Translate this page into:
Pulmonary Hyalinizing Granuloma Associated with Idiopathic Thrombocytopenic Purpura
Address for correspondence: Dr. Christopher Coleman, Department of Diagnostic Radiology, Mayo Clinic, Mayo 2-131S, 4500 San Pablo Road, Jacksonville, FL 32224, USA. E-mail: Coleman.christopher@mayo.edu
-
Received: ,
Accepted: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Pulmonary hyalinizing granuloma (PHG) is a rare, benign lung disease of unknown etiology. It manifests as discrete, rounded nodules within the lung parenchyma. A 39-year-old woman presented for investigation after pulmonary nodules were found incidentally. Chest computed tomography showed multiple, discrete, non-enhancing pulmonary nodules bilaterally. Positron emission tomography (PET) was negative. Biopsy demonstrated a non-specific lymphoplasmacytic infiltrate. Open resection yielded two nodules consistent with hyalinizing granulomas. The differential for multiple pulmonary nodules is broad. PET scan can help rule out metastatic disease, although some cancers are not hypermetabolic on PET. Furthermore, some non-malignant conditions, including hyalinizing granuloma, can show increased activity on PET. PHG should be included in the differential of multiple pulmonary nodules, especially if nodule stability can be demonstrated and/or needle biopsies are non-diagnostic. Associated immune-mediated conditions, such as idiopathic thrombocytopenic purpura (ITP) in our patient, may also favor HG. In this case report we find an association between PHG and ITP.
Keywords
Hyalinizing granuloma
nodule
pulmonary
INTRODUCTION

Pulmonary hyalinizing granuloma (PHG) is a rare, benign lung disease that was first described by Engleman et al., in 1977.[1] The disease presents as slowly enlarging solitary or multiple nodules, which can simulate metastatic disease.[2] No gender or racial preference has been shown and reported age range of presentation is 17-61 years. Up to 25% of patients with PHG are asymptomatic at the time of presentation.[3] The remainder may present with mild symptoms of chest pain, dry cough, fever, dyspnea, sinusitis, and occasionally hemoptysis. The exact etiology of PHG is unknown, although a proposed mechanism is an exaggerated immune response to the antigenic stimuli associated with various infections and immune-mediated conditions that affect the lung. Associations have also been made with some non-pulmonary processes, but no causal or physiologic link has been established. In an article in 2005, Satti et al., have reported a case of a patient with both PHG and idiopathic thrombocytopenic purpura (ITP), but raised the possibility in their conclusion that the association might be coincidental.[4] This case supports an association between PHG and ITP.
CASE REPORT
The present case report is about a 39-year-old woman who presented with pulmonary nodules incidentally detected on abdominal computed tomography (CT) performed for renal calculi [Figure 1]. Further work-up revealed a 2-year history of minimally progressive, non-specific symptoms, which included fatigue, generalized weakness, dyspnea on exertion, and occasional cough associated with recumbent positioning. The patient denied fever, weight loss, chest pain, hemoptysis, or tobacco use. The patient's past medical history was significant for idiopathic thrombocytopenic purpura (ITP) for at least 4 years prior to presentation, for which she had never received any treatment. Additional history indicated chronic sinusitis and medical marijuana use for intractable pain and presumed ilioinguinal neuralgia that followed robotic hysterectomy. The patient also had a left oophrectomy for dysmenorhea 3 years prior to presentation. A chronic organizing abdominal wall hematoma resulting from an intraoperative inferior epigastric vessel injury probably also contributed to her chronic abdominal pain. A family history of malignancy included leukemia, lymphoma, and renal and prostate cancers.
- 39-year-old woman with pulmonary nodules discovered incidentally diagnosed as pulmonary hyalinizing granuloma associated with idiopathic thrombocytopenic purpura. Lung windows from computed tomography of the abdomen and pelvis demonstrate nodules in the left (red arrow) and right (yellow arrow) lower lobes.
