Translate this page into:
Transradial versus transfemoral arterial access in DEB-TACE for hepatocellular carcinoma

*Corresponding author: Nabeel Mohsin Akhter, Department of Interventional Radiology, University of Maryland, Baltimore, Maryland, United States. nakhter@umm.edu
-
Received: ,
Accepted: ,
How to cite this article: Ghosh A, Gupta V, Khalifah AA, Akhter NM. Transradial versus transfemoral arterial access in DEB-TACE for hepatocellular carcinoma. J Clin Imaging Sci 2022;12:38.
Abstract
Objectives
Transradial access has become increasingly popular in body interventional procedures but has not been ubiquitously adapted. This retrospective study compares the efficacy of this approach versus transfemoral access in hepatocellular carcinoma (HCC) patients who underwent drug-eluting bead transarterial chemoembolization (DEB-TACE).
Materials and Methods
A total of 130 HCC patients underwent 146 DEB-TACE procedures within our institution from June 2015 to May 2020. About 90 and 56 procedures were logged for the transradial and transfemoral cohorts, respectively. Peak skin dose, fluoroscopy time, administered contrast volume, total procedure time, and equipment cost data for each procedure were reviewed to evaluate for statistical differences between the two groups.
Results
All 146 cases were technically successful without major complications or access failures in either group. No statistical differences were present between the two access groups in regards to peak skin dose or fluoroscopy time. Transradial access recorded a significantly higher contrast volume (P < 0.05), and a significantly longer procedural time than transfemoral access (P < 0.01). However, transradial access also displayed a significantly lower procedural equipment cost (P < 0.01) between the two groups.
Conclusion
Transradial DEB-TACE has similar trends to transfemoral DEB-TACE in several pertinent radiation parameters and is also significantly more cost-efficacious. The results of this investigation suggest the consideration of transradial access whenever viable as an alternative to transfemoral access in the DEB-TACE treatment of HCC patients.
Keywords
DEB-TACE
TACE
transradial access
transfemoral access
hepatocellular carcinoma
INTRODUCTION
Drug-eluting bead transarterial chemoembolization (DEB-TACE) is an important treatment modality for liver-directed therapy of malignancy, providing the benefit of ischemia and focal drug delivery. Hepatic malignancies cause significant morbidity and mortality, representing the second-highest cause of cancer-related deaths worldwide.[1] Of these fatalities, hepatocellular carcinoma (HCC) is the most common primary hepatic malignancy. Due to this increasing incidence of liver disease (and consequently, cirrhosis), there has been a persistent rise in HCC incidence, reaching 6.2 /100,000 cases per year.[2]
Although definitive therapy for HCC consists of liver transplantation, focal therapy is important to manage the disease burden, and provide a bridge to transplantation.[3,4] The efficacy of down-staging patients using TACE to meet Milan criteria has been well documented.[5] Consequently, TACE remains the first-line treatment for many clinical scenarios such as large or multifocal HCC. Conventional TACE consists of the administration of a chemotherapeutic agent (i.e., doxorubicin in an oil-based emulsion agent like lipiodol) with subsequent vessel embolization. Although this therapy combines the cytotoxic effects of chemotherapy with tumoral ischemia, previous literature has noted the inconsistencies in therapeutic efficacy associated with this technique.[6] DEB-TACE represents a newer technique where microparticles already laced with the chemotherapeutic agent are administered, providing cytotoxic and ischemic effects simultaneously in one step.[7] Prior research has documented a decrease in adverse events with DEB-TACE as compared to conventional TACE, while also documenting non-inferiority in clinical efficacy.[7-9] Additionally, other studies have noted a decreased requirement in the number of treatments needed with DEB-TACE versus conventional TACE.[6]
In congruence with other IR (interventional radiology) procedures, both conventional TACE and DEB-TACE have historically been performed using transfemoral arterial access (TFA). However, growing evidence in body interventional literature has demonstrated the many advantages of transradial artery access (TRA) over TFA. These include faster recovery times, earlier sheath removal, and fewer entry site complications.[10-14] Multiple studies have also reported increased patient satisfaction and comfort with TRA over TFA.[15-17] Similarly, TRA has also been shown to be more cost efficacious in many scenarios.[18] Despite these advantages, prior studies have implicated TRA with higher radiation use, which has undoubtedly limited its adoption for novel procedures.[11,19]
Currently, there are limited studies in the body interventional literature that evaluate transradial (TR) versus transfemoral access in TACE pertaining to essential radiation variables: radiation dose, fluoroscopy time, contrast volume, procedure time, and equipment cost. The aim of this study is to compare the potential benefits and pitfalls of the two vascular approaches (TRA and TFA) in the specific population of HCC patients who underwent DEB-TACE.
