Translate this page into:
Radiologic-Pathologic Correlation: Metastatic Pulmonary Epithelioid Hemangioendothelioma of Bone Primary
Address for correspondence: Dr. Christine U Lee, Department of Radiology, Mayo Clinic, 200 First Street SW, Rochester - 55905, Minnesota, USA. E-mail: lee.christine@mayo.edu
-
Received: ,
Accepted: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Epithelioid hemangioendothelioma is a rare vascular malignancy often characterized by a clinically indolent course and delayed diagnosis. The authors present the radiologic and pathologic features of a case of pulmonary epithelioid hemangioendothelioma which was initially thought to be calcified granulomas.
Keywords
Calcified pulmonary nodules
epithelioid hemangioendothelioma
epithelioid vascular tumors
intravascular bronchioloalveolar tumors

[SHOW_RELATED_PUBMED_ARTICLES]

INTRODUCTION
Epithelioid hemangioendothelioma (EHE) is a rare low-to-intermediate grade vascular malignancy that occurs in multiple organs and tissues with a predilection for soft tissue of the extremities, bone, liver, and lung. It can be considered part of the morphologic spectrum of epithelioid vascular tumors, encompassing benign epithelioid hemangioma, low-to-intermediate grade EHE, and high-grade epithelioid angiosarcoma, with EHE being histologically distinct.[1] The primary sites observed in a recent cohort of 39 EHE cases included lung in 18%, bone in 15%, liver in 5%, and the remainder in other soft tissue sites, with about 21% localized to the head and neck.[2] Pulmonary EHE is rare with small-numbered case series in the literature[345] since its first description as “intravascular bronchioloalveolar tumors” nearly 40 years ago.[6] It has no characteristic presentation, but a predilection for middle-aged females (mean age of 40.1 ± 17.5 years).
Although the biological behavior of EHE is typically more indolent than angiosarcoma, metastases occur in 20–30% of cases and the 5-year survival approaches 60%.[7] Significant independent risk factors in patients with pulmonary EHE include pleural effusion, pulmonary symptoms, weight loss, and anemia, with significantly worse survival in patients with hemorrhagic symptoms such as hemoptysis and hemorrhagic pleural effusions.
Given its rarity, relatively slow growth, and nonspecific clinical and radiologic findings, EHE requires confirmatory tissue diagnosis. Typical clinical presentation is an indolent course with delayed diagnosis and several non-definitive intervening diagnostic tests. Treatment of pulmonary EHE is on a case-by-case basis with considerations of surgical resection, chemotherapy including hormonal therapy, radiotherapy, and lung transplantation.
A 61-year-old woman presented to our institution for definitive treatment of recently biopsy-proven EHE of the vertebral bodies and ribs. This patient is a lifelong nonsmoker who lives in an area where sequelae of prior granulomatous disease of the lungs are relatively common. Her clinical history started 6 years ago with empiric treatment for progressive thoracic back pain suspected to be neuropathic in origin. Her symptoms persisted and further work-up 3 years later revealed radiographic and computed tomography (CT) abnormalities in the left posterior 8th and 9th ribs; bilateral pulmonary nodules were described as bilateral calcified granulomas. Biopsy of the 9th rib showed Streptococcus bovis osteomyelitis, for which she was treated with surgical debridement and antimicrobial therapy. Over the last year, her pain worsened in the same region, and imaging revealed a soft tissue mass at the T7–T10 vertebral bodies and extending to the ribs; this was biopsied and pathology showed EHE. On presentation to our institution, additional imaging was performed to re-evaluate and determine if surgical resection with perioperative radiotherapy for local control was a viable option. This article focuses on the pulmonary radiologic and pathologic findings which ultimately determined clinical management.
RADIOLOGIC FEATURES
A non-contrast enhanced CT revealed multiple bilateral lobulated variable-sized pulmonary nodules, many with coarse calcifications [Figure 1a]. The largest nodule measured about 14 mm in the left upper lobe and showed very minimal enlargement compared to prior CT. Positron emission tomography/computed tomography (PET/CT) demonstrated minimal, if any, fluorodeoxyglucose (FDG) uptake of the pulmonary nodules [Figure 1b] and moderate FDG uptake of the rib lesions.