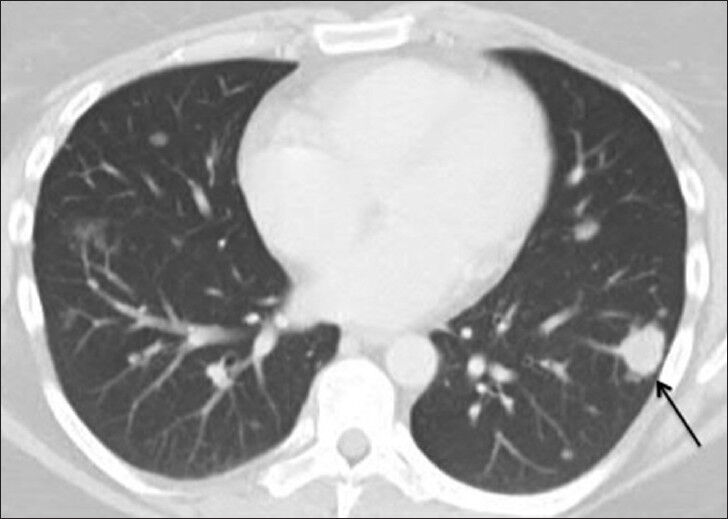
Additional immune, infectious, and malignancy test results were negative. Specifically, fungal serologies, transglutanin antibody, phospholipid antibody, rapid plasma reagin, antinuclear antibody, anti-mitochondrial antibody, double-stranded deoxyribonucleic acid antibody, anti-Ro (SS-A/B) antibodies, anti-smith antibody, ribonucleoprotein antibody, striated muscle antibody, anti-smooth muscle antibody, anti-collapsin response mediator antibody, voltage-gated potassium channel antibody, N-type calcium channel antibody, and angiotensin-converting enzyme testing were within normal limits. A chest radiograph demonstrated bilateral rounded pulmonary opacities of various sizes [Figure 2]. A contrast enhanced chest CT showed multiple rounded, discrete pulmonary nodules bilaterally with relative lower lobe and peripheral predominance, which is a common presentation for metastases [Figure 3]. The largest nodule was 2 cm in diameter. Neither enlarged mediastinal or hilar nodes, nor pleural effusions were demonstrated. Positron emission tomography (PET) CT scan was negative [Figure 4]. CT-guided left lower lobe nodule biopsy yielded non-specific lymphoplasmacytic infiltrate, areas of fresh alveolar hemorrhage and no evidence of well-formed granuloma or malignancy. Transbronchial left lower lobe nodule biopsy 6 months later demonstrated fragments of normal lung parenchyma without evidence of a well-formed granuloma or malignancy.
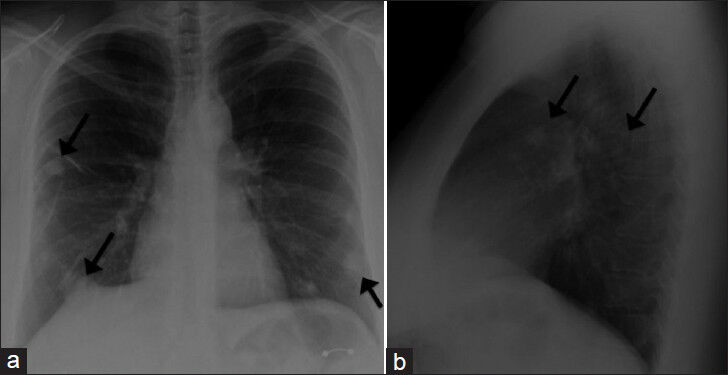
- 39-year-old woman with pulmonary nodules discovered incidentally diagnosed as pulmonary hyalinizing granuloma associated with idiopathic thrombocytopenic purpura. (a) Posteroanterior (PA) and b) lateral radiographs demonstrate multiple pulmonary nodules (several of which are identified by arrows) bilaterally with relative peripheral and lower lung zone predominance.
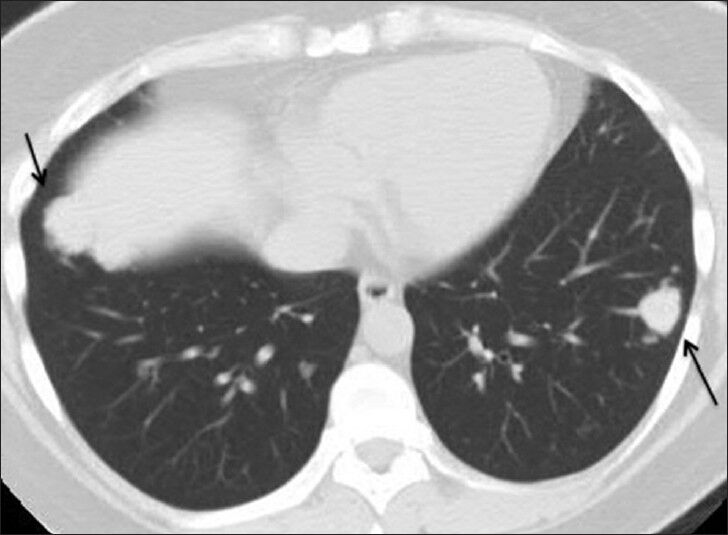
- 39-year-old woman with pulmonary nodules discovered incidentally diagnosed as pulmonary hyalinizing granuloma associated with idiopathic thrombocytopenic purpura. Follow-up computed tomography chest on lung window demonstrates stability of one of the larger nodules in the left lower lobe (red arrow). Additional nodules (yellow arrows) are seen at slightly different levels than on prior study due to differences in positioning and level of inspiration.
