Translate this page into:
Quantitative classification of invasive and noninvasive breast cancer using dynamic magnetic resonance imaging of the mammary gland

*Corresponding author: Yoshiaki Miyazaki, Department of Radiological Technology, National Cancer Center, Chuo-ku, Tokyo, Japan. yomiyaza@ncc.go.jp
-
Received: ,
Accepted: ,
How to cite this article: Miyazaki Y, Shimizu J, Kubo Y, Tabata N, Aso T. Quantitative classification of invasive and noninvasive breast cancer using dynamic magnetic resonance imaging of the mammary gland. J Clin Imaging Sci 2022;12:45.
Abstract
Objectives
Breast cancers are classified as invasive or noninvasive based on histopathological findings. Although time-intensity curve (TIC) analysis using magnetic resonance imaging (MRI) can differentiate benign from malignant disease, its diagnostic ability to quantitatively distinguish between invasive and noninvasive breast cancers has not been determined. In this study, we evaluated the ability of TIC analysis of dynamic MRI data (MRI-TIC) to distinguish between invasive and noninvasive breast cancers.
Material and Methods
We collected and analyzed data for 429 cases of epithelial invasive and noninvasive breast carcinomas. TIC features were extracted in washout areas suggestive of malignancy.
Results
The graph determining the positive diagnosis rate for invasive and noninvasive cases revealed that the cut-off θi/ni value was 21.6° (invasive: θw > 21.6°, noninvasive: θw ≤ 21.6°). Tissues were classified as invasive or noninvasive using this cut-off value, and each result was compared with the histopathological diagnosis. Using this method, the accuracy of tissue classification by MRI-TIC was 88.6% (380/429), which was higher than that using ultrasound (73.4%, 315/429).
Conclusion
MRI-TIC is effective for the classification of invasive vs. noninvasive breast cancer.
Keywords
Breast cancer
Invasive ductal carcinoma
Noninvasive ductal carcinoma
Computer-aided diagnosis
Time-intensity curve
INTRODUCTION
Mammography and ultrasonography are the first-line diagnostic imaging methods for breast cancer. Magnetic resonance imaging (MRI) is also an important tool for precise preoperative examination of breast tumors because of its high spatial resolution and ability to evaluate blood flow, provide a qualitative diagnosis, and assess the spread of breast tumors. According to Berg et al. MRI detects malignancies with a sensitivity of over 95% (167/177), except in cases of special tumors and microscopic lesions; this detection rate was higher than that of mammography (68%; 120/177) and ultrasound (83%; 147/177).[1]
The time-intensity curve (TIC) [Figure 1] plots the contrast enhancement effect of dynamic MRI over time and is expected to provide a qualitative diagnosis because it reflects the state of blood flow within the tumor.[2] The diagnostic ability of the TIC to distinguish benign from malignant tumors has been demonstrated in several previous studies.[3-10]
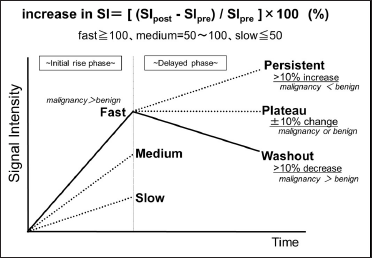
- Overview of the time-intensity curve analysis.
Malignant mammary gland tumors are divided into two types: invasive cancer, in which the cancer cells break through the ducts and lobules to invade the surrounding tissues in the breast, and noninvasive cancer, in which the cancer cells grow only in the ducts and lobules.[11,12]
When cancer cells leave the ducts and lobules of the mammary gland, they may metastasize through the blood and lymphatic vessels, greatly affecting the treatment plan and prognosis for life.
Brennan et al. reported[13] that 25.9% of cases diagnosed as noninvasive breast cancer were misdiagnosed, indicating that invasive breast cancer or other cancers were undetected postoperatively. In addition, Sagara et al. reported[14] that in some “noninvasive cancers,” resection and nonresection revealed characteristics that did not significantly differ in survival rates. In other words, the importance of more accurate differentiation between “invasive” and “noninvasive” cancer as the initial management of breast cancer is extremely higher than in the two reports. However, in MRI, qualitative evaluation of the enhancement effect[2] is the main method for differentiating between invasive and noninvasive lesions, and quantitative evaluation methods have not yet been established.