MATERIALS AND METHODS
This retrospective study was performed under clinical study guidelines and found to be IRB exempt. The demographic and radiation-related data were collected from the electronic medical record and radiation safety work files associated with each case. A total of 130 HCC patients underwent 146 DEB-TACE procedures at our institute from June 2015-May 2020. All patients were staged either T1aN0M0 or T1bN0M0 with the tumor sizes ranging between 2 and 5 cm. In regards to liver function, all patients had preserved liver function status with BCLC stage A. Further, the Child-Pugh scores were either A5, A6, or B7 among the entire study subset. The MELD score was <15 for all patients.
These patients were retrospectively reviewed in two groups: TRA (90 procedures) and TFA (56 procedures). 16 patients underwent more than one round of treatment, explaining the discrepancy in number of patients versus total procedures. Precisely, 12 patients underwent two different rounds of TRA DEB-TACE while another fou patients underwent two nonconsecutive rounds of TFA DEB-TACE. No significant patterns or observations were extrapolated from the multiple procedures undergone by these patients. Each treatment session was recorded as a new entry regardless of its correspondence to the same patient.
The choice of access was based on the preferences of seven different operators with experience ranging from 2 to 20+ years serving as IR faculty at a tertiary care hospital. All seven operators learned TRA in the same year with its introduction at our hospital in 2014. Additionally, they all conducted an equal amount of TRA and TFA procedures as one another throughout this entire investigation. All 146 procedures were conducted in the same up-to-date angiography system (Siemens Healthineers, Malvern, PA).
It is important to mention that our operators favored TRA over time given their day-to-day clinical experiences suggesting easier accessibility, and greater visible patient comfort, which is in congruence with existing literature. TRA was also chosen over TFA whenever possible to minimize complication risks associated with arteriovenous fistulas, and pseudo-aneurysms. For cases with extreme vessel tortuosity, and where navigation of the aortic arch/radial artery seemed quite difficult based on preprocedural angiograms, TFA was chosen over TRA. Patients with prior failed TRA attempts, or with notable bleeding or coagulopathic considerations were additionally considered for TFA.
In regards to each recorded procedure, no more than one treatment session was conducted at a given date with no more than one segment or one lobe being targeted per session. Total treatment time for delivering the chemotherapeutic pharmaceutical was constant among all procedures.
Transradial artery approach
TRA was performed by first accessing the radial artery with a 21-gauge needle via ultrasound (US) guidance, and then placing a 5F vascular access sheath through the left radial artery. A 5F Jacky angled tip hydrophilic glidecath (Terumo, Tokyo, Japan) was next carried through this to the proper hepatic artery. After, a Renegade Hi-Flo microcatheter was advanced (Boston Scientific, Natick, MA), and used for subselective branch therapy [Figure 1]. All patients were evaluated prior to the procedure for collateral flow sufficiency to the hand via Barbeau’s tests.[20] Patients with a type D response (absent radial artery pulse tracing after 2 minutes of compression, indicating insufficient ulnar artery collateral flow and a lack of patency from the deep/superficial palmar arch) were moved to TFA. For each patient, an US image revealed a radial artery diameter of 2 mm. Prior to every procedure, the skin overlying the left radial artery was anesthetized with lidocaine and nitroglycerin paste. For each patient, a preprocedural radial artery “cocktail” was utilized consisting of 200 ug nitroglycerin, 2.5 mg verapamil, and 3000 units of heparin.
