Translate this page into:
Adverse Events in Coronavirus Disease Patients Management: A Pictorial Essay

*Corresponding author: Letizia Di Meglio, Postgraduation School Radiodiagnostics, Univerisità Degli Studi di Milano, Via Festa Del Perdono 7, Milan, Italy. letiziadimeglio@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Angileri SA, Petrillo M, Meglio LD, Arrichiello A, Roda GM, Ierardi AM, et al. Adverse Events in Coronavirus Disease Patients Management: A Pictorial Essay. J Clin Imaging Sci 2020;10:42.
Abstract
Clinical manifestation of coronavirus disease (COVID-19) varies from asymptomatic to severe clinical forms that can result in acute respiratory distress syndrome or in multiple organ dysfunction syndromes. There are no guidelines, based on randomized controlled trials, for the treatment of COVID-19 patients. The treatment is based on antiviral drugs, invasive and non-invasive ventilation supports, and anticoagulant therapy. This is a pictorial essay covering the multiple adverse events encountered during the treatment of COVID-19 patients in an area with a high pandemic incidence. Adverse events are defined as unexpected events following treatment for the infection. The cases described would be useful in aiding early diagnosis, limiting and improving the management of serious complications for patients, and allowing rapid and appropriate treatment.
Keywords
Coronavirus disease
Adverse events
Barotrauma
Coagulopathy
Bleeding
INTRODUCTION
On December 31, China alerted the World Health Organization (WHO) of an increased number of pneumonia cases, and on February 7, these were recognized to be caused by a novel coronavirus, SARS-CoV-2.
On February 21, the first cases of SARS-CoV-2 were confirmed in Italy and from that moment the numbers increased rapidly. The main cluster was identified in Lombardy, particularly in the area of Codogno (LO).
SARS-CoV-2-positive cases were then also subsequently confirmed in all continents, so the WHO, on March 11, declared a pandemic.
Coronavirus disease (COVID-19) clinical manifestations vary from asymptomatic or paucisymptomatic patients to severe clinical forms that can result in respiratory failure, acute respiratory distress syndrome (ARDS), and in multiple organ dysfunction syndromes. Other severe manifestations of COVID-19 involve coagulopathy, thromboembolic events, and disseminated intravascular coagulation.[1]
The management of COVID-19 patients is complex and may require mechanical ventilation and support in the intensive care unit (ICU).[2]
There is little experience in the imaging and interventional management of this novel virus pandemic and consequently no published evidence-based guidelines. Treatment recommendations currently are essentially based on antiviral drugs, invasive and non-invasive ventilation supports, and anticoagulant therapy: In fact, preliminary studies have shown the potential beneficial effect of heparin treatment in reducing the mortality of COVID-19 patients, due to its anticoagulant effect and an anti-inflammatory action.[3]
Although these treatments have a beneficial effect in terms of reducing the mortality of COVID-19 patients, they are not without complications and adverse events. This review is intended to aid clinicians in allowing the early diagnosis and management of patients with complications of COVID-19. These include life- threatening hemorrhage, barotrauma, and thromboembolic disease, leading to multiorgan failure and stroke.
CLINICAL EXAMPLES
Bleeding complications
Bleeding is the principal adverse event of treatment with any anticoagulants which can be non-major or major [Figure 1], potentially life threatening and result in long-term morbidity.[4]
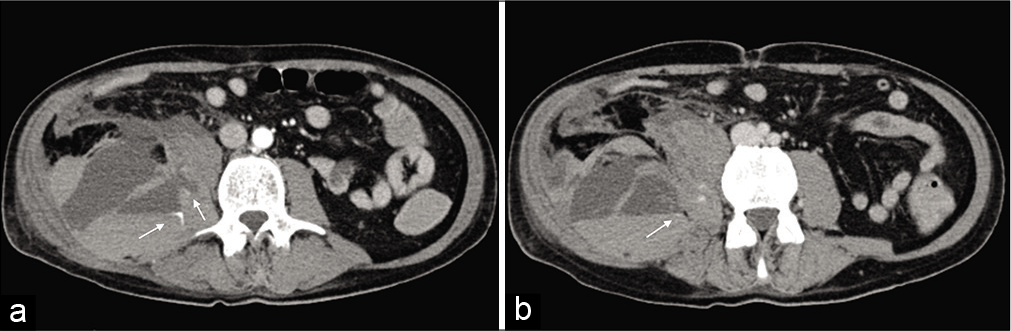
- Axial view of the CT angiogram showing a spontaneous heterogeneous 18.7 × 8.8 × 10.8 cm right iliopsoas hematoma with two areas of active arterial bleeding (white arrows) (a and b), possibly originating from the ipsilateral lumbar artery.