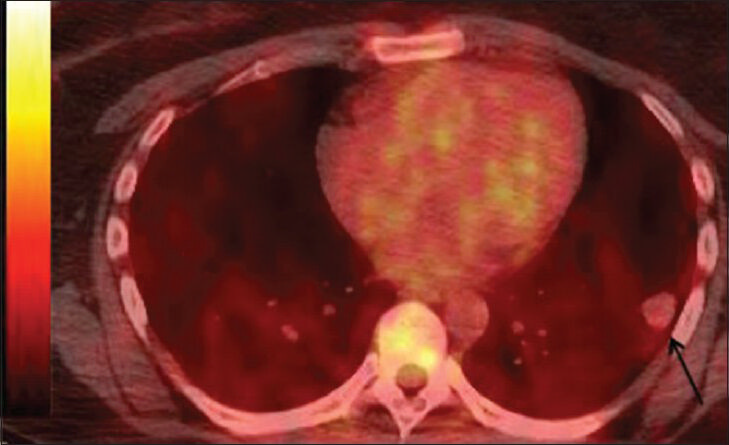
- 39-year-old woman with pulmonary nodules discovered incidentally diagnosed as pulmonary hyalinizing granuloma associated with idiopathic thrombocytopenic purpura. Fused images from F-18 fluorodeoxyglucose positron emission tomography- computed tomography demonstrate the index lesion without abnormally increased glucose metabolism.
Follow-up CT scans performed 8- and 16-months after the initial abdominal CT demonstrated stability of the existing nodules and no new nodules. However, continued diagnostic uncertainty led to a thoracoscopic wedge resection of nodules in the superior segment of the left lower lobe and anterior segment of the left upper lobe. Microscopic analysis of each of the two resected round, well-circumscribed, indurated, tan nodules demonstrated some defining features for hyalinizing granuloma, including layers of collagen concentrically arranged in a whorled fashion around small blood vessels [Figure 5]. Inflammatory infiltrate both within and around the nodules consisted primarily of lymphocytes and plasma cells with a few neutrophils and histiocytes. No treatment has been initiated at this point in view of the documented stability of the lung lesions and a lack of significant evidence to suggest that the nodules were responsible for the patient's reported clinical symptoms. Steroids have been reported to be successful for symptom relief and nodule regression in patients with PHG.
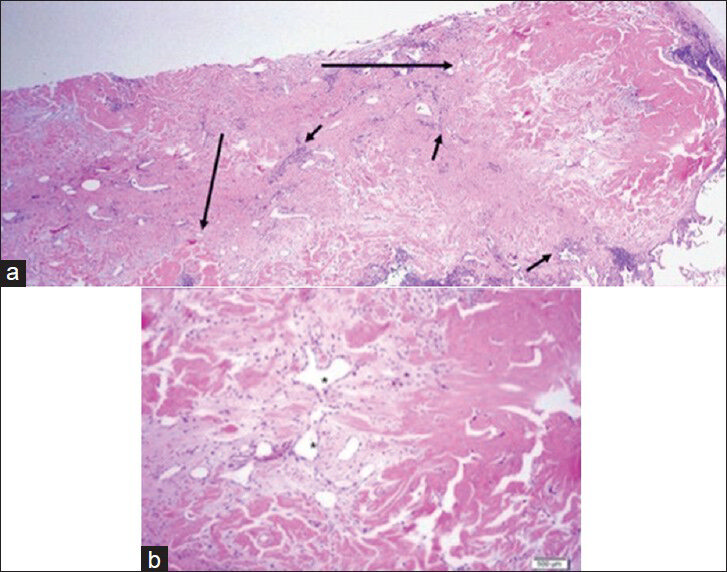
- 39-year-old woman with pulmonary nodules discovered incidentally diagnosed as pulmonary hyalinizing granuloma (PHG) associated with idiopathic thrombocytopenic purpura. a) ×10 magnified H and E, stained microscopy of the two nodules from wedge resection (long arrows) shows clusters of lymphoplasmacytic infiltrate (short arrows) peripherally. b) ×20 magnified view demonstrates whirled layers of collagen (dark pink) around small blood vessels (*), consistent with PHG. No multinucleated giant cells are present.
