Translate this page into:
Malignant Transformation of Hepatic Adenoma in Glycogen Storage Disease Type-1a: Report of an Exceptional Case Diagnosed on Surveillance Imaging
Address for correspondence: Dr. Neeraj Lalwani, University of Washington, Seattle, Washington - 98104-2499, USA. E-mail: neerajl@u.washington.edu
-
Received: ,
Accepted: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Hepatocellular adenoma is a heterogeneous group of benign neoplasms arising from hepatocellular cells and can be subclassified into four major groups based on genotypic and phenotypic characteristics. These four subtypes are hepatocyte nuclear factor (HNF) 1α-inactivated, β-catenin–activated, inflammatory, and unclassified adenomas. Immunohistochemistry studies have demonstrated that since β-catenin–activated adenomas have a higher risk of malignant transformation, the identification of the subtype of adenoma remains crucial in patient management. However, malignant transformation of hepatic adenoma without β-catenin overexpression can be seen in 30–65% cases. We report a case of malignant transformation of hepatic adenoma without overexpression of β-catenin in a 31-year-old man with a known glycogen storage disease (GSD) Type-1a, which was diagnosed on surveillance magnetic resonance imaging (MRI). The MRI showed a mild interval increase in one lesion with relative stability of the other adenomas. The lesion was presumed to be suspicious for a hepatocellular carcinoma (HCC) and was confirmed on pathology.
Keywords
β-catenin mutation
glycogen storage disease
hepatic adenoma
hepatocellular carcinoma
malignant transformation

INTRODUCTION
Glycogen storage disease (GSD) Type-1a (or von Gierke's disease) is an uncommon disease caused by the absence of glucose-6-phosphatase, an enzyme necessary for gluconeogenesis and glycogenolysis. It often manifests with episodes of hypoglycemia and marked hepatomegaly during the first year of life.
Hepatic adenomas (HCAs) are classically associated with GSD Type-1a. Presence of multiple adenomas (arbitrarily, more than 10) without known risk factors (including GSD) is termed as hepatic adenomatosis. HCAs can be subclassified based on the following genotype/phenotype characteristics: a) Inactivating mutations in the HNF1α gene (30–35%), b) activating β-catenin mutation (10–15%), c) inflammatory (40–50%), and d) unclassified (<10%).[1]
HCAs developing on the background of GSD-1a are unique and characterized by a lack of HNF1α inactivation, which can likely be associated with similar metabolic defects observed with HNF1α inactivation and glucose-6-phosphatase deficiency.[2] Most of the HCAs developing on the background of GSD-1a can be subgrouped as inflammatory (52%), β-catenin activated (28%), or unclassified (20%).[2] Identifying the HCA subtype remains crucial in patient management because β-catenin–activated HCAs have a higher frequency of malignant transformation.
Malignant transformation of an HCA in a patient with hepatic adenomatosis has been described in the literature.[3] Type I GSD has a predilection for HCAs, with malignant transformation reported in about 10% of patients.[4]
We report a case of GSD-1a–associated HCA and the malignant transformation of one of the HCAs diagnosed on surveillance imaging. This adenoma had no underlying β-catenin activation, which is relatively less common.
CASE REPORT
A 31-year-old patient of type GSD-1a (von Gierke's disease) with known multiple HCAs presented for MR imaging. The contrast-enhanced multiphasic liver magnetic resonance imaging (MRI) was performed with gadopentetate dimeglumine in an outside facility in May 2014 and demonstrated multiple HCAs ranging in size from less than 1 cm up to 5 cm, some of which were hemorrhagic. The lesions showed variable heterogeneity and arterial enhancement with subtle washout [Figure 1]. Given the higher frequency of β-catenin–activated HCA associated with GSD-1a, regular surveillance imaging was recommended.
