Translate this page into:
Adding Value to the Magnetic Resonance Examination in a Case of Brachial Plexus Birth Palsy
Address for correspondence: Dr. Ajay Prashanth Dsouza, Department of Diagnostic Imaging, Al Jalila Children's Hospital. P.O Box. 7662, Dubai, UAE. E-mail: APDsouza@ajch.ae
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
We report a case of brachial plexus birth palsy in an infant with the inability to move the left upper limb since birth. There was neither history of birth trauma nor any complications during delivery. Magnetic resonance imaging (MRI) of brachial plexus showed postganglionic injury with musculoskeletal abnormalities. The child underwent surgical repair of the plexus and is on physical rehabilitation. In this case report, we discuss the utility of a single MRI examination with an elaborate discussion on various MRI signs of brachial plexus injury including secondary musculoskeletal manifestations. The case reiterates the significance of two-in-one approach while imaging these cases with MRI. Apart from reporting the damage to the brachial plexus, the radiologist should actively search for glenohumeral dysplasia. Awareness of classification and assessment of glenohumeral dysplasia should be routinely included as an integral part of imaging report as it adds incremental value to the overall patient management and functional outcome.
Keywords
Brachial plexus birth palsy
glenohumeral dysplasia
magnetic resonance imaging brachial plexus
periscalene soft-tissue sign
pseudomeningocele

INTRODUCTION
Brachial plexus birth palsy (BPBP) is an uncommon condition. Imaging is performed to confirm the diagnosis and to make treatment decisions. Ample consideration is given to the status of the plexus, while often the importance of glenohumeral dysplasia is undermined. This case is presented to highlight the importance of reporting glenohumeral dysplasia. In a child with BPBP, awaiting neurological recovery, the functional recovery depends on the secondary musculoskeletal abnormalities. Positive results of physiotherapy and rehabilitation are directly related to early detection of musculoskeletal abnormalities, especially glenohumeral dysplasia (GHD).
CASE REPORT
A 10-week infant presented with an inability to move the left upper limb since birth. The infant was born at 38 weeks of gestation by vaginal delivery with birth weight of 3.8 kg. The perinatal and antenatal history was uneventful. The radiographic examination did not reveal any fractures or bony deformities. On physical examination, the left upper limb was abducted, internally rotated at the shoulder, and extended at the elbow joint. No handgrip was noted. The deep tendon reflexes were absent. The contralateral limb was normal on examination. BPBP was considered.
Magnetic resonance imaging (MRI) examination of the brachial plexus was done under general anesthesia using 3T MRI. The thecal sac was unremarkable without pseudomeningocele, and abnormal periscalene soft-tissue signal was noted on the left side with thickening and the increased signal from the left C5–C7 cervical nerves. Diffuse swelling and increased signals were seen in the left shoulder girdle muscles with evidence of posterior subluxation of the left humeral head [Figures 1a–f and 2a–f].
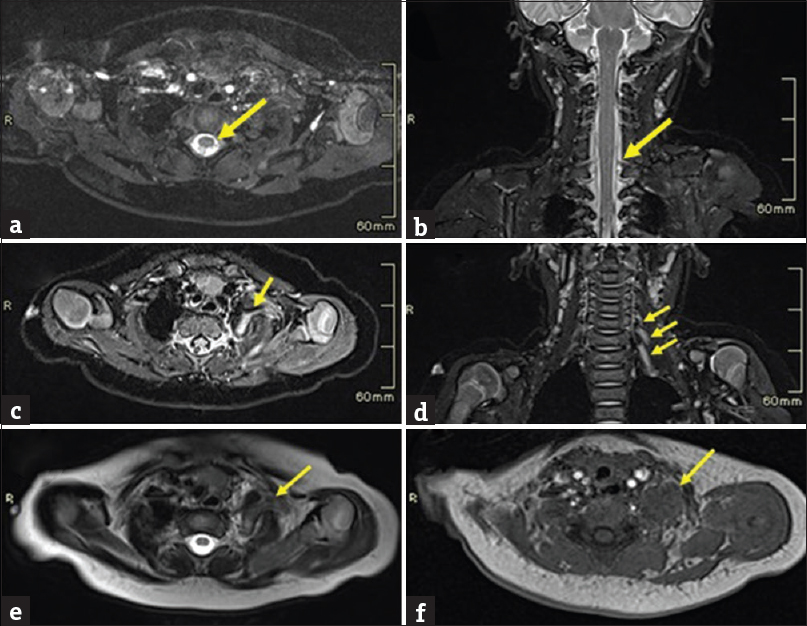
- A case of left brachial plexus birth palsy. Images (a-f) show MRI of brachial plexus in a three-month-old infant. (a, b) Axial T2W CISS sequence and Coronal T2W image shows thecal sac without pseudomeningocele. (c) Axial STIR image shows thickening and increased signals from C5 cervical nerve (arrow) on the left side. (d) Coronal STIR image shows increased signals from the C5, C6 and C7 cervical nerves (arrow) on the left side. (e) Axial T2W image shows swelling of left scalene muscles with an abnormal T2 bright (arrow) periscalene signal. (f) Axial T1W images show isointense signal giving a mass like appearance (arrow).
