Translate this page into:
Emergency room imaging findings in patients presenting after COVID-19 vaccination

*Corresponding author: Nadia Solomon, Department of Radiology and Biomedical Imaging, Yale University School of Medicine, New Haven, Connecticut, United States. nadia.solomon@yale.edu
-
Received: ,
Accepted: ,
How to cite this article: Solomon N, Sailer A, Patel A, Revzin MV. Emergency room imaging findings in patients presenting after COVID-19 vaccination. J Clin Imaging Sci 2022;12:33.
Abstract
Objectives
Data on potential side effects of COVID-19 vaccines remains limited. This study aims to evaluate the relationship between the clinical presentations and imaging findings of emergency room (ER) patients presenting with suspected side effects or complications of recent COVID-19 vaccination.
Materials and Methods
An Institutional Review Board-approved retrospective analysis of vaccinated patients who underwent imaging studies in the ER between December 2020 and August 2021 was conducted. Reports were analyzed for imaging modality, chief complaints, and imaging findings.
Results
A total of 173 studies on 161 patients were included: 73 X-rays, 57 computed tomographys, 12 magnetic resonance imagings, and 31 ultrasounds. Analysis of the 168 reports dictated in these 173 studies revealed chest pain (27%), shortness of breath (17%), headache (12.5%), fever (10%), and cough (11.9%) as the most common presenting signs/symptoms. About 57.7% of reports showed no post-vaccine complications. Of the 42.3% of reports with findings, lung opacities/consolidation (36.6%) and cervical and/or axillary adenopathy (35.2%) were most commonly seen; other major findings included saddle embolus (1.4%) and vertebral artery occlusion (1.4%).
Conclusion
Chest pain, cough, shortness of breath, and headache were the most common presenting symptoms in the ER after COVID-19 vaccination, and chest X-ray and computed tomography chest angiography were the most commonly ordered studies to assess vaccine-related complications. Lung opacities/consolidations were the most common findings. Given that vascular post-vaccine complications are considered the most dangerous and 2.8% of reports demonstrated positive vascular findings, concern for vascular complications should initiate appropriate imaging to ensure prompt diagnosis and management.
Keywords
COVID-19
Vaccine
Side effect
Complication
Emergency radiology
INTRODUCTION
COVID-19, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was officially declared a pandemic by the World Health Organization on March 11, 2020, and has since been reported around the world.[1] Mortality associated with COVID-19 is currently estimated at 1.6% in the United States, and most commonly occurs secondary to respiratory failure from acute lung injury and complicating acute respiratory distress syndrome.[2-4] Clinical presentation, however, is not limited to flu-like (fever, malaise) and pulmonary symptoms: patients have also been observed to present with altered mental status, headache, loss of taste/smell, and various gastrointestinal symptoms, and even asymptomatic patients have been identified.[5-8] Reported post-COVID-19 manifestations/complications are also varied, including fatigue, pulmonary fibrosis, renal failure, myocarditis, and stroke.[9]
To meet the emergent need to reduce spread and mortalities, several vaccines have been developed and are being administered rapidly and in mass: as of July 12, 2021, 184.4 million people in the United States had received at least one vaccine dose, and 159.5 million were fully vaccinated (48.0% of the population); in Connecticut alone, 2.42 million people had received one dose, and 2.2 million were fully vaccinated (61.8% of the state’s population).[10] Because of the rapid rollout, data and literature on the full range of vaccine side effects and potential complications remain limited.[11,12] Just as the disease itself is associated with a wide variety of clinical presentations, post-disease manifestations, and unexpected complications, the COVID-19 vaccine has been accompanied by some unexpected side effects, some of which have resulted in emergency room (ER) presentations.
The following study aims to evaluate the relationship between the clinical presentations and imaging findings of ER patients (1) presenting with symptoms suspected to be side-effects of recent COVID-19 vaccination, or (2) with imaging findings thought to be due to recent vaccination.