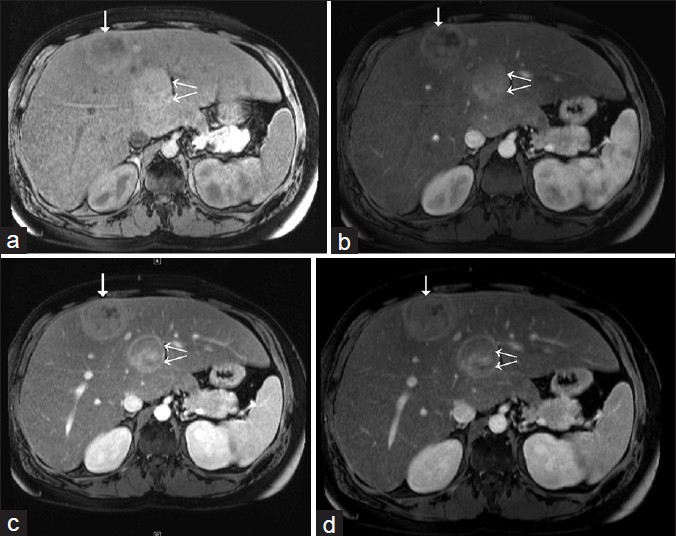
- 31-year-old man with known glycogen storage disease Type-1a undergoing surveillance imaging. Dynamic multiphase contrast-enhanced MRI obtained in May 2014. (a) Pre-contrast T1W axial image shows two hepatic lesions in segments 4 (double arrows) and 8 (single arrow). (b) T1W axial image (arterial phase) shows variable heterogeneity foci of both of the lesions and arterial enhancement. (c) T1W axial image (venous phase) shows persistent enhancement of hepatic lesions. (d) T1W axial image (delayed or equilibrium phase) shows subtle washout in both of the lesions; posterior lesion also shows peripheral rim enhancement (double arrows).
Follow-up MRI with gadoxetate disodium (gadolinium ethoxybenzyl diethylenetriamine pentaacetic acid/Gd-EOB-DTPA) in our institution was performed after 6 months in Nov 2014. It showed a mild interval increase in the size of one lesion from 4.0 × 3.9 cm to 4.9 × 4.2 cm. The lesion demonstrated predominantly isointense to slightly increased intensity compared with the rest of the liver parenchyma on the T2-weighted (T2W) image, with an eccentric, ill-defined, T2-hyperintense possible scar, and did not reveal intralesional lipid. There was no T1 hyperintensity to suggest interval hemorrhage [Figure 2]. It was arterial enhanced with washout, with an enhancing rim on the venous phase, and did not show uptake of hepatocyte-specific contrast on the delayed phase images [Figure 3]. The size of the remaining adenomas either marginally decreased or was stable [Figure 4]. In view of this isolated increase in size, the lesion was presumed to be suspicious.
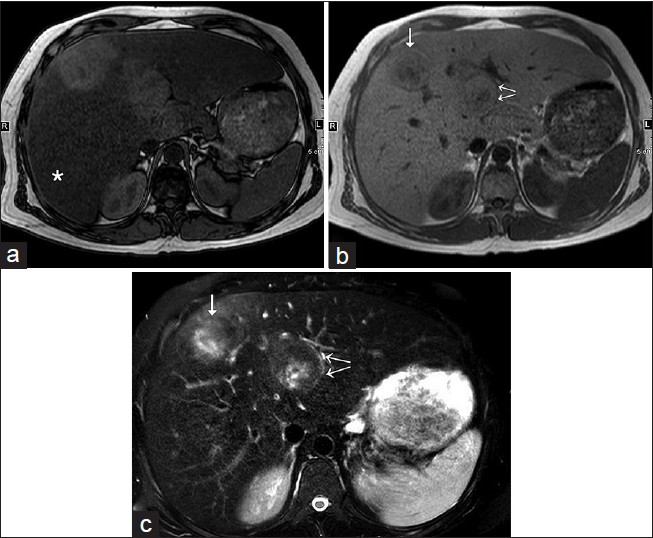
- 31-year-old man with known glycogen storage disease Type-1a undergoing surveillance imaging diagnosed with hepatic adenoma. (a) T1W opposed-phase axial image shows diffuse loss of intensity of hepatic parenchyma (asterisk), which suggests the presence of diffuse hepatic steatosis. Two hepatic lesions in segments 4 and 8 are relatively hyperintense on the background of steatosis and lack microscopic fat. (b) T1W in-phase axial image shows two hepatic lesions in segments 4 (double arrows) and 8 (single arrow). (c) Fat-suppressed T2W axial image demonstrates isointense to slightly increased intensity of the above-described hepatic lesions (see the corresponding arrows), with an eccentric, ill-defined, T2-hyperintense possible scar.