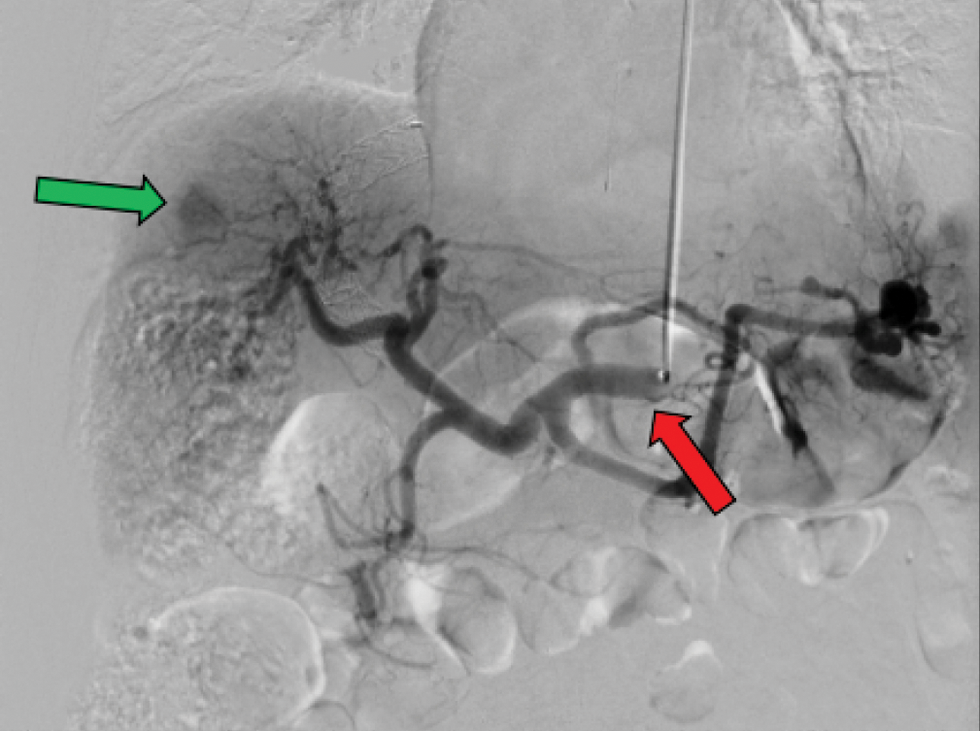
- Transradial access for DEB-TACE55 y/o M with HCC presenting for TRA DEB-TACE. Imaging modality shown is a digital subtracted angiogram (DSA) of the liver with AP supine view. Large tumoral blush (green arrow) from the HCC is noted. The above figure shows a typical case using a 5-F hydrophilic-coated Glidecath (Terumo, Tokyo, Japan) to access the celiac artery (red arrow).
After chemoembolization with Oncozene microspheres (Varian Medical Systems, Palo Alto, CA), all wires and catheters were removed. Before sheath removal, an ateriogram was conducted to assess for radial artery patency. Following this, vascular closure was obtained by the application of a TR compression band (Terumo, Somerset, NJ) over the arteriotomy site. The hemostasis was subsequently maintained for 60 minutes. Arterial hemostasis was reconfirmed as the cuff was incrementally deflated. Upon cuff removal, the patient was observed for an additional 30 minutes before discharge.
Transfemoral artery approach
Using US guidance, the right common femoral artery was accessed with a 21-gauge needle through which a 5F vascular access sheath was advanced. A Simmons one hydrophilic glidecath (Terumo, Tokyo Japan) was then carried through this to the proper hepatic artery. Subsequently, a Renegade Hi-Flo microcatheter was advanced (Boston Scientific, Natick MA), and used for sub-selective branch therapy [Figure 2]. Typical femoral vascular access closure was obtained by either STARCLOSE (Abbott Vascular, Chicago, IL), MYNXGRIP (Cardinal Health, Dublin, OH), ANGIO-SEAL (Terumo, Somerset, NJ) or manual compression. The patient was then transferred to the recovery area with their lower extremity straight for 2 hours before discharge.