Recent studies have shown that prophylactic doses of low-molecular-weight heparin (LMWH) and unfractionated heparin are associated with a reduced 28-day mortality in sepsis-induced coagulopathy in COVID-19 patients.[1] The precise dosages, types of anticoagulant, and their routes of administration are yet to be defined to ensure effective and safe treatments.
Spontaneous iliopsoas hematoma [Figure 1]
A 59-year-old man hospitalized for a COVID-19 pneumonia on invasive mechanical ventilation for 11 days, in treatment with hydroxychloroquine (hydroxychloroquine 200 mg) twice daily, anakinra (anakinra 100 mg) 3 times daily, and enoxaparin sodium (enoxaparin sodium 6000 IU) twice daily. Two days after being extubated he suffered an episode of hypotension. The patient was hemodynamically unstable, and blood analysis showed a hemoglobin value of 5.5 g/dl so a computer tomography (CT) angiogram was requested
This showed a spontaneous heterogeneous 18.7 × 8.8 × 10.8 cm right iliopsoas hematoma with two areas of active arterial bleeding (white arrows) [Figure 1a and b], possibly originating from the ipsilateral lumbar artery.
Barotrauma
Every patient on positive pressure ventilation risks developing pulmonary barotrauma. This is usually due to alveolar rupture, which results in leakage of air in extra-alveolar spaces which can in severe cases result in pneumothorax [Figure 2], pneumomediastinum [Figure 3], subcutaneous emphysema, and rarely pneumoperitoneum [Figure 4]. In 2004, a prospective cohort study conducted in 361 ICU on 5184 patients estimated that incidence of barotrauma was 2.9% in general, with a different prevalence depending on the clinical indication of ventilation. The prevalence in ARDS patients was 6.5%, showing that ARDS may predispose to pulmonary barotrauma. Most patients with ARDS due to COVID-19 require intubation and mechanical ventilation. Therefore, the risk of barotrauma for these patients is high.[5]
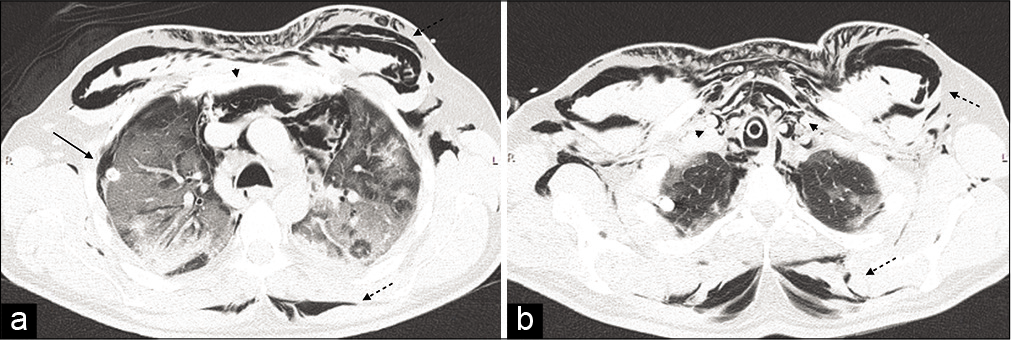
- Non-contrast computer tomography was carried out showing a massive subcutaneous emphysema (dashed black arrows), extending from the thoracic outlet and the axillae to the laterocervical areas, surrounding the rhomboids muscles posteriorly. It also demonstrated compressive pneumomediastinum (black arrow heads), located predominantly in the anterior and superior mediastinum, and a right pneumothorax (black arrows), located in the anterolateral region of the apical segment of the right superior lobe (a and b).