![31-year-old man with known glycogen storage disease Type-1a undergoing surveillance imaging. Dynamic multiphase contrast-enhanced MRI obtained in Nov 2014 with gadolinium-EOB-DTPA. (a) Pre-contrast fat-suppressed T1W axial image shows two hepatic lesions in segments 4 (double arrows) and 8 (single arrow). The anterior lesion (single arrow) shows interval decrease in size, whereas the posterior lesion is slightly increased in size compared to MR dated May 2014 [Figure 2]. (b) Fat-suppressed T1W axial image (arterial phase) shows arterial enhancement of both hepatic lesions. (c) Fat-suppressed T1W axial image (venous phase) shows persistent enhancement of hepatic lesions; however, the anterior lesion (single arrow) appears fainter. (d) Fat-suppressed T1W axial image (delayed or equilibrium phase) shows remarkable washout in the posterior lesion (double arrows) with well-evident peripheral rim enhancement. (e and f) Fat-suppressed T1W axial image (hepatobiliary phases at 10 and 20 min, respectively) shows peripheral retention of the contrast in the anterior lesion (image f, star), which can be seen with inflammatory adenomas. The posterior lesion gradually becomes hypointense to the liver parenchyma and shows no uptake of contrast.](/content/12/2015/5/1/img/JCIS-5-47-g004.png)
- 31-year-old man with known glycogen storage disease Type-1a undergoing surveillance imaging. Dynamic multiphase contrast-enhanced MRI obtained in Nov 2014 with gadolinium-EOB-DTPA. (a) Pre-contrast fat-suppressed T1W axial image shows two hepatic lesions in segments 4 (double arrows) and 8 (single arrow). The anterior lesion (single arrow) shows interval decrease in size, whereas the posterior lesion is slightly increased in size compared to MR dated May 2014 [Figure 2]. (b) Fat-suppressed T1W axial image (arterial phase) shows arterial enhancement of both hepatic lesions. (c) Fat-suppressed T1W axial image (venous phase) shows persistent enhancement of hepatic lesions; however, the anterior lesion (single arrow) appears fainter. (d) Fat-suppressed T1W axial image (delayed or equilibrium phase) shows remarkable washout in the posterior lesion (double arrows) with well-evident peripheral rim enhancement. (e and f) Fat-suppressed T1W axial image (hepatobiliary phases at 10 and 20 min, respectively) shows peripheral retention of the contrast in the anterior lesion (image f, star), which can be seen with inflammatory adenomas. The posterior lesion gradually becomes hypointense to the liver parenchyma and shows no uptake of contrast.
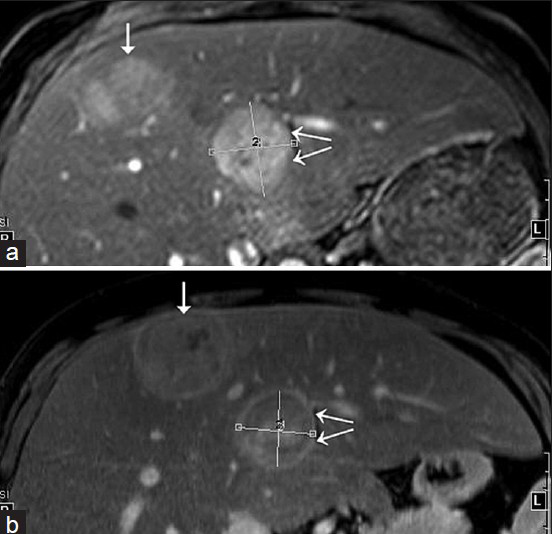
- 31-year-old man with known glycogen storage disease Type-1a undergoing surveillance imaging. Index images of two hepatic lesions with an interval of 6 months. (a) Fat-suppressed T1W fat post-contrast image acquired in Nov 2014. (b) Fat-suppressed T1W fat post-contrast image obtained in May 2014. The anterior lesion (single arrow) shows interval decrease in size on follow-up imaging, whereas the posterior lesion (double arrows) is slightly increased in size.
Accordingly, a short-interval follow-up scan or a biopsy was recommended to rule out malignant transformation. An ultrasound (US)-guided biopsy was performed as per patient preference. The biopsy revealed absent normal portal tracts in the tissue, increased unpaired arteries, and moderate steatosis, with many ballooned cells, some containing Mallory–Denk bodies. A reticulin stain showed focal loss of the reticulin framework [Figure 5]. The hepatocytes did not show increased Nuclear: Cytoplasmic (N: C) ratio, and there were no overtly prominent nucleoli or mitotic figures. Although there was no pseudoglandular formation and β-catenin was negative for nuclear staining, focal Glypican-3 staining was present. Taken together, while the differential diagnosis included hepatocellular adenoma and well-differentiated carcinoma, the focal Glypican -3 stain and focal loss of reticulin stain led to the final diagnosis of a well-differentiated hepatocellular carcinoma (HCC). The patient continues to be asymptomatic and has been put on the hepatic transplant list, with close monitoring of the HCC on imaging pending the transplantation.