- Transfemoral access for DEB-TACE57 y/o M with HCC presenting for TFA DEB-TACE. Imaging modality shown is a digital subtracted angiogram (DSA) of the liver with AP supine view. Large tumoral blush (green arrow) from the HCC is noted. The above figure shows a typical case using a 5-F Sim 1 hydrophilic-coated Glidecath (Terumo, Tokyo, Japan) to access the right hepatic artery (red arrow).
Postprocedure discharge and clinic follow-up
Repeat evaluation of the access site, and pulses (radial or femoral/ dorsalis pedis) were performed for each patient before discharge. All patients were reassessed in clinic between 8 and 12 weeks from the date of their operation for postprocedural complications.
Statistical analysis
Patient characteristics were compared between the two groups using the Wilcoxon rank sum test for demographic characteristics. Wilcoxon rank sum test was also used to evaluate for any statistical differences between the two groups in regards to the desired study variables. P-values < 0.05 were considered to be statistically significant. The following statistic software was utilized for all analyses: SigmaStat version 2.03, SPSS Inc.
RESULTS
In this study, 130 patients underwent a combined total of 146 DEB-TACE procedures [Table 1]. About 58% of the patient population was <65 y/o with the median reported at 63 y/o [Table 1]. 85% of the patient population was male [Table 1]. Both groups demonstrated a 100% technical success rate without any complications or access failures. No statistical differences were found between the TRA and TFA groups in regards to radiation dose [Table 2, 1578.9 mGy vs.1383.0 mGy, P > 0.05] or fluoroscopy time [Table 2, 26.8 min vs. 24.8 min, P > 0.05]. TRA recorded significantly higher contrast volumes [Table 3, 162.6 mL vs. 113.1 mL, P < 0.05], and significantly longer procedural times [Table 4, 139.7 min vs. 106.1 min, P < 0.01] versus TFA. However, TRA did amount to a significantly lower average procedural equipment cost than TFA [Table 4, $6666.9 vs. $7105.6, P < 0.01].
| Age | Count |
|---|---|
| <65 Y | 76 |
| ≥65 Y | 54 |
| Total: 130 Median Age: 63 Y |
|
| Sex | Count |
| Male | 111 |
| Female | 19 |
| Total: 130 |
| All (n = 146) | TRA (n = 90) | TFA (n = 56) | P-value | |
|---|---|---|---|---|
| Radiation Dose (mGy PSD) | 1503.8 | 1578.9 | 1383.0 | 0.08 |
| Fluoroscopy time (min) | 26.0 | 26.8 | 24.8 | 0.23 |
| All patients (n = 146) | TRA (n = 90) | TFA (n = 56) | P-value | |
|---|---|---|---|---|
| Contrast (mL) | 143.6 | 162.6 | 113.1 | 0.04 |
| All (n = 146) | TRA (n = 90) | TFA (n = 56) | P-value | |
|---|---|---|---|---|
| In suite procedure time (min) | 126.8 | 139.7 | 106.1 | <0.01 |
| Material cost (USD) | 6666.9 | 6393.9 | 7105.6 | <0.01 |
DISCUSSION
Hepatic malignancies continue to cause a significant burden of disease, and are projected to continue increasing in prevalence.[2,21,22] Consequently, loco-regional therapy used to bridge or downstage patients to transplant is an important component toward definitive treatment. The advent of DEB-TACE represents a technical advancement over conventional TACE by providing more predictable dose delivery and reliable ischemia.[6-9,23] As aforementioned, prior body interventional research has highlighted numerous benefits of TRA for catherization over TFA, including fewer access site complications, faster recovery times, and greater patient comfort.[10-17] Few studies, however, have assessed these two vascular techniques (TRA and TFA) in the context of DEB-TACE. This investigation not only compares these two approaches in the DEB-TACE treatment of HCC, but also finds additional supportive data in favor of TRA utilization.
All patients within this investigation underwent mapping angiograms prior to their respective treatment sessions. This data was excluded due to the wide variability in times present in navigating unknown vessels, embolizing nontarget branch vessels, and utilizing cone-beam computed tomography for multiple branch vessels. The need to eliminate confounding parameters, and to analyze the main variable of access modality in regards to known vessels drove our decision to include only procedural data. Furthermore, this study’s retrospective nature also limited the incorporation of mapping angiograms into our design.
