilateral stent placement at 30 days and 6 months follow-up. We did not encounter any incidence of HPS in any of the patients in our series, and we agree with these authors regarding the safety and efficacy of SBCAS with good outcome in selected patients.
The second important major concern regarding SBCAS is carotid sinus reaction triggering hypotension and bradycardia. Franz leisch et al.,[14] studied in detail the carotid sinus reaction during CAS in a study of 108 patients during a 2-year period. In their experience, carotid sinus reaction occurred in 42 (40%) patients who underwent unilateral CAS (hypotension defined as systolic blood pressure <90 or bradycardia i.e. heart rate <50 beats/minute). In our study, incidence of CSR causing HD (hemodynamic depression) in SBCAS was higher (66%) than that in the reported series of unilateral CAS.[15–18] But, none of our patients had any adverse effects due to this, probably because post-dilatation was strictly avoided after the first stent and all the 6 patients were aggressively managed with intra-procedure atropine, intra- and post-procedure dopamine infusion. None of our patients had any post-procedural effects because of the HD, usually considered a common and benign event,[13] which does not increase the procedural risk of CAS.
The main limitation of the present study is that it is a retrospective analysis with a small series of patients. A randomized controlled prospective study is required with large numbers to get more insight about the difference in the outcomes. Additional limitation is that there is no comparison with endarterctomy procedure as was done in CREST trial.
CONCLUSION
Simultaneous bilateral carotid artery stenting (SBCAS) is an elegant method of treatment for bilateral carotid artery disease with acceptable risks in select group of patients. However, a study with large number of patients is required to prove the same in high-risk patients.
Available FREE in open access from: http://www.clinicalimagingscience.org/text.asp?2012/2/1/72/104305
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- Simultaneous bilateral carotid stenting for restenosis after endarterectomy. Catheter Cardiovasc Diagn. 1998;45:11-5.
- [Google Scholar]
- Feasibility of simultaneous bilateral carotid artery stenting. Catheter Cardiovasc Interv. 2004;61:437-42.
- [Google Scholar]
- Is simultaneous bilateral carotid artery stenting safe? J Am Coll Cardiol. 2001;37:1188-217.
- [Google Scholar]
- Staged bilateral carotid stenting, an effective strategy in high-risk patients: Insights from a prospective multicenter trial. J Vasc Surg. 2008;47:1227-34.
- [Google Scholar]
- Bilateral carotid angioplasty and stenting. Catheter Cardiovasc Interv. 2005;64:275-82.
- [Google Scholar]
- Simultaneous bilateral carotid stenting for postendarterectomy restenosis. Catheter Cardiovasc Interv. 2003;58:147-50.
- [Google Scholar]
- Simultaneous bilateral carotid stenting in one session in high-risk patients. J Neuroimaging. 2008;18:252-5.
- [Google Scholar]
- Endovascular intervention for symptomatic bilateral carotid artery stenosis in an octogenarian. Age Ageing. 2007;36:102-4.
- [Google Scholar]
- Intracranial hemorrhage and hyperperfusionsyndrome following carotid artery stenting: Risk factors, prevention, and treatment. J Am Coll Cardiol. 2004;43:1596-601.
- [Google Scholar]
- Simultaneous hybrid revascularization by bilateral carotid stenting and coronary artery bypass grafting. Catheter Cardiovasc Interv 2011:1522-726.
- [Google Scholar]
- Simultaneous hybrid revascularization by carotid stenting and coronary artery bypass grafting: The SHARP study. JACC Cardiovasc Interv. 2009;2:393-401.
- [Google Scholar]
- Simultaneous hybrid revascularization by carotid stenting and coronary artery bypass grafting. Ann Thorc Surg. 2006;81:1883-5.
- [Google Scholar]
- Simultaneous bilateral carotid stenting in high-risk patients. AJNR Am J Neuroradiol. 2010;31:1113-7.
- [Google Scholar]
- Carotid sinus reactions during carotid artery stenting: Predictors, incidence, and influence on clinical outcome. Catheter Cardiovasc Interv. 2003;58:516-23.
- [Google Scholar]
- Frequency and determinants of postprocedural hemodynamic instability after carotid angioplasty and stenting. Stroke. 1999;30:2086-93.
- [Google Scholar]
- Is haemodynamic depression during Carotid stenting a predictor of peri-procedural complications? Eur J Vasc Endovasc Surg. 2008;35:399-404.
- [Google Scholar]
- Periprocedural hemodynamic instability with carotid angioplasty and stenting. Surg Neurol. 2008;70:279-85.
- [Google Scholar]
- Analysis of parameters associated with hypotension requiring vasopressor support after carotid angioplasty and stenting. J Vasc Surg. 2006;43:714-20.
- [Google Scholar]