The MRI findings were consistent with postganglionic left brachial plexus injury, involving C5–C7 nerve roots with acute postdenervation pattern in shoulder girdle muscles and posterior subluxation of the humeral head. The patient was on physiotherapy and underwent surgical repair of the plexus at 6 months. Six-month postsurgical follow-up shows regaining of movements at fingers, wrist, elbow, and shoulder although limited. The infant is currently on physiotherapy and occupational therapy.
DISCUSSION
Brachial plexus is formed by a network of nerves derived from the ventral rami of C5–T1 spinal nerves [Figure 3].
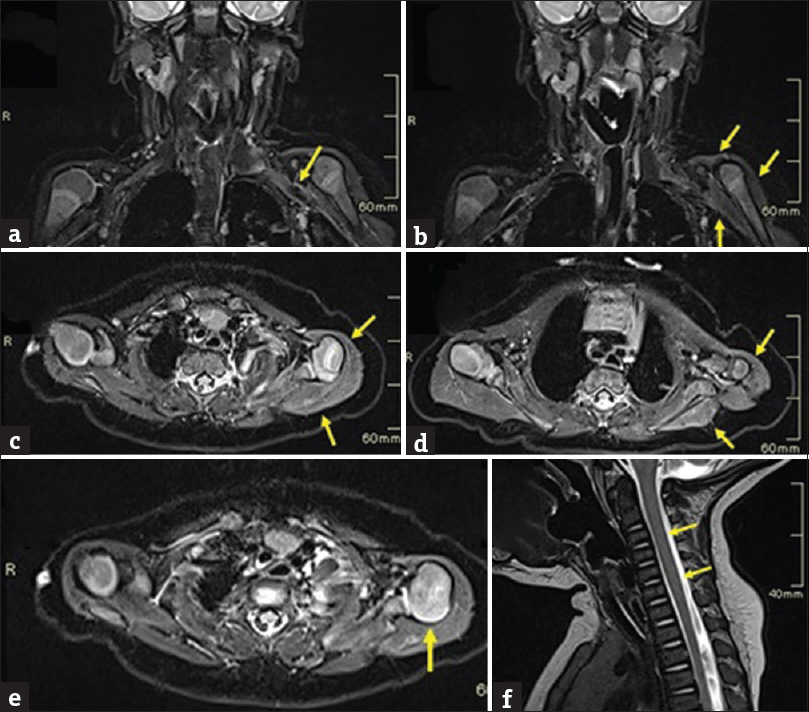
- A case of left brachial plexus birth palsy. Image (a-f) shows MRI of brachial plexus in a three-month-old infant with a postganglionic injury and musculoskeletal abnormalities. (a) Coronal STIR image shows generalized thickening of left brachial plexus (arrow) beyond lateral border of the first rib. (b, c & d) Coronal and Axial STIR images showing diffuse swelling and increased signals involving the shoulder girdle muscles (arrows). (e) Axial STIR image shows posterior subluxation of the humeral head (arrow). (f) Sagittal T2W image shows no abnormal signal intensity lesions in the cervicothoracic spinal cord (arrows).