In concordance with other investigations, our study documented no complications for TRA.[12-14] TFA also recorded no complications; the overall complication rate for the entire study was 0/146 or 0%. Similarly, no access failures were recorded in either group. In theory, TFA carries a higher risk for complications than TRA given that the femoral artery is ×3 larger in diameter than the radial artery. This is primarily due to larger vessels posing a greater risk for complications such as bleeding, AV fistulas, and pseudo-aneurysms. A follow-up study with a larger sample size could definitely accentuate these differences, and provide further support for the use of TRA over TFA in high-risk scenarios.
In contrast to previous research implicating TRA with greater radiation usage versus TFA, this study showed no significant differences in radiation dose or fluoroscopy time between the two access techniques.[11,19,24] A plausible rationale for this observation can be attributed to the directionality of the advancing catheter versus the body’s natural current of blood flow. A bulk of existing literature on TRA focuses on cardiac interventions where the TR catheter is advanced up against the normal flow of the ascending aorta. The TR catheter in DEB-TACE is seeded down the descending aorta which is in line with both gravity, and the body’s natural direction of blood flow. Meanwhile, the transfemoral catheter in DEB-TACE (and other body interventional procedures) is advanced against the flow of the femoral artery, which hypothetically increases radiation use compared to TRA due to added catheter resistance. The above reasoning helps explain why DEB-TACE TRA did not show significantly higher radiation doses or fluoroscopy times versus DEB-TACE TFA in this study. Given that our operators have more experience with TFA and only started learning TRA in 2014, TRA has the potential to have even lower radiation totals/shorter fluoroscopy times compared to TFA. A follow-up investigation is certainly needed to elucidate whether a lack of technical experience did not overestimate the recorded radiation data for TRA DEB-TACE in this study.
Unlike previous studies which showed no differences in contrast usage between TRA and TFA, our investigation revealed significantly greater contrast volumes for TR DEB-TACE.[25] Although a concrete mechanism cannot be postulated to explain this discrepancy at this time, previous studies have noted a decrease in contrast use among operators with more training and familiarity.[25] Given the relatively recent introduction of TRA within our institution, a follow-up study will truly help ascertain if the current contrast data is a byproduct of inexperience or specific to TRA DEB-TACE. Needless to say, it is crucial to minimize overall contrast usage, as multiple investigations have reported on the dose-dependent nature of contrast-induced nephropathy.[9,26]
Our investigation also reported a significant increase in procedural time for TRA, which has not been previously mentioned in existing literature.[26,27] Most likely, this can be explained by the initial technical challenges associated with TRA, and the lack of familiarity with the procedure among our operators in the early years of this study. In theory, TRA should be faster than TFA due to being a more convenient mode of initial vascular entry; in addition, the catheter undergoes less total resistance via TRA compared to TFA as aforementioned which only speeds up the time to hepatic access. Moreover, this reduced time does not take into account TRA’s more efficient hemostasis times compared to TFA due to the radial artery requiring ×9 less of an area in need of clamping versus the femoral artery. This only further encourages more efficient ambulation and discharge of the patient, decreasing overall recovery time and subsequent hospital costs related to prolonged length of stay. Given these findings, a follow-up study is necessitated to determine if the total procedure times for TRA in this study can be further mitigated with increased technical expertise.