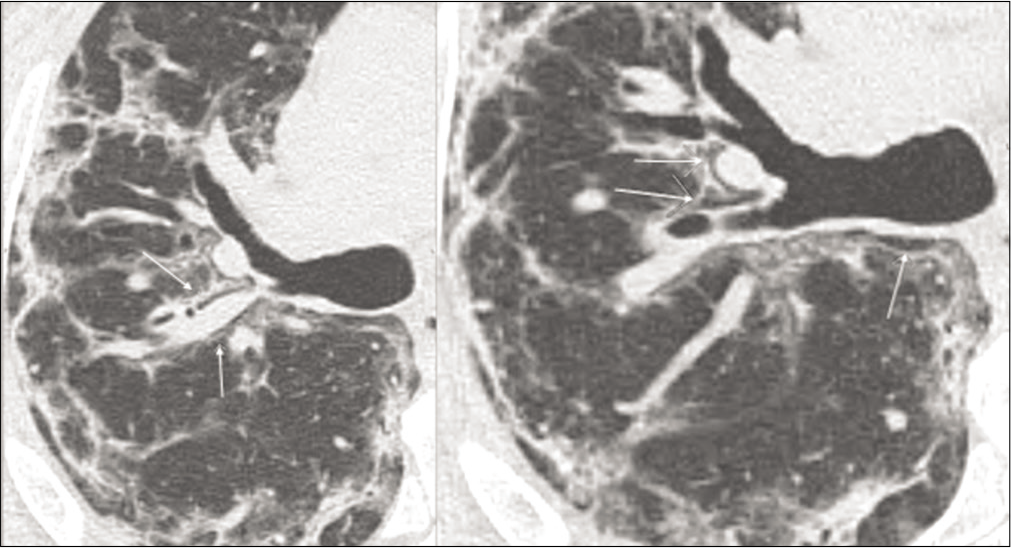
- Axial view of a non contrast CT scan showing progressive pneumomediastinum (white arrows). This completely resolved after CPAP suspension.
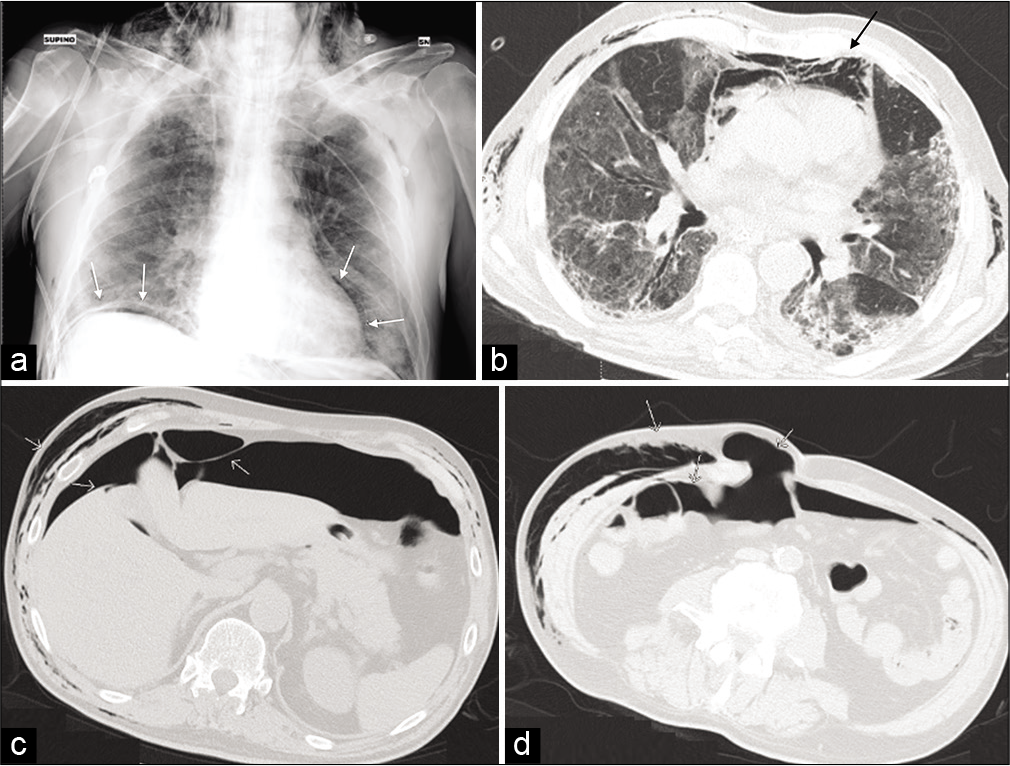
- Bedside Chest-XR showing a massive subcutaneous emphysema and a small quantity of subphrenic air (a) (white arrows). Axial view of CT-scan demonstrating an extensive compressive pneumomediastinum (black arrow) (b) and pneumoperitoneum located predominantly under the anterior wall, around the liver and spleen (c and d).