Therefore, in this study, to evaluate the ability of TIC analysis of dynamic MRI data (MRI-TIC) to distinguish between invasive and noninvasive breast cancers, we extracted TIC features and performed quantitative, pathology-based classification of invasive/noninvasive cancers. Furthermore, we compared the accuracy of MRI-TIC with ultrasonography, which can evaluate blood flow.[15]
MATERIAL AND METHODS
Study design
This retrospective study included data for 429 cases of invasive (364 cases) and noninvasive (65 cases) breast cancers diagnosed and treated from April 2016 to March 2018. The inclusion criteria were as follows:[1] dynamic MRI and ultrasonography were performed,[2] no chemotherapy was administered, and[3] a definite diagnosis of invasive or noninvasive cancer was established by pathological examination of the excised lesion.
A 1.5-Tesla MRI device (Siemens Magnetom Symphony; Erlangen, Germany) with a four-channel breast array coil was used to obtain dynamic MRI fat-suppressed T1-weighted images using the gadolinium contrast agent Magnevist IV (Schering Berlin, Germany) and the following parameters: matrix size, 512 × 256; pixel size, 0.6 × 0.8 mm; slice thickness, 1.0 mm; time to repeat, 5.42 s; time to echo, 2.11 s; flip angle, 20°; bandwidth, 300 Hz/pixel, and parallel imaging generalized autocalibrating partial parallel acquisition (GRAPPA; accel. factor PE 2 and ref. lines PE 50). A Sonic Shot GX device (Nemoto Kyorindo Co., Ltd, Tokyo, Japan) was used to inject the contrast medium. The contrast agent (0.2 mL/kg) was injected at a rate of 2.0 mL/s, and 20 mL of physiological saline was boosted at a rate of 2.0 mL/s.
Dynamic MRI was performed using four-time points: before injection of the contrast agent (prephase), immediately after injection of the contrast agent (injection phase), at 60 s (peak phase), and at 300 s (delay phase). The imaging time was 60 s per phase, and there were 96 images taken per phase (384 images total).
All personal information related to the clinical images used in this study was anonymized, except for the MRI, pathological diagnoses, and tumor locations. This study was conducted in accordance with the Declaration of Helsinki. The Ethics Review Committee of the National Hospital Organization Kyushu Cancer Center approved the utilization of data (Approval no. 2016-52) and waived the need for patient consent because of the retrospective nature of the study.
Figure 2 shows the method for distinguishing between invasive and noninvasive breast cancers using MRI-TIC. We investigated the part of the lesion that showed a washout effect, which suggests malignancy. Figure 3 shows the process of extraction of the region with a washout effect in a breast cancer lesion. Automatic extraction was performed as previously reported.[16]
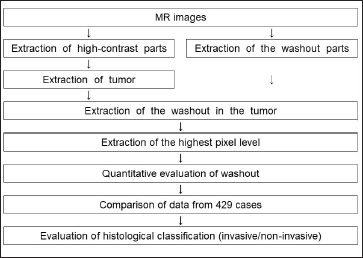
- Flowchart for histological classification of invasive/noninvasive cancer.
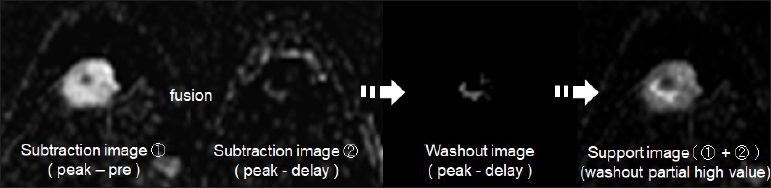
- Extraction of the region with a washout effect in breast cancer using images processed by a computer-aided diagnosis program in dynamic magnetic resonance imaging Invasive breast cancer tissue from a 42-year-old woman with a 1.0 cm mass is shown.