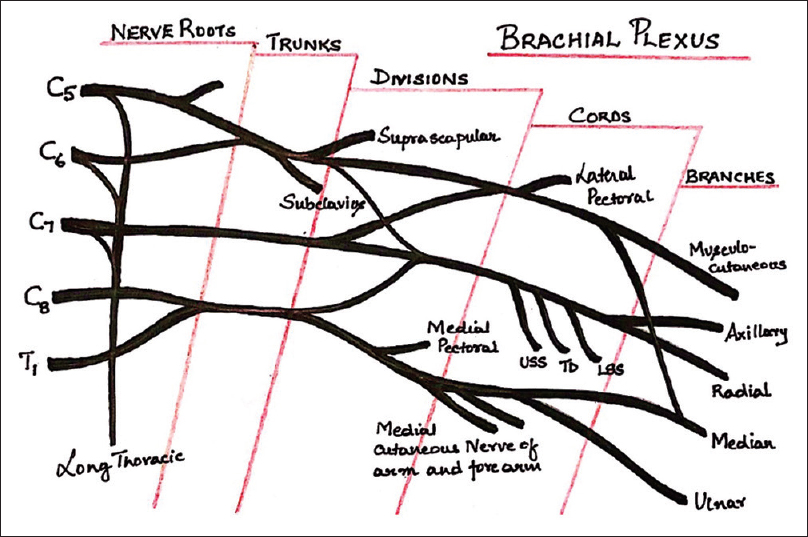
- Schematic diagram of brachial plexus.
The brachial plexus supplies sensory and motor innervation to the ipsilateral chest, shoulder, arm, and hand. BPBP has a reported incidence of 3 per 1000 livebirths. It has significant morbidity concerning nerve and muscle dysfunction, weakness, and disability.[1]
From an imaging perspective, BPBP can be classified into two patterns: pre- and post-ganglionic injuries as it has implications for management and prognosis.[2] Preganglionic injury indicates avulsion of nerve roots from the spinal cord [Figure 4]. In a postganglionic pattern, the injury to the nerve occurs distal to the dorsal root ganglion [Figure 5].
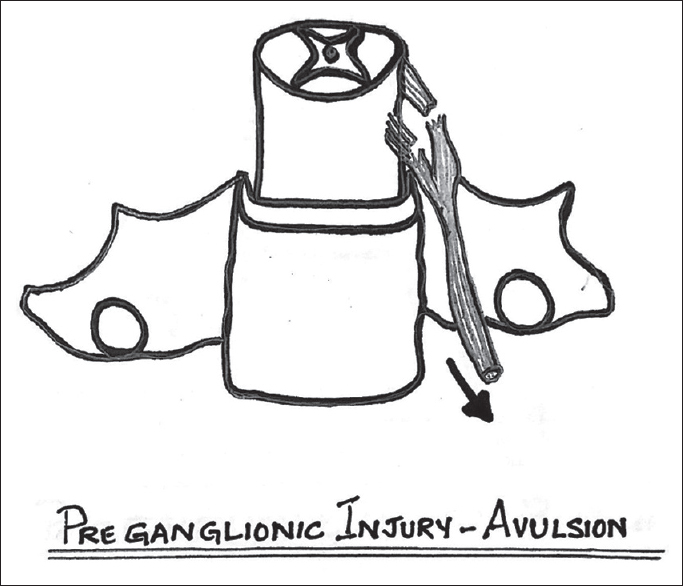
- Schematic diagram of preganglionic avulsion of the rootlets from the spinal cord.

- Schematic diagram of postganglionic rupture of the nerve beyond the dorsal root ganglion.
Postganglionic lesions are of three types.[3] In neuropraxia, there is stretching of an intact nerve, whereas in axonotmesis, there is rupture of some of the axons with nerve intact. In neurotmesis, the entire nerve is severed. Both the extent (number of nerve roots) and the severity (avulsion, rupture, and stretch) of the injury influence the prognosis. Fractures of clavicle, humerus, cervical spine and shoulder joint subluxation, facial nerve palsy, and spinal cord injury have been reported in BPBP.[4]
MRI is the investigation of choice as it can image spinal cord, brachial plexus, and both shoulder joints in a single sitting. MRI defines the extent and severity and shows the associated injuries.[2] MRI protocol should include imaging of both shoulders along with cervical spine and brachial plexus. Heavily T2-weighted, three-dimensional, constructive interference in steady-state sequence should be used to assess the thecal sac, rootlets, and the exiting nerve roots.[5]
Neuropraxia, which is the commonest of BPBP, manifests as thickening, T2-signal prolongation and diffuse contrast enhancement of the plexus on MRI. It resolves without treatment.[25]
Pseudomeningocele is a contained cerebrospinal fluid (CSF) leak surrounding the avulsed nerve root extending along the dural sleeve. It is an imaging finding that appears as a CSF intensity, fluid-filled structure adjacent to the injured nerve root extending along its course.[1] Despite the surgical finding of avulsion of the C7 nerve root, a pseudomeningocele was not seen in our case. In children <18 months, MRI finding of pseudomeningocele has a low sensitivity and high specificity for nerve root avulsion.[6] In this age group, there is a limitation in evaluating the exiting nerve roots on MRI due to their smaller size and poor spatial resolution.[6]
Periscalene soft-tissue sign has been described in cases of Erb's palsy,[7] which denotes the imaging manifestation of posttraumatic reparative neuroma of the brachial plexus. Periscalene soft-tissue sign manifests as a signal abnormality around the scalene muscle when compared to the normal side. The reported sensitivity and specificity of this sign is 97% and 100%, respectively.[7] Periscalene soft-tissue sign was observed in our case [Figure 1e and f]. In spite of high sensitivity and specificity, periscalene soft-tissue signs fail to determine the level of the injury.[67] We had a similar experience, as the reparative neuroma could be identified on MRI and surgery, but could not precisely localize the level within the plexus.