MATERIAL AND METHODS
This is an Institutional Review Board (IRB) approved retrospective analysis of patients who underwent imaging studies in the ER between December 2020 and August 2021 to evaluate for potential vaccine complications. A search was performed for studies containing the keywords “vaccine” or “vaccination” in their reports. Studies performed to evaluate for potential vaccine complications or demonstrate imaging findings thought to be related to vaccination were included in the data analysis. Studies performed on an inpatient or outpatient basis were excluded from the analysis. Studies with reports noting no COVID-19 vaccination or referring to vaccination other than for COVID-19 were also excluded. The radiology reports included in the study had been read either by an attending radiologist or had been preliminarily read by a resident radiologist before being finalized by an attending radiologist. Reports were analyzed for imaging modality, chief complaints, and imaging findings. A subset of studies was further analyzed for clinical and laboratory data. Descriptive analysis was carried out via calculation of mean and range for quantitative variables and frequency and percentage for qualitative variables. Statistical analysis was performed using Excel software.
RESULTS AND STATISTICAL ANALYSES
A search of the Yale-New Haven Health System imaging database for reports containing the keywords “vaccine” or “vaccination” ordered since the first COVID-19 vaccine was administered in the United States returned 2249 reports for the time period of December 14, 2020, through July 12, 2021. After excluding inpatient and outpatient studies, 180 ER studies remained. After filtering out studies on patients who had not been vaccinated for COVID, the final sample comprised 173 studies performed on 161 patients (six of the 173 patients received two imaging studies). Of the 161 patients, 58 were male (36.0%) and 103 were female (64.0%). Patient ages ranged from 12 to 97 years [Figure 1], with a mean age of 47 years. Eight patients (5%) were under 15 years of age, 37 (23%) were between 16 and 30 years of age, 37 (23%) were between 31 and 45 years of age, 29 (18% were between 61 and 75 years of age, and 20 (12.4%) were over 75 years of age.
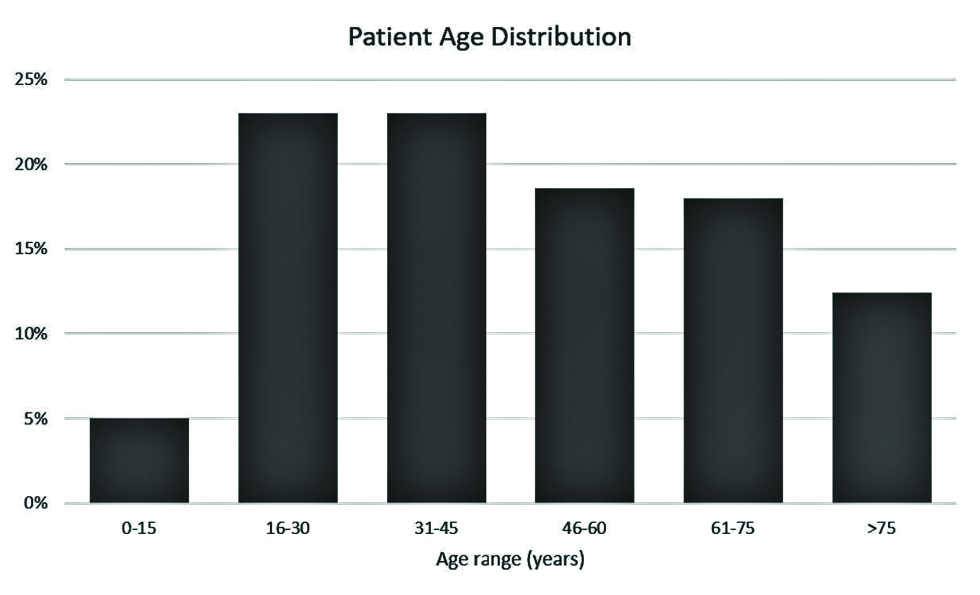
- Age distribution of the included 161 COVID-19-vaccinated ER patients.
Of the 173 included studies performed on 161 patients, 73 were radiographic studies, 57 were computed tomography (CT) studies, 