Invasive/noninvasive classification
Images of lesions with features suggestive of malignancy were automatically extracted from the imaged slices using a 2×2-pixel region of interest (ROI). We defined θinvasive/noninvasive (θi/ni) as the angle between straight lines A and B in the TIC (A: a straight line parallel to the time axis and extending from the peak phase, B: a straight line passing through the two points of the peak phase and the delay phase) [Figure 4]. The highest θ value in each case was defined as θwashout (θw), and the classification of invasiveness/noninvasiveness was made according to this value. For all 429 cases, θi/ni was calculated as the borderline value of θw where the pathological diagnosis was most consistent with the MRI-TIC-based classification by θw.
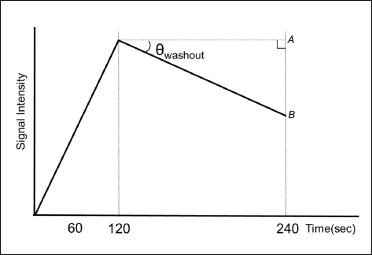
- Definition of θwashout in invasive/noninvasive classification using TIC.
Statistical analysis
All statistical analyses were performed using Excel® 2016 (Microsoft Corporation; Redmond, WA, USA).
Evaluation of the MRI-TIC-based classification by comparison with the postsurgical pathological diagnosis
The sensitivity, specificity, positive predictive value, and negative predictive value for the TIC classification using θi/ni were calculated for each histological type (invasive/noninvasive). In addition, accuracy rates for MRI-TIC, and ultrasound results were calculated.
RESULTS
Case details
The patients were women aged 21-88 years (mean 58.2 ± 12.8 years). The tumor diameters were between 0.6 and 9.2 cm (mean 2.1 ± 1.8 cm). Table 1 shows the detailed classification of the target cases according to the World Health Organization (WHO) Classification of Tumors of the Breast.[11]
| Organizational type | Number of cases | % of cases |
|---|---|---|
| Invasive breast carcinoma | ||
| Invasive breast carcinoma of no special type | 312 | 73.1 |
| Microinvasive carcinoma | 4 | 0.9 |
| Invasive lobular carcinoma | 17 | 4.0 |
| Tubular carcinoma | 1 | 0.2 |
| Cribriform carcinoma | 0 | 0.0 |
| Mucinous carcinoma | 18 | 4.2 |
| Mucinous cystadenocarcinoma | 0 | 0.0 |
| Invasive micropapillary carcinoma | 4 | 0.9 |
| Carcinoma with apocrine differentiation | 6 | 1.4 |
| Metaplastic carcinoma | 2 | 0.5 |
| Noninvasive lobular neoplasia | ||
| Atypical lobular hyperplasia | 0 | 0.0 |
| Lobular carcinoma in situ | 3 | 0.7 |
| Ductal carcinoma in situ | ||
| Ductal carcinoma in situ | 62 | 14.5 |
| Sum total | 429 | 100.0 |
WHO Classification of Tumours Editorial Board. Breast tumors (medicine). 5th ed. Lyon: IARC Press; 2019. p.68-138.
Determination of θi/ni for distinguishing between invasive and noninvasive lesions
For all 429 cases, the signal intensity in the delay phase was reduced compared to that in the peak phase, and a plateau or washout was observed in MRI-TIC. The graph in Figure 5 shows the θw of cases classified as invasive or noninvasive based on the final pathological diagnosis. The θw for all cases ranged from 0.7° to 51.8°, with values of 11.2°-51.8° for invasive and 0.7°-32.1° for noninvasive cases. The relationship between the two was P = 9.7 × 10-52.

- Statistics of θwashout in pathological diagnosis (invasive/noninvasive).