Pneumothorax, pneumomediastinum, and subcutaneous emphysema [Figure 2]
A 48-year-old man was admitted with worsening COVID-19 pneumonia. The patient was initially on Venturi mask but, CPAP ventilation had to be commenced for 5 days.
The patient was initially on Venturi mask, due to the worsening of his pneumonia, CPAP ventilation was started and kept for 5 days, eventually, the ventilation support was switched into invasive mechanical ventilation. He developed extensive subcutaneous emphysema and a non-contrast CT was carried out which showed massive subcutaneous emphysema (dashed black arrows), extending from the thoracic outlet and the axillae to the laterocervical areas, surrounding the rhomboids muscles posteriorly.
It also demonstrated compressive pneumomediastinum (black arrow heads), located predominantly in the anterior and superior mediastinum, and a right pneumothorax (black arrows), located in the anterolateral region of the apical segment of the right superior lobe [Figure 2a and b].
Pneumomediastinum [Figure 3]
A 50-year-old female patient underwent CPAP support for persistent hypoxia but developed a progressive pneumomediastinum (white arrows). This completely resolved after CPAP suspension.
Pneumoperitoneum [Figure 4]
A 65-year-old man was admitted for COVID-19 pneumonia. The patient underwent early intubation invasive mechanical ventilation due to fast worsening of the oxygen saturation despite CPAP ventilation with high oxygen flow. After the finding of subcutaneous emphysema, a CXR confirmed massive subcutaneous emphysema and a small quantity of subphrenic air [Figure 4a] (white arrows).
In addition, the CT scan showed an extensive compressive pneumomediastinum (black arrow) [Figure 4b] and pneumoperitoneum located predominantly under the anterior wall, around the liver and spleen [Figure 4c and d]. There is also gas within an abdominal wall hernia. The absence of rising WBC and CRP without signs of peritoneal reaction suggested that this was related to ventilation. Conservative management resulted in complete, spontaneous resolution after 10 days after stopping ventilation.
Thromboembolic events
Although most patients with COVID-19 infections will have a mild disease, some progress to respiratory distress, sepsis, and septic shock predisposing patients to thrombotic disease, both in the venous and arterial circulations. This is the result of severe inflammation, platelet activation, endothelial dysfunction, and stasis. Many authors have reported derangements in coagulation function in severely infected patients including elevated D-dimer, fibrin/fibrinogen degradation products, and fibrinogen levels, resembling coagulopathy.[6] A significant correlation between elevated D-dimer levels on admission and in-hospital COVID-19 mortality has been shown by different authors.[7,8] Pulmonary embolism [Figure 5] is considered one of the main reasons for the rapid clinical worsening of their lung function and sudden death. Prophylactic LMWH doses are usually given in many countries with the COVID pandemic to mitigate this risk of thromboembolism. However, the most appropriate type of anticoagulation, the dosage levels, and frequency of administration are not clearly understood in this cohort of patients.