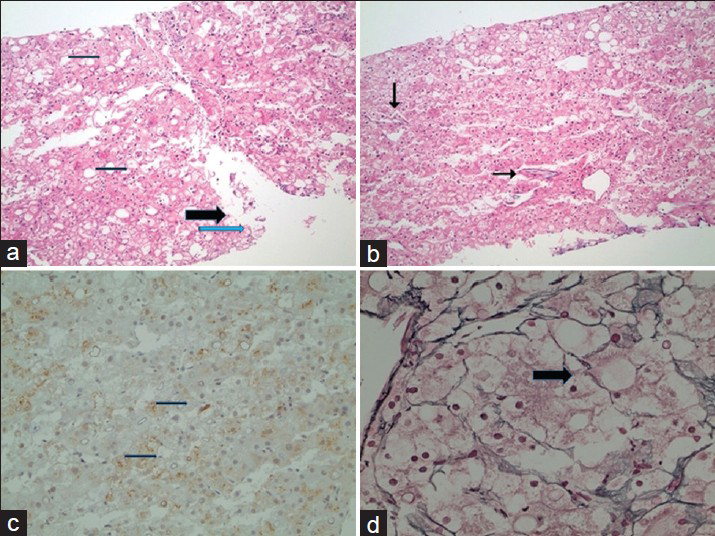
- 31-year-old man with known glycogen storage disease Type-1a. Post-biopsy histopathology of suspicious lesion seen on imaging. There is lack of portal tracts in all fragments. (a) In low-power magnification (hematoxylin and eosin stain), there is moderate steatosis (thin arrows) with many ballooned cells (thick arrow), some containing Mallory–Denk bodies (blue arrow). (b) In low-power magnification (hematoxylin and eosin stain), there are increased unpaired arteries (arrows). (c) In low-power magnification, there is focal Glypican-3 staining of the tumor cells. (d) In higher magnification, a reticulin stain shows focal loss of the reticulin framework (arrow).
DISCUSSION
HCAs are traditionally considered as uncommon benign hepatic tumors that commonly occur in women on oral contraceptives. With an evolving understanding of these entities, it was realized that adenomas represent a heterogeneous group of tumors with specific genetic abnormalities and clinical and prognostic features.[4] Accordingly, adenomas are now subclassified into four types.
Inflammatory adenomas are the most common, accounting for 40–50% of all adenomas. They demonstrate activation of the Janus kinase-signal transducer and activator of transcription (JAK-STAT3) pathway. Glycoprotein 130 forms the final common pathway for the activating mutations, which ultimately lead to increased C-reactive protein, serum amyloid-A, and other acute phase reactants, and inflammatory cell infiltration into the adenoma. Telangiectatic focal nodule hyperplasia, previously considered a subtype of focal nodule hyperplasia, was reclassified as an inflammatory adenoma due to similar pathology.
HNF1α-mutated (hepatocyte nuclear factor 1α-mutated) adenomas are the second most common subtype, constituting 30–35% of all adenomas. These adenomas demonstrate inactivating mutations of the HNF1α gene, a tumor suppressor gene.[4] This leads to increased lipogenesis due to multiple mechanisms, ultimately leading to increased intracellular lipid deposition or steatosis. Immunohistochemistry analysis reveals lack of expression of liver fatty acid binding protein (LFABP). These adenomas occur almost always in females, are multiple in half the cases, and can be associated with familial hepatic adenomatosis and maturity-onset diabetes of the young (MODY).[4]
The β-catenin–mutated adenomas have activating mutations of the catenin β1 gene (CTNNB1), leading to unrestrained hepatocyte proliferation and adenoma formation. These constitute about 10–15% of all adenomas and have a higher association with GSD, familial adenomatous polyposis, and androgen administration.[4] HCCs and hepatoblastomas may also show β-catenin activation, and β-catenin adenomas have a higher risk of malignant transformation.[4]
Unclassified adenomas are the fourth subtype, accounting for 10% of the cases. These are poorly understood, but do have the potential to become malignant.[456]
Patients with GSDs are more vulnerable to HCA, which can develop in up to 75% of cases and are often multiple.[4] Frequency of β-catenin–mutated adenomas is higher in this subgroup, due to which these patients undergo regular surveillance imaging.[567]
Diagnosis of malignant transformation of adenoma in GSD1 remains challenging. No effective biomarker exists to predict malignant transformation; α-fetoprotein (AFP) and carcinoembryonic antigen (CEA) levels are often normal even in the setting of HCC.[8] There is limited literature on the imaging findings of malignant transformation of adenomas in such patients. Meager evidence exists to support the worth of surveillance imaging.