Figure 6 shows the relationship between the positive diagnosis rate at each θw value for cases classified as invasive or noninvasive from pathological diagnoses. For invasive cancer, the positive diagnosis rate showed a gradual decrease from 0° to 20°, after which the slope increased and the positive diagnosis rate decreased. For noninvasive cancer, the positive diagnosis rate reached 52% at 10°, 78% at 20°, and 96% at 30°. The graph determining the positive diagnosis rate for invasive and noninvasive cases revealed that the cut-off θi/ni value was 21.6° (invasive: θw > 21.6°, noninvasive: θw ≤ 21.6°).

- Determination of θinvasive/noninvasive to classify invasive/noninvasive (Discriminant accuracy in θwashout).
Effectiveness of MRI-TIC in distinguishing invasive and noninvasive breast carcinoma
Table 2 shows the classification of breast cancer tumors using the θi/ni cut-off value of 21.6° for invasiveness/noninvasiveness, which was the most consistent with the pathological diagnoses. The sensitivity, specificity, positive predictive value, and negative predictive value for each tissue are shown in Table 3; these values were 89.3%, 84.6%, 97.0%, and 58.5%, respectively, for invasive tumors and 84.6%, 89.3%, 58.5%, and 97.0%, respectively, for noninvasive tumors. The accuracy of discrimination between invasive and noninvasive cancers using MRI-TIC was 88.6%. Figure 7 shows the classification of invasive (θi/ni > 21.6°) and noninvasive (θi/ni ≤ 21.6°) breast carcinoma using MRI-TIC and 4-pixel pathological images (size of the ROI, 1.2×1.6 mm).
| Pathological diagnoses | θinvasive/noninvasive ≤ 21.6° (noninvasive) | θinvasive/noninvasive > 21.6° (invasive) |
|---|---|---|
| Invasive breast carcinoma: 364 | 39/364 | 325/364 |
| Noninvasive breast carcinoma: 65 | 55/65 | 10/65 |
| Sensitivity | Specificity | Positive predictive value | Negative predictive value | Discriminative predictive value | |
|---|---|---|---|---|---|
| Invasive breast carcinoma: 364 | 89.3 | 84.6 | 97.0 | 58.5 | 88.6 |
| Noninvasive breast carcinoma: 65 | 84.6 | 89.3 | 58.5 | 97.0 |
Values shown are percentages

- Classification of invasive/noninvasive breast carcinoma using time-intensity curve analysis of magnetic resonance imaging data and pathological images The size of the region of interest (4 pixels:1.2 × 1.6 mm) is the same as the size of the pathology image.
Comparison of MRI-TIC and ultrasonography
The results of MRI-TIC, and ultrasonography are compared with the pathology results in Table 4. For invasive carcinoma (364 cases), the diagnoses for 325 and 280 cases analyzed by MRI-TIC and ultrasound, respectively, were consistent with the pathological diagnoses. For noninvasive carcinoma (65 cases), the diagnoses for 55 and 35 cases analyzed by MRI-TIC and ultrasound, respectively, were consistent with the pathological diagnoses. The discrimination accuracies of MRI-TIC and ultrasound were 88.6% (380/429) and 73.4% (315/429), respectively, and MRI-TIC was 15.2% (65/429) more accurate than ultrasound in terms of consistency with the pathological diagnosis.
| MRI-TIC results consistent with pathological diagnosis | Ultrasonography results consistent with pathological diagnosis | |
|---|---|---|
| Invasive breast carcinoma: 364 | 325 | 280 |
| Noninvasive breast carcinoma: 65 | 55 | 35 |
| Discriminative predictive value | 88.6% | 73.4% |
TIC: time-intensity curve, MRI: magnetic resonance imaging, Ultrasound results included the first and second differential diagnoses
DISCUSSION
In this study, we developed and validated an algorithm for distinguishing between invasive and noninvasive breast cancers using quantitative MRI-TIC for 429 cases. Classification using MRI-TIC was based on θi/ni, with a cut-off value of 21.6°.