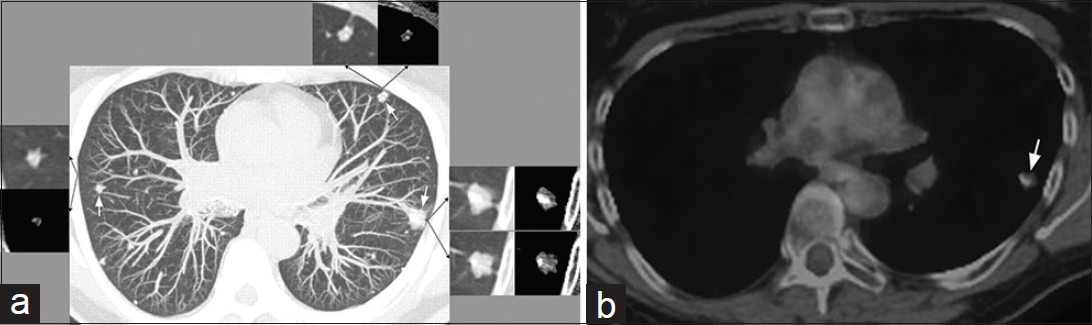
- 61-year-old woman with endothelial hemangioendothelioma presented for consideration of surgical resection, but was found to have pulmonary metastases which were previously thought to be calcified granulomas. (a) Non-contrast enhanced maximum intensity projection CT image shows several bilateral variable-sized peripheral-predominant nodules (white arrows pointing to the three largest nodules). Lung and mediastinal soft tissue window-levels of the three largest nodules are shown (black arrows point to the corresponding lung and mediastinal soft tissue window-levels). These larger nodules are associated with an irregularly marginated soft tissue component and eccentric calcifications. (b) PET/CT of the largest pulmonary nodule in the left upper lobe demonstrates minimal, if any, FDG uptake (arrow). This nodule in the left upper lobe was subsequently biopsied with the pathologic diagnosis of EHE.
A literature review of 93 cases of pulmonary EHE described the usual radiologic feature of pulmonary EHE as bilateral pulmonary nodules of variable size ranging from 5 to 15 mm, but generally less than 20 mm.[7] A study evaluating CT features of 178 nodules or masses in six patients with pulmonary EHE showed a subpleural (93% within 2 cm from the pleura) and perivascular (100%) predilection, with typical pulmonary EHE appearing as multiple irregular nodules with punctate calcifications (90% were <10 mm in diameter with the largest nodule or mass in each patient having a mean size of 27 mm).[8]
Multiple calcified pulmonary nodules most commonly arise as sequelae of prior granulomatous diseases, such as histoplasmosis, and less often from healed varicella pneumonia and tuberculosis. Other differential considerations of calcified pulmonary nodules with variability in distribution, occurrence, and association with calcified lymph nodes include pneumoconioses such as coal workers’ pneumoconiosis and silicosis, secondary hyperparathyroidism, hamartomas, and metastatic diseases such as those from osteosarcoma, colon cancer, or those in association with chronic renal failure, hyperparathyroidism, sarcoidosis, and multiple myeloma.
Radiologic clues that may favor etiologies other than infectious or inflammatory include the irregular morphology of the soft tissue component of the pulmonary nodules as well as their distribution. In this case, the distribution was predominantly subpleural and perivascular. The pattern of solitary pulmonary nodule calcifications can be useful in refining the differential diagnosis with “popcorn”, “central”, “concentric”, or “homogeneous or diffuse” patterns of calcifications being classically benign. Other patterns of calcifications are more indeterminate and raise the suspicion for malignancy. The largely eccentric pattern of calcifications in the larger nodules in this case could represent engulfed adjacent calcified granulomas; however, this was not observed in the biopsy material.
Associated non-pulmonary findings such as the expansile lytic lesions in the left posterior 8th and 9th ribs [Figure 2] and the incidentally detected hepatic mass [Figure 3] in this case may provide additional clues to consider another differential diagnosis. While the generally nonspecific imaging features of EHE in the lungs are also true in the bone and in the liver, the multi-system distribution of the disease would be consistent with a vascular process.