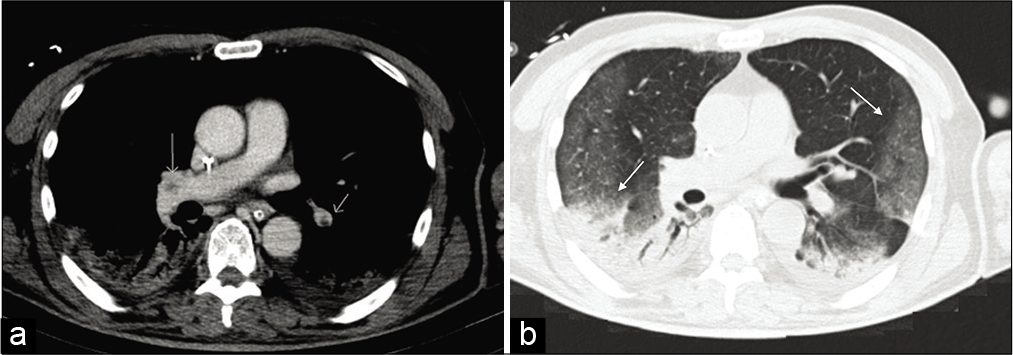
- A contrast-enhanced CT of the chest was undertaken, confirming multiple filling defects of the middle lobar artery on both sides (a) indicating acute pulmonary embolism (white arrows). Axial view of CT scan showing with lung window bilateral peripheral ground glass opacities and consolidations in the inferior lobes (b) (black arrows).
Pulmonary thromboembolism [Figure 5]
This is a 69-year-old COVID-positive man admitted to a subacute ICU for sudden worsening of his respiratory symptoms. He had been managed with a Venturi mask before a sudden loss of the oxygen saturation and his D-dimer values were elevated to 2231 ng/mL.
A contrast-enhanced CT of the chest was undertaken. This confirmed multiple filling defects of the middle lobar artery on both sides [Figure 5a] indicating acute pulmonary embolism (white arrows). – Axial view of CT scan showing with lung window bilateral peripheral ground glass opacities and consolidations in the inferior lobes [Figure 5b] (black arrows).
Brain ischemia/infarction [Figure 6]
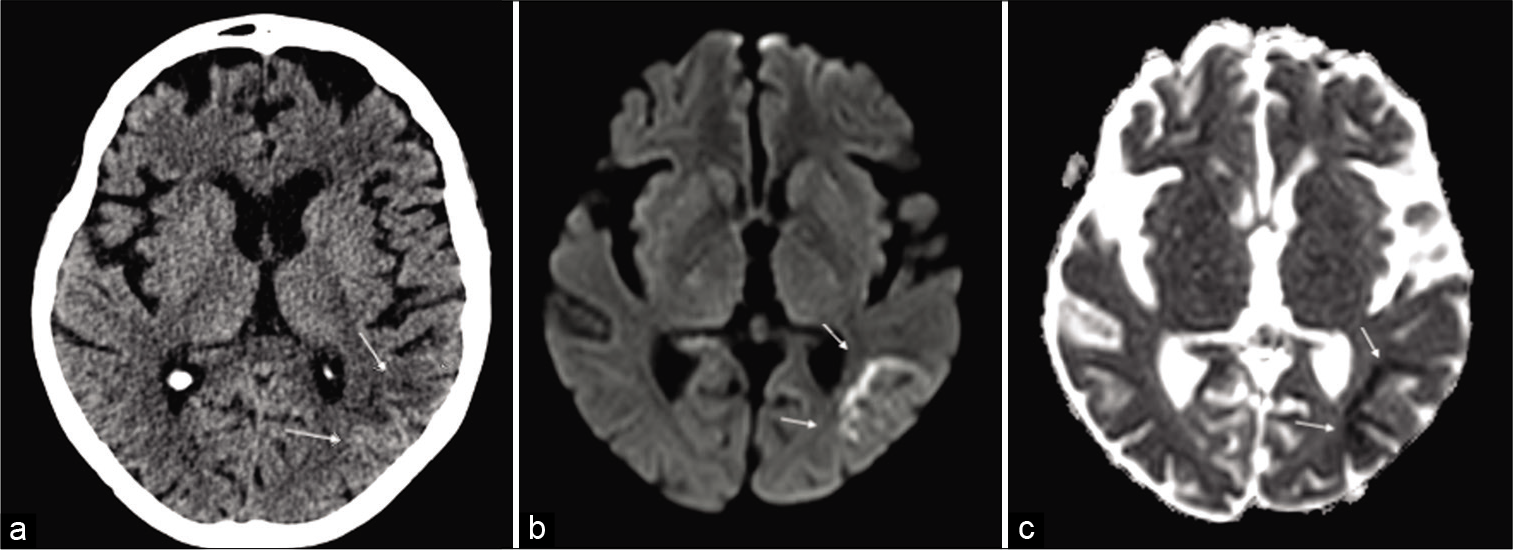
- A brain CT and then magnetic resonance imaging scan were undertaken to rule out acute ischemic stroke or viral encephalitis. The axial CT scans showed mild parietal subcortical hypodensity (a) confirmed on the subsequent MRI (b and c) scan, showing multiple linear areas of subcortical restricted diffusion (DWI) in the same territory and hypointensity on correspondent ADC maps (b and c) (white arrows). An acute ischemic stroke was diagnosed.