The final parameter assessed in this study was the procedural equipment cost between TRA and TFA. Our cost model did not include each patient’s postprocedural hospital stay, and was strictly limited to the charges of items (i.e., catheters, guide wires, and syringes) utilized in each operation. Angiography suite time was not built into our design. Our data revealed that TRA was approximately $700 cheaper per operation than TFA, which translates to a net savings of $21,000 across 30 HCC DEB-TACE procedures conducted at our institution every year. A large portion of this cost discrepancy is due to the use of expensive TFA closure devices (i.e., STARCLOSE, MYNXGRIP, and ANGIOSEAL), which are roughly 200$ more than the TR Band utilized for TRA. Given our hospital’s tremendously large patient volume, these transfemoral closure devices are quite necessary for allowing for more efficient postprocedural turnover compared to the alternative of manual and/or band compression. Moreover, these transfemoral closure devices are more convenient for the operator, more comfortable for the patient, and exhibit higher efficiency at achieving effective hemostasis. This argument only further adds to the rationale for utilizing TRA whenever feasible to limit the cost associated with required closure devices. The other roughly 500$ component in the cost discrepancy above can be attributed to the technical challenges of, and the subsequent equipment required for accessing and navigating the femoral artery- which requires more deliberation given its larger size and higher risk for bleeding compared to the radial artery. The present cost analysis of this study is limited, however, because it does not account for postprocedural savings of TRA. In one systematic review, TRA saved hospitals, on average, $275 more per patient than TFA when considering factors such as hemostasis time, and supplemental costs due to procedural complications.[28] These observations only further augment our institution’s findings that suggest TRA is more cost efficacious than TFA.
The primary limitation of this investigation is its retrospective nature, and its evaluation from a single institution’s perspective. A prospective study would’ve been more ideal in order to mitigate biases with regards to data collection, providing further confirmation of this study’s results. Our investigation noted a higher male percentage in both access cohorts, which was not previously able to be accounted for. This is most attributable to a higher male fraction making up the surrounding patient population which our hospital serves. Retrospective analysis and sampling also contributed to an uneven number of TRA and TFA cases, mostly attributable to the predominance of TRA within our institution beginning in 2014, and its gradual preference by our operators over time as aforementioned. Understandably, a larger prospective, randomized, and multicenter clinical trial would allow for more equitable sampling of both access groups, and help mitigate any biases present in TRA utilization.
Everything considered, the findings of this study demonstrate that the well-documented benefits of TRA can be applied to DEB-TACE, an important modality for local regional therapy of hepatic metastasis. While TRA was associated with longer procedural times and higher contrast volumes compared to TFA, TRA showed no significant differences in regards to radiation use or fluoroscopy time versus TFA. Additionally, this investigation provided further evidence to suggest that TRA is more cost efficacious than TFA in body interventional procedures. The results of this study certainly advocate for the utilization of TRA whenever viable as an alternative to TFA in the DEB-TACE treatment of HCC patients.
Declaration of patient consent
Institutional Review Board (IRB) permission obtained for the study.
Financial support and sponsorship
Nil.
Conflict of interest
There are no conflicts of interest.
References
- Mortality assessment of patients with hepatocellular carcinoma according to underlying disease and treatment modalities. Medicine (Baltimore). 2017;96:E5904.
- [CrossRef] [PubMed] [Google Scholar]
- Review of hepatocellular carcinoma: Epidemiology, etiology, and carcinogenesis. J Carcinog. 2017;16:1.
- [CrossRef] [PubMed] [Google Scholar]
- Transarterial chemoembolization with drug-eluting beads is effective for the maintenance of the Milan-in status in patients with a small hepatocellular carcinoma. Liver Transplant. 2015;21:1259-69.
- [CrossRef] [PubMed] [Google Scholar]
- Bridging and downstaging treatments for hepatocellular carcinoma in patients on the waiting list for liver transplantation. World J Gastroenterol. 2013;19:7515-30.
- [CrossRef] [PubMed] [Google Scholar]
- The treatment of intermediate stage tumours beyond TACE: From surgery to systemic therapy. J Hepatol. 2017;67:173-83.
- [CrossRef] [PubMed] [Google Scholar]
- Conventional transarterial chemoembolization versus drug-eluting bead transarterial chemoembolization for the treatment of hepatocellular carcinoma. BMC Cancer. 2015;15:465.
- [CrossRef] [PubMed] [Google Scholar]
- Drug-eluting beads transarterial chemoembolization for hepatocellular carcinoma: Current state of the art. World J Gastroenterol. 2018;24:161-9.
- [CrossRef] [PubMed] [Google Scholar]
- Doxorubicin-eluting bead versus conventional TACE for unresectable hepatocellular carcinoma: A meta-analysis. Hepatogastroenterology. 2013;60:813-20.