Our patient showed additional musculoskeletal findings that are interesting and equally significant. Fluid-sensitive sequences showed swelling and increased signal in the left deltoid and rotator cuff muscles [Figure 2b–d]. On T1- and T2-weighted images, edema and swelling were seen in muscles. These findings suggested an acute pattern of postdenervation change in muscles. Postdenervation changes can be timed on an MRI in skeletal muscles. Acute changes manifest within a month and overlap into a subacute phase that is seen up to 12–20 months.[8] Acute phase manifests as abnormal muscle enhancement, T2 hyperintense signals, and an increase in muscle volume without fatty infiltration. Detection of fatty changes indicates subacute phase.[8] In our case, MRI was done at the early 3rd month of the postnatal life, with corresponding changes of acute denervation [Figure 2c].
BPBP patients are prone to GHD. GHD represents a spectrum of findings comprising of glenoid and humeral head articular incompatibilities, deformity of glenoid, and subluxation or dislocation of the glenohumeral joint.[9] GHD can manifest as early as 3 months.[1] The injury to brachial plexus leads to nerve dysfunction, which in turn predisposes to an imbalance of muscle power. The internal rotators of the shoulder joint are unopposed by the weak external rotators; this combined with the child's growth spurt causes contractures, deformity of joint, posterior subluxation, and dislocation of humeral head.[1]
GHD is manifested on imaging by an abnormal glenoid version with poor head coverage, resulting in abnormal translation of humeral head or posterior subluxation.[1] These findings should be identified as early as possible. Classification of the severity of glenohumeral deformity is essential in the treatment planning and follow-up.[1] MRI examination allows quantitative assessment of GHD simultaneously while imaging the brachial plexus.
Several morphometric features of GHD have been described.[1] The most common, accurate, and reproducible measurements are Glenoid Version described by Chagas-Neto et al.,[10] and the measurement of the degree of posterior subluxation of humeral head by Chagas-Neto et al.[10]
The Glenoid Version can be calculated as described in Figure 6.
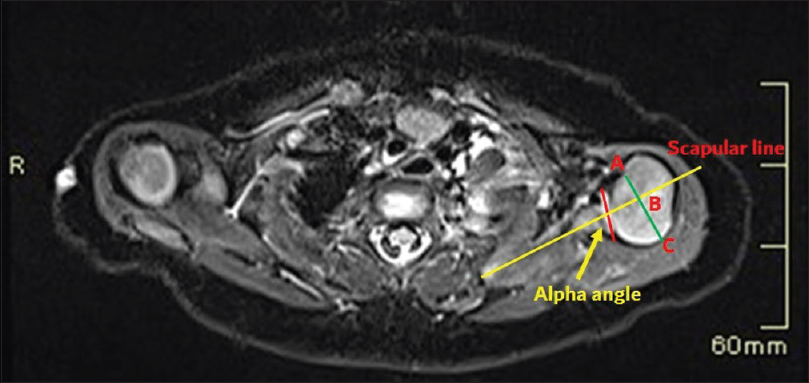
- A case of brachial plexus birth palsy. MRI in a three-month-old infant at the level of left shoulder joint with drawings to assess GHD. Axial image shows scapular line (yellow) drawn on the body of scapula bisecting the midpoint of the glenoid surface (red line). The alpha angle is subtracted from 90° to get an angle of glenoid version. Humeral head coverage is measured by extending scapular line through the humeral head and a bisecting line drawn at midpoint of the humeral head (green line). The humeral head anterior to scapular line is the percentage of humeral head coverage calculated by the formula: AB/AC X 100%.