Respiratory-related infection is a recognized independent risk factor for acute cerebrovascular disease.[9,10] The cytokine storm during the infection of COVID-19 can cause coagulopathy in critically ill patients with elevated levels of D-dimer and severe platelet reduction, may expose these patients to sudden acute cerebrovascular events.[11] A 76-year- old man was admitted to the emergency department with a high fever of 39.5° and sudden worsening of his dyspnea. Blood gases showed a pH of 7.5 with a pCO2 31 and pO2 58 and he was commenced on hi-flow oxygen on a Venturi mask. After 2 days, the patient showed a sudden onset of confusion and slurred speech. On neurologic examination, there was no focal neurology. Laboratory studies showed lymphopenia and eosinophilic granulocytopenia with two throat swab samples, one negative and one positive for SARS- CoV-2 nucleic acid. A brain CT and then magnetic resonance imaging scan were undertaken to rule out acute ischemic stroke or viral encephalitis.
The axial CT scans showed mild parietal subcortical hypodensity [Figure 6a] confirmed on the subsequent MRI [Figure 6b and c] scan, showing multiple linear areas of subcortical restricted diffusion (DWI) in the same territory and hypointensity on correspondent ADC maps [Figure 6b and c] (white arrows). An acute ischemic stroke was diagnosed and clopidogrel (75 mg) was commenced orally. After 25 days of treatment, the patient was discharged without neurological impairment and near- normal speech.
CONCLUSION
There are currently no guidelines for the optimal management of COVID-19 patients. It is a complex disease with the majority of patients who have minor symptoms. However, in a small minority of patients, symptoms and complications of the disease can be severe and life threatening. COVID-19 patients often require ICU management with invasive ventilation support and multidrug treatment which can in themselves result in complications. Sharing the experiences from centers such as ours with a high incidence of this pandemic is a first step in helping to improve patient care and outcomes.
Given our experience in a COVID-19 center, we think that it is important to show the potential adverse events that can occur during the management of these patients, to ensure rapid diagnosis and proper treatment, due to the life- threatening nature of some of these events.
Acknowledgments
The opinions are those of the authors in their personal capacity.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18:1094-9.
- [CrossRef] [Google Scholar]
- Features, Evaluation and Treatment Coronavirus (COVID-19) Treasure Island, FL: Stat Pearls Publishing; 2020.
- [Google Scholar]
- COVID-19 and haemostasis: A position paper from italian society on thrombosis and haemostasis (SISET) Blood Transfus. 2020;18:167-9.
- [Google Scholar]
- Treatment of bleeding complications in patients on anticoagulant therapy. Blood. 2019;133:425-35.
- [CrossRef] [PubMed] [Google Scholar]
- Barotrauma and Mechanical Ventilation Treasure Island, FL: Stat Pearls Publishing; 2020.
- [Google Scholar]
- Prominent changes in blood coagulation of patients with SARS-CoV-2 infection. Clin Chem Lab Med. 2020;58:1116-20.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet. 2020;395:1054-62.
- [CrossRef] [Google Scholar]
- Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844-7.
- [CrossRef] [PubMed] [Google Scholar]
- Why now? Moving from stroke risk factors to stroke triggers. Curr Opin Neurol. 2007;20:51-7.
- [CrossRef] [PubMed] [Google Scholar]
- Laboratory-confirmed respiratory infections as triggers for acute myocardial infarction and stroke: A self-controlled case series analysis of national linked datasets from Scotland. Eur Respir J. 2018;51:1701794.
- [CrossRef] [PubMed] [Google Scholar]
- Unique epidemiological and clinical features of the emerging 2019 novel coronavirus pneumonia (COVID-19) implicate special control measures. J Med Virol. 2020;92:568-76.
- [CrossRef] [PubMed] [Google Scholar]