- [CrossRef] [PubMed] [Google Scholar]
- Conventional versus drug-eluting beads chemoembolization for hepatocellular carcinoma: Emphasis on the impact of tumor size. J Gastroenterol Hepatol. 2017;32:487-96.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of transradial and femoral approaches for percutaneous coronary interventions: A systematic review and hierarchical Bayesian meta-analysis. Am Heart J. 2012;163:632-48.
- [CrossRef] [PubMed] [Google Scholar]
- Radiation exposure in relation to the arterial access site used for diagnostic coronary angiography and percutaneous coronary intervention: A systematic review and meta-analysis. Lancet. 2015;386:2192-203.
- [CrossRef] [PubMed] [Google Scholar]
- Radial versus femoral approach for percutaneous coronary diagnostic and inter- ventional procedures; Systematic overview and meta-analysis of randomized trials. J Am Coll Cardiol. 2004;44:349-56.
- [CrossRef] [PubMed] [Google Scholar]
- Meta-analysis of ten trials on the effectiveness of the radial versus the femoral approach in primary percutaneous coronary intervention. Am J Cardiol. 2012;109:813-8.
- [CrossRef] [PubMed] [Google Scholar]
- Radial versus femoral access in patients with acute coronary syndromes undergoing invasive management: A randomised multicentre trial. Lancet. 2015;385:2465-76.
- [CrossRef] [PubMed] [Google Scholar]
- Patient experience and preference in transradial versus transfemoral access during transarterial radioembolization: A randomized single-center trial. J Vasc Interv Radiol. 2019;30:414-20.
- [CrossRef] [PubMed] [Google Scholar]
- Patient preference for radial versus femoral vascular access for elective coronary procedures: The PREVAS study. Catheter Cardiovasc Interv. 2018;91:17-24.
- [CrossRef] [PubMed] [Google Scholar]
- Patient preference for transradial access over transfemoral access for cerebrovascular procedures. J Vasc Interv Neurol. 2017;9:1-5.
- [PubMed] [PubMed Central] [Google Scholar]
- Transradial vs transfemoral percutaneous coronary intervention in st-segment elevation myocardial infarction: A systemic review and meta-analysis. Can J Cardiol. 2016;32:777-90.
- [CrossRef] [PubMed] [Google Scholar]
- Transradial vs. transfemoral approach in cardiac catheterization: A literature review. Cureus. 2017;9:e1309.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of the ulnopalmar arterial arches with pulse oximetry and plethysmography: Comparison with the Allen’s test in 1010 patients. Am Heart J. 2004;147:489-93.
- [CrossRef] [PubMed] [Google Scholar]
- Epidemiology of hepatocellular carcinoma in the United States: Where are we? Where do we go? Hepatology. 2014;60:1767-75.
- [CrossRef] [PubMed] [Google Scholar]
- The learning curve for transradial percutaneous coronary intervention among operators in the United States: A study from the National Cardiovascular Data Registry®. Circulation. 2014;129:2277-86.
- [CrossRef] [PubMed] [Google Scholar]
- Chemoembolization of hepatocellular carcinoma. Semin Intervent Radiol. 2013;30:3-11.
- [CrossRef] [PubMed] [Google Scholar]
- Radiation exposure during coronary angiography via transradial or transfemoral approaches when performed by experienced operators. Am Heart J. 2013;165:286-92.
- [CrossRef] [PubMed] [Google Scholar]
- A randomized comparison of transradial versus transfemoral approach for coronary angiography and angioplasty. JACC Cardiovasc Interv. 2009;2:1047-54.
- [CrossRef] [PubMed] [Google Scholar]
- Contrast-induced acute kidney injury: How much contrast is safe? Nephrol Dial Transplant. 2013;28:1376-83.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison between transradial and transfemoral percutaneous coronary intervention in acute ST-elevation myocardial infarction. Am J Cardiol. 2012;110:1262-5.
- [CrossRef] [PubMed] [Google Scholar]
- Systematic review and cost- benefit analysis of radial artery access for coronary angiography and intervention. Circ Cardiovasc Qual Outcomes. 2012;5:454-62.
- [CrossRef] [PubMed] [Google Scholar]