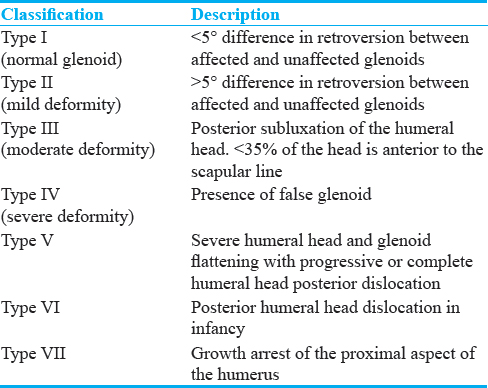
A negative angle is a retroversion and a positive angle is an anteversion.[10] The normal Glenoid version in children <2 years is − 6.3 (±6.5 SD) degrees and − 2.1 (±5.9 SD) degrees in children >2 years.[11] Humeral head coverage can be measured by extending the scapular line as shown in Figure 6. Normal humeral head coverage is between 40% and 55%. Posterior subluxation of the humeral head is defined when the measurement is between 0% and 35%. Dislocation is considered when the entire humeral head is posterior to the scapular line.[10] Once the measurements are made, GHD can be graded based on Waters criteria[112] as mentioned in Table 1. Retrospectively, both these measurements were abnormal in our case, thus classifying the left shoulder joint having moderate GHD.
Physiotherapy and surgery are the available treatment options for BPBP.[13] Physiotherapy forms the mainstay during the anticipation period of spontaneous recovery. Physiotherapy should be started as early as possible to improve the functional outcomes and to maintain the flexibility of soft tissues and joints and avoid future contractures.[13] Contractures begin as early as 2–3 weeks in postnatal life, especially at the glenohumeral joint;[14] hence, detection of GHD should take precedence. Surgery is indicated at 3–9 months in the absence of functional recovery.[13]
Through this case report, we emphasize the concurrent assessment of GHD and brachial plexus on a single MRI examination as it adds incremental value in case management. Implementing an MRI protocol for brachial plexus should include both shoulder joints, enabling radiologists to assess GHD on routine diagnostic workstations.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Available FREE in open access from: http://www.clinicalimagingscience.org/text.asp?2018/8/1/38/239704
REFERENCES
- Brachial plexus birth palsy: Multimodality imaging of spine and shoulder abnormalities in children. AJR Am J Roentgenol. 2015;204:W199-206.
- [Google Scholar]
- Current management of obstetrical brachial plexus injuries at Texas children's hospital brachial plexus center and Baylor college of medicine. Semin Plast Surg. 2005;19:42-55.
- [Google Scholar]
- High-resolution MRI evaluation of neonatal brachial plexus palsy: A promising alternative to traditional CT myelography. AJNR Am J Neuroradiol. 2014;35:1209-13.
- [Google Scholar]
- Diagnostic performance of MRI and MR myelography in infants with a brachial plexus birth injury. Pediatr Radiol. 2006;36:1295-9.
- [Google Scholar]
- Periscalene soft tissue: The new imaging hallmark in Erb's palsy. AJNR Am J Neuroradiol. 2010;31:882-5.
- [Google Scholar]
- MR imaging in two cases of subacute denervation change in the muscles of facial expression. AJNR Am J Neuroradiol. 2001;22:880-4.
- [Google Scholar]
- Glenohumeral deformity in children with brachial plexus birth injuries. Bull NYU Hosp Jt Dis. 2011;69:36-43.
- [Google Scholar]
- Imaging assessment of glenohumeral dysplasia secondary to brachial plexus birth palsy. Radiol Bras. 2016;49:144-9.
- [Google Scholar]
- Glenohumeral deformity secondary to brachial plexus birth palsy. J Bone Joint Surg Am. 1998;80:668-77.
- [Google Scholar]
- Neonatal brachial plexus palsy – Management and prognostic factors. Semin Perinatol. 2014;38:222-34.
- [Google Scholar]
- A review of brachial plexus birth palsy: Injury and rehabilitation. R I Med J (2013). 2017;100:17-21.
- [Google Scholar]