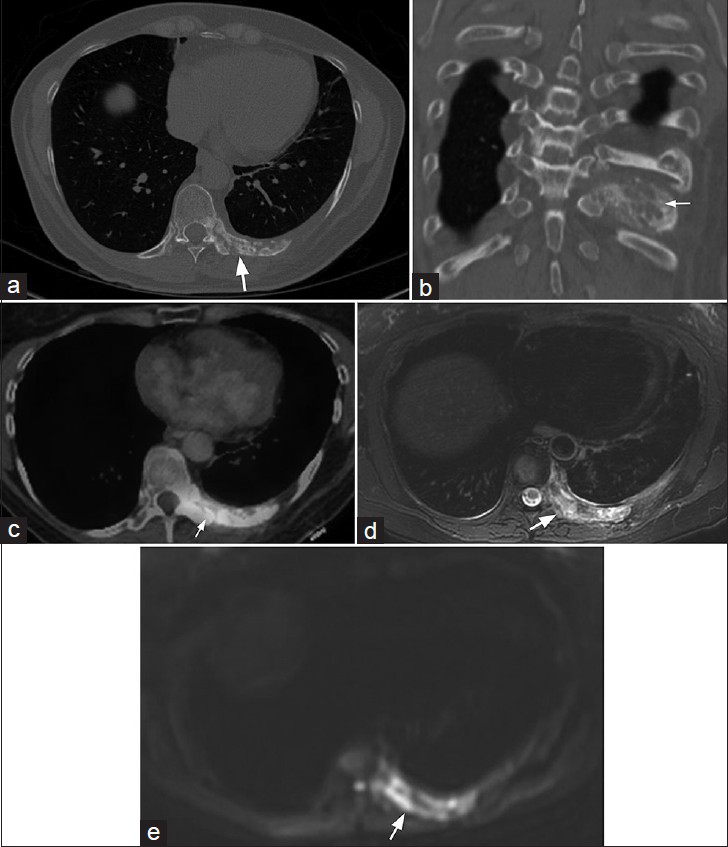
- 61-year-old woman with endothelial hemangioendothelioma presented for consideration of surgical resection, but was found to have pulmonary metastases which were previously thought to be calcified granulomas. (a) CT of the chest in the axial plane and (b) CT of the chest reformatted in the coronal plane with bony window-level settings of the left 9th rib show predominantly lytic lesions with osseous expansion of the posterior left rib(s), and associated sclerosis with extension to the articulating thoracic vertebral body and pedicle (arrows pointing to the rib lesion). (c) PET/CT demonstrates moderate FDG uptake (arrow). (d) Magnetic resonance T2-weighted image and (e) MR diffusion-weighted image of the lower chest included during magnetic resonance imaging of the abdomen show heterogeneous T2 signal hyperintensity and restricted diffusion associated with the rib involvement (arrows). This had been previously biopsied and shown to be EHE.