DISCUSSION
PHG is a rare, benign lung condition of unknown etiology that presents as pulmonary nodules. The defining histologic features of PHG are the presence of densely fibrotic nodules with patchy chronic inflammation both around and within them. The nodules are composed of thick, hyalinized bands of collagen that were arranged in whorls and cartwheels. They may be associated with prominent perivasculitis and germinal center formation. The inflammatory infiltrate is predominantly lymphocytes and plasma cells, but the nodules can also contain neutrophils and histiocytes. Multinucleated giant cells typical of a true granuloma are generally scarce or absent and well-formed granulomas are not a feature. The term “granuloma” is thus a relative misnomer.
The mean age of presentation of PHG is 44 years, with an age range of 19-77 years.[5] No gender or racial predilection has been shown. PHG often presents with vague chest symptoms or is found on incidental chest imaging, as in this case. Pulmonary nodules are often multiple, bilateral, and of various sizes on radiographs. Solitary nodules are rare.[6] Nodules range from a few millimeters to several centimeters in diameter and may coalesce over time. Adenopathy is not a feature in the absence of a concomitant infection.
The tendency for multiplicity and growth of PHG nodules limits the ability to distinguish Pulmonary hyalinizing granuloma (PGH) from metastatic disease in many cases. Solitary lesions can simulate primary lung carcinoma. The differential diagnosis also includes a wide variety of other entities in various cases, including fungal and tuberculous infections, non-infectious granulomatous diseases (sarcoidosis, Wegener granulomatosis, lymphomatoid granulomatosis, and plasma cell granuloma) and rheumatoid, amyloid and septic emboli. Lesions that contain calcification can simulate sarcomatous and carcinomatous metastases. PET-CT may or may not reveal increased metabolic activity in PHG lesions, although it can be useful in the workup for metastatic disease. Slow growth on serial follow-up studies may also help distinguish PHG from metastatic disease and non-diagnostic needle biopsies should raise the possibility of PHG. It is not uncommon for the definitive diagnosis of PHG to require excisional biopsy with gross pathological evaluation.
The etiology of PHG has not been fully elucidated. It has been hypothesized that the nodules result from an abnormally elevated immune response to one of a variety of inciting infectious or autoimmune processes. Specifically, associations have been reported with fungal and mycobacterial lung disease as well as with rheumatoid arthritis, uveitis, lupus-like anticoagulant, Morvan's syndrome, sclerosing mediastinitis, idiopathic systemic fibrosis, sarcoidosis and IgG4-related sclerosing disease.[789] An association of PHG with lymphoproliferative disease (lymphoma and Castleman's) has also been demonstrated.[7]
PHG has no known malignant potential, although it may recur following surgical removal.[3] On the other hand, a solitary lesion may remain relatively stable over a long interval. Multiple lesions have a worse prognosis. Enlargement and coalescence of lesions may result in impaired pulmonary function over time. There is no established medical treatment for PHG and no treatment has been instituted in the current patient to date. Glucocorticoids have been reported to provide symptomatic relief and nodule regression in patients with PHG and enlarging or compressive nodules.[10]
CONCLUSION
This case shows an association between Pulmonary hyalinizing granuloma (PGH) and immune-mediated conditions and specifically supports a previously reported association of PGH with Idiopathic Thrombocytopenic Purpura (ITP).
Available FREE in open access from: http://www.clinicalimagingscience.org/text.asp?2014/4/1/8/127835
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- Hyalinizing granuloma: An unusual case of a pulmonary mass. Case Rep Med 2010 2010 984765
- [Google Scholar]
- Pulmonary hyalinizing granuloma. Bilateral pulmonary nodules associated with chronic idiopathic thrombocytopenic purpura. Saudi Med J. 2005;26:1459-63.
- [Google Scholar]
- Radiology-pathology conference: Pulmonary hyalinizing granuloma associated with lupus-like anticoagulant and Morvan's Syndrome. Clin Imaging. 2007;31:264-8.
- [Google Scholar]
- Pulmonary hyalinizing granuloma: A rare cause of a solitary pulmonary nodule. J Thorac Imaging. 1991;6:54-6.
- [Google Scholar]
- Pulmonary hyalinizing granuloma with Castleman's disease. Intern Med. 1994;33:689-91.
- [Google Scholar]
- Multiple pulmonary hyalinizing granulomas associated with systemic idiopathic fibrosis. Acta Pathol Jpn. 1991;41:375-82.
- [Google Scholar]
- Pulmonary hyalinizing granuloma with associated elevation in serum and tissue IgG4 occurring in a patient with a history of sarcoidosis. Am J Surg Pathol. 2012;36:774-8.
- [Google Scholar]
- A case of steroid responsive pulmonary hyalinising granuloma: Complicated by deep venous thrombosis. Eur Respir J. 2004;23:954-6.
- [Google Scholar]