We found that θi/ni > 21.6° indicated invasive cancer and θi/ni ≤ 21.6° indicated noninvasive cancer. They are related by the degree of contrast agent loss after the peak signal intensity, similar to the findings of Kamitani et al.[17] and indicate that the post-peak TIC has a steep trend for invasive cancer and a gradual trend for noninvasive cancer. The difference in θi/ni for invasive vs. noninvasive tumors in this study may be the result of differences in histological construction.
In cases of invasive cancer, as the blood concentration of the contrast agent increases, the contrast agent seeps from the blood vessels into the interstitium, resulting in a strong thick stain; this is due to the high vascular density[18] and increased vascular permeability.[19] The contrast agent that has leaked into the extravascular interstitium is readily expelled into the vasculature as its blood concentration decreases, and its concentration within the tumor decreases. Because there is little interstitium,[11,12] the concentration of contrast agent in the tumor tends to decrease quickly and is likely to exhibit a steep washout curve.
In noninvasive cancers, tumor vessels with increased vascular permeability rarely grow.[19,20] The periductal stroma is wide, and it takes time for the concentration of the contrast agent to increase,[21] resulting in a poor contrast effect early in the imaging process (slow pattern). The periductal stroma gradually gains contrast, but the contrast agent temporarily pools and drains slowly. Largely because of tumor angiogenesis in the interstitium,[20] washout is less likely to occur within the examination time, and a plateau or slow washout is seen. In brief, the differences in TIC characteristics are related to the density and permeability of the tumor vessels (noninvasive < invasive) and the size of the stroma (invasive < noninvasive).
Using the pathological diagnoses as a reference, the accuracy of MRI-TIC in distinguishing between invasive and noninvasive breast cancers in this study (364 invasive and 65 noninvasive breast cancers) was 88.6%. Thus, the TIC results did not completely agree with the pathological diagnoses, possibly because data were collected at only four-time points (prephase, injection phase, peak phase, and delay phase). More time points were not used because the timing technique was not fine-tuned, and this may have caused a discrepancy between the exact peak point and the values at the 2-minute point, which is assumed to be the peak point.[2] The same can also be said for the washout point; this issue should be investigated further in future research.
Another reason for the lack of complete agreement between MRI-TIC and pathological results was that the invasive/noninvasive tissue classification was determined by observation. It is difficult to observe the entirety of the malignant tissues of a lesion, and judgments based on partial observation may lead to bias among observers and discrepancies in diagnostic results. However, the method of classification of invasive/noninvasive cancers in this study did achieve good quantitative results and compensated for possible subjectivity by scanning all imaging data (396 images: 96 images × 4 phases) and extracting the washout areas in the tumors. The usefulness of our method is demonstrated by the fact that MRI-TIC-based invasive/noninvasive classifications were consistent with the pathological diagnoses in 88.6% of the 429 cases.
There was a large difference of more than 15.0% in the discrimination accuracy between ultrasound examination, where the first and second differential diagnoses were considered, and our MRI-TIC method. We believe that there are three reasons for this difference. First, ultrasonography findings are affected by the skill of the operator. Second, it is difficult to image the entire lesion. Third, it is difficult to evaluate the lesion quantitatively.
Accurate diagnosis of invasive/noninvasive breast cancer is thought to play a significant role not only in the evaluation of possible metastasis but also in surgical decisions.[13,14] Since the most accurate information possible from multiple examinations is essential for the diagnosis of breast cancer, this study demonstrates the validity of the quantitative evaluation of invasion/noninvasion in MRI of breast cancer.
We believe that this will help improve pathological diagnosis and will be of great utility in clinical practice.
CONCLUSION
The findings of this study suggest that quantitative MRI-TIC of the mammary glands is effective in distinguishing between invasive and noninvasive breast cancers. We believe that this method can assist physicians in determining the most appropriate treatments for patients with malignant carcinomas.
Declaration of patient consent
Institutional Review Board (IRB) permission obtained for the study.
Financial support and sponsorship
Nil.
Conflict of interest
There are no conflicts of interest.
References
- Diagnostic accuracy of mammography, clinical examination, US, and MR imaging in preoperative assessment of breast cancer. Radiology. 2004;233:830-49.