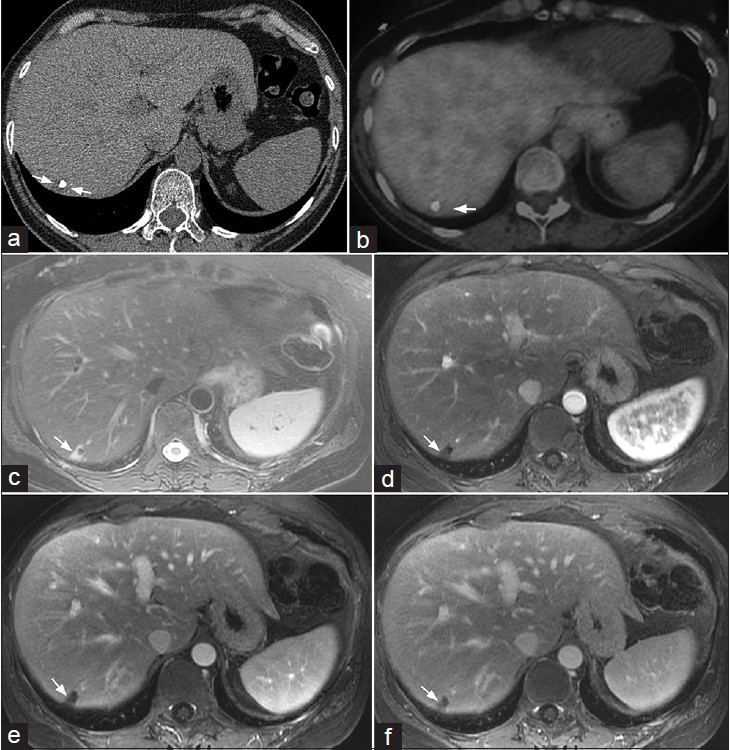
- 61-year-old woman with endothelial hemangioendothelioma presented for consideration of surgical resection, but was found to have pulmonary metastases which were previously thought to be calcified granulomas. (a) Axial non-contrast enhanced CT demonstrates a low attenuation mass with an associated coarse calcification (arrows delineating the mass). (b) PET/CT shows no appreciable FDG uptake in the peripheral right hepatic lobe. (c) MR T2-weighted image of the liver shows a wedge-shaped peripheral right hepatic lobe mass with increased T2 signal (arrow). (d–f) MR post-gadolinium axial 3D spoiled gradient recalled acquisition in steady state (SPGR) images of the liver acquired during the (d) arterial, (e) portal venous, and (f) delayed phases demonstrate minimal delayed peripheral enhancement of this mass (arrows). Imaging differential considerations include EHE and hemangioma. Biopsy of this mass was not performed.
PATHOLOGIC FEATURES
Review of prior rib biopsies concurred with nonspecific chronic inflammation. A CT-guided biopsy of the largest pulmonary nodule demonstrated a predominantly myxohyaline matrix with embedded bland epithelioid tumor cells arranged in short cords. The neoplastic cells contained occasional intracytoplasmic vacuoles with fragmented red blood cells and intranuclear cytoplasmic inclusions. The neoplastic cells were strongly positive for the vascular markers CD31 and Fli-1, and weakly positive for keratin AE1/AE3 [Figure 4].
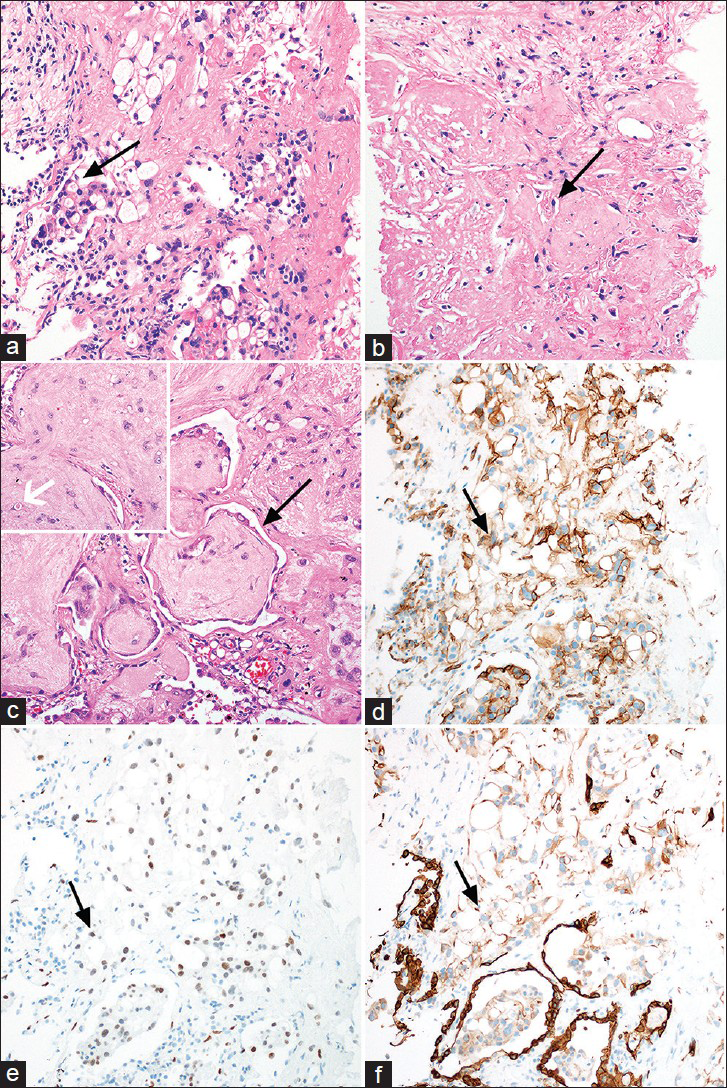
- 61-year-old woman with EHE presented for consideration of surgical resection, but was found to have pulmonary metastases which were previously thought to be calcified granulomas. (a–c) Photomicrographs of the lung mass biopsy, using hematoxylin and eosin (100× magnification) staining, reveal groups and cords of EHE cells with low-grade cytology and frequent intracytoplasmic vacuoles infiltrating the lung parenchyma (a, arrow pointing to an EHE cell with an intracytoplamsic vacuole), and embedded in a hyalinized matrix (b, arrow pointing to an EHE cell embedded within a hyalinized matrix). At the periphery of tumor nodules, EHE displays a micropolypoid growth pattern, in which the tumor protrudes into adjacent alveolar spaces (c, black arrow pointing to the tumor displaying a micropolypoid growth pattern). Occasionally, tumor cells have a fragmented erythrocyte within the intracytoplasmic vacuole (c, inset with white arrow pointing to an EHE cell containing a