HCA subtypes demonstrate specific imaging features corresponding to the underlying pathogenesis, with one MRI study describing accurate pathological classification in 85% of all cases.[9] Inflammatory adenomas are T2 hyperintense and demonstrate strong arterial enhancement that persists on the delayed phase, consistent with their inflammatory pathology. Inflammatory adenomas may occasionally show hyperintensity on hepatobiliary phase and can mimic focal nodular hyperplasia [Figure 3e and f].[10] On the one hand, HNF1a-mutated adenomas demonstrate intracellular fat, leading to loss of signal on the out-of-phase images, and show moderate arterial enhancement that does not persist in the delayed phase (but without washing out). On the other hand, β-catenin–mutated adenomas do not have very specific imaging features. They may appear homogeneous or heterogeneous, lack intratumoral fat (compared to classical adenomas), and demonstrate a vaguely defined T2-hyperintense scar. They may also show strong arterial enhancement, which may or may not persist on the delayed-phase images or show washout.[46] Although presence of washout has been reported in them, it is not a specific finding and has been described in unclassified adenomas as also in a subset of inflammatory adenomas with associated β-catenin activation.[9] Unclassified adenomas do not have any definite radiologic features.[4]
Accordingly, current guidelines by the American College of Medical Genetics and Genomic recommend that these patients undergo screening computed tomography (CT)/MRI every 6–12 months.[8] Considering that adenomas can demonstrate delayed washout and have a higher risk of malignant transformation in GSD patients, it is difficult to distinguish them from HCC on surveillance imaging in these patients.[7] No original series has been published on this issue to the best of our knowledge. In our case, the slight interval increase in size despite the lack of hemorrhage, in contradistinction to the stability of the other lesions, led to the suspicion of malignant transformation. Based on this case, it might be prudent to keep a low threshold to biopsy an adenoma that demonstrates suspicious imaging feature. Imaging features such as relatively increasing size compared to other lesions and development of washout and peripheral enhancing rim are often of concern.
The mainstay of management in GSD-1a remains diet modification; hepatic transplantation is rarely performed. However, excessive HCA burden, bulky size of neoplasm, or malignant transformation of HCA may qualify the patient for a hepatic transplantation.
CONCLUSION
Malignant transformation of HCA in GSD-1a has been linked with relatively higher frequency of β-catenin overexpression. Malignant transformation without β-catenin mutation may also occur and suggests that an alternative unknown genetic pathway of malignant transformation of HCA also exists in such patients. Surveillance imaging is recommended in all GSD patients to monitor HCAs and their growth pattern, and can be helpful to make an early diagnosis of malignant transformation. Increase in size of HCA in contrast to other stable lesions should be viewed with suspicion. Malignant transformation of HCA in this subgroup of patients may qualify them for a hepatic transplantation.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We sincerely acknowledge the contribution and help rendered by Carolyn Wang, MD (Assistant Professor, Radiology, University of Washington, Seattle).
Available FREE in open access from: http://www.clinicalimagingscience.org/text.asp?2015/5/1/47/163991
REFERENCES
- Genotype-phenotype correlation in hepatocellular adenoma: New classification and relationship with HCC. Hepatology. 2006;43:515-24.
- [Google Scholar]
- Molecular characterization of hepatocellular adenomas developed in patients with glycogen storage disease type I. J Hepatol. 2013;58:350-7.
- [Google Scholar]
- Genetics and imaging of hepatocellular adenomas: 2011 update. Radiographics. 2011;31:1529-43.
- [Google Scholar]
- Hepatocellular adenomas in glycogen storage disease type I and III: A series of 43 patients and review of the literature. J Pediatr Gastroenterol Nutr. 1997;24:276-9.
- [Google Scholar]
- Hepatocellular adenomas: Correlation of MR imaging findings with pathologic subtype classification. Radiology. 2011;261:172-81.
- [Google Scholar]
- CT scan diagnosis of hepatic adenoma in a case of von Gierke disease. Indian J Radiol Imaging. 2012;22:54-7.
- [Google Scholar]
- American College of Medical Genetics and Genomics. Diagnosis and management of glycogen storage disease type I: A practice guideline of the American College of Medical Genetics and Genomics. Genet Med. 2014;16:e1.
- [Google Scholar]
- Hepatocellular adenomas: Accuracy of magnetic resonance imaging and liver biopsy in subtype classification. Hepatology. 2011;53:1182-91.
- [Google Scholar]
- Inflammatory hepatocellular adenomas can mimic focal nodular hyperplasia on gadoxetic acid-enhanced MRI. AJR Am J Roentgenol. 2014;203:W408-14.
- [Google Scholar]