- [CrossRef] [PubMed] [Google Scholar]
- ACR BI-RADS ATLAS breast imaging reporting and data system In: In: Magnetic Resonance Imaging (5th ed.). United States: American College of Radiology; 2013.
- [Google Scholar]
- Benign and malignant breast lesions: Differentiation with echo-planar MR imaging. Radiology. 1995;197:33-8.
- [CrossRef] [PubMed] [Google Scholar]
- Dynamic breast MR imaging: Are signal intensity time course data useful for differential diagnosis of enhancing lesions? Radiology. 1999;211:101-10.
- [CrossRef] [PubMed] [Google Scholar]
- Differentiation between benign and malignant breast lesions detected by bilateral dynamic contrast-enhanced MRI: A sensitivity and specificity study. Magn Reson Med. 2008;59:747-54.
- [CrossRef] [PubMed] [Google Scholar]
- Cancerous breast lesions on dynamic contrast-enhanced MR images: Computerized characterization for image-based prognostic markers. Radiology. 2010;254:680-90.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- The diverse pathology and kinetics of mass, nonmass, and focus enhancement on MR imaging of the breast. J Magn Reson Imaging. 2011;33:1382-9.
- [CrossRef] [PubMed] [Google Scholar]
- Computer-aided diagnosis for dynamic contrast-enhanced breast MRI of mass-like lesions using a multiparametric model combining a selection of morphological, kinetic, and spatiotemporal features. Med Phys. 2012;39:1704-15.
- [CrossRef] [PubMed] [Google Scholar]
- A novel approach to contrast-enhanced breast magnetic resonance imaging for screening: High-resolution ultrafast dynamic imaging. Invest Radiol. 2014;49:579-85.
- [CrossRef] [PubMed] [Google Scholar]
- Kinetic analysis of benign and malignant breast lesions with ultrafast dynamic contrast-enhanced MRI: Comparison with standard kinetic assessment. AJR Am J Roentgenol. 2016;207:1159-66.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- General rules for clinical and pathological recording of breast cancer (18th ed). Tokyo: Kanehara Shuppan; 2018. p. :24-30.
- [Google Scholar]
- Ductal carcinoma in situ at core-needle biopsy: Meta-analysis of underestimation and predictors of invasive breast cancer. Radiology. 2011;260:119-28.
- [CrossRef] [PubMed] [Google Scholar]
- Survival benefit of breast surgery for low-grade ductal carcinoma in situ: A population-based cohort study. JAMA Surg. 2015;150:739-45.
- [CrossRef] [PubMed] [Google Scholar]
- Role of ultrasonic flow imaging diagnosis in differential classification of Ductal Carcinoma in situ. Japanese J Med Ultrasound. 2014;39:449-57.
- [Google Scholar]
- [Development of a computer-aided diagnosis system to distinguish between benign and malignant mammary tumors in Dynamic magnetic resonance images: Automatic detection of the position with the strongest washout effect in the tumor] Nihon Hoshasen Gijutsu Gakkai Zasshi. 2108;74:251-61.
- [CrossRef] [Google Scholar]
- Dynamic MR mammography. Differences of time-intensity curve among each histology. Jpn J Breast Cancer. 2003;18:240-5.
- [Google Scholar]
- Magnetic resonance imaging of breast cancer: Correlation between contrast enhancement and tumor angiogenesis. Nippon Acta Radiol. 1999;59:682-8.
- [PubMed] [Google Scholar]
- Vascular permeability changes involved in tumor metastasis. Cancer Lett. 2013;335:259-69.
- [CrossRef] [PubMed] [Google Scholar]
- Be active or not: The relative contribution of active and passive tumor targeting of nanomaterials. Nanotheranostics. ;1:346-357.
- [CrossRef] [PubMed] [Google Scholar]
- Ductal carcinoma in situ: MR imaging-histopathologic correlation. Radiology. 1995;196:415-9.
- [CrossRef] [PubMed] [Google Scholar]