fragmented erythrocyte within an intracytoplasmic vacuole). tissue stained for (d) vascular markers CD31, (e) Fli-1 and (f) keratin AE1/AE3 show EHE cells are strongly positive for the vascular markers CD31 (black arrow pointing to a positive cell in d, 100× magnification) and Fli-1 (e, black arrow pointing to a positive cell, 100× magnification), and are often positive for keratin AE1/AE3 (black arrow pointing to a positive cell in f, 100× magnification), weak staining compared to the strongly staining alveolar epithelium, which is a common diagnostic pitfall.
Microscopically on low power, the growth pattern of EHE often correlates with the lesions seen on chest imaging studies. The tumor frequently forms multiple pulmonary nodules, growing in a “lymphangitic” pattern in close proximity to bronchovascular bundles, interlobular septa, and the pleural surface.[19] Intravascular growth is also common. The tumor nodules are often hyalinized and less cellular centrally. The EHE cells are typically embedded in a characteristic myxohyaline or myxochondroid matrix and are arranged in short cords. Although EHE is a vascular neoplasm, the cells do not form extracellular vascular structures. Instead, some EHE cells have a single intracytoplasmic vacuole that represents an intracytoplasmic vascular lumen. Occasionally, a fragmented red blood cell will be observed within the vacuole.[19] In the lungs, a micropolypoid growth pattern may be observed at the periphery of the tumor nodules, in which plugs of tumor protrude into the adjacent alveolar spaces and bronchioles.[19] The majority of EHE cases have bland nuclei with occasional intranuclear cytoplasmic inclusions; however, intermediate-grade tumors will display increased cellularity, necrosis, and increased nuclear pleomorphism.[10]
Upon immunohistochemistry, the cells of EHE typically express vascular markers such as CD31, Friend Leukemia Integration 1 (Fli1), Ets Related Gene (ERG), and CD34, and up to 40% of the cells express low-molecular-weight cytokeratin or Epithelial Membrane Antigen (EMA).[110] Recently, a subset of both low- and intermediate-grade EHEs has been shown to harbor t (1;3)(p36;q23-25) resulting in the WWTR1–CAMTA1 fusion gene (WW Domain Containing Transcription Regulator 1 and Calmodulin Binding Transcription Activator 1, respectively). This recurrent genetic finding may be useful in differentiating EHE from other entities in the differential diagnosis, since it seems to be specific for EHE based on early studies[10] [Figure 4].
The histopathologic differential diagnosis of EHE is broad. Granulomatous inflammation is often considered because of the central hyalinization and occasional necrosis seen in EHE, and also because EHE cells may resemble epithelioid histiocytes. Another common pitfall is the expression of keratin by EHE, which may lead to consideration of metastatic carcinoma if a lymphangitic or intravascular growth pattern is observed, or consideration of malignant mesothelioma if pleural growth is prominent.[10] EHE can be differentiated from these entities morphologically by careful attention to the characteristic extracellular matrix and intracytoplasmic vacuoles, and strong expression of vascular markers by immunohistochemistry. Finally, epithelioid angiosarcoma should be considered in the differential diagnosis when nuclear atypia, necrosis, and increased mitotic activity are observed. Identification of the characteristic matrix and intracytoplasmic lumen formation favors the diagnosis of EHE, while formation of extracellular vascular structures, papillary growth, blood lakes, and prominent nucleoli favors epithelioid angiosarcoma.[10] In difficult cases, testing for the WWTR1–CAMTA1 fusion may be helpful in differentiating EHE from epithelioid angiosarcoma, which can be performed by Reverse Transcription Polymerase Chain Reaction (RT-PCR) or Fluorescence In Situ Hybridization (FISH).
DISCUSSION
In isolation, the clinical and radiologic features of EHE are nonspecific; however, multi-system involvement can provide a clue to a vascular etiology. This case reflects the usual indolent course of EHE with delayed diagnosis confounded here by the diagnosis of osteomyelitis in the ribs and the initial radiologic interpretation of bilateral calcified granulomas in the lungs. The distinction between pulmonary calcified granulomas and metastatic pulmonary EHE can be radiologically challenging and tissue diagnosis may be needed, as was the case here. Here, osteomyelitis was almost certainly a false-positive in the setting of a pre-existing EHE. To our knowledge, there are no reports describing EHE arising from sites of treated osteomyelitis.
CONCLUSION
In this patient, tentative management of the osseous lesions was initially surgical excision with high probability of significant morbidity, followed by local radiation therapy. After the diagnosis of metastatic pulmonary EHE, however, the treatment approach was changed. She instead underwent local radiation to her ribs with concurrent Taxol treatment. Four-month follow-up PET/CT showed reduced metabolic activity in the left posterior thoracic hemangioendothelioma; the multiple lung nodules previously associated with minimal metabolic activity were relatively unchanged; and there were no new lesions identified.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Available FREE in open access from: http://www.clinicalimagingscience.org/text.asp?2015/5/1/50/163994
REFERENCES
- WHO Classification of Tumours of the Lung, Pleura, Thymus and Heart. In: Bosman FT, Jaffe ES, Lakhani SR, Ohgaki H, eds. WHO Classification. Lyon: IARC Press; 2015. p. :468.
- [Google Scholar]
- Epithelioid Hemangioendothelioma: Clinicopathologic, immunhistochemical, and molecular genetic analysis of 39 cases. Diagn Pathol. 2014;9:131.
- [Google Scholar]
- Pulmonary epithelioid hemangioendothelioma: An unusual case and a review of the literature. Chest. 2004;125:789-93.
- [Google Scholar]
- Clinicopathological characteristics of pulmonary epithelioid hemangioendothelioma: A report of four cases and review of the literature. Oncol Lett. 2014;8:2517-22.
- [Google Scholar]
- Risk factors and independent predictors of survival in patients with pulmonary epithelioid haemangioendothelioma. Review of the literature and a case report. Respirology. 2006;11:818-25.
- [Google Scholar]
- The computed tomographic findings of pulmonary epithelioid hemangioendothelioma. Radiol Med. 2014;119:705-13.
- [Google Scholar]
- Intravascular, bronchiolar, and alveolar tumor of the lung (IVBAT). An analysis of twenty cases of a peculiar sclerosing endothelial tumor. Cancer. 1983;51:452-64.
- [Google Scholar]
- Thoracic epithelioid malignant vascular tumors: A clinicopathologic study of 52 cases with emphasis on pathologic grading and molecular studies of WWTR1-CAMTA1 fusions. Am J Surg Pathol. 2015;39:132-9.
- [Google Scholar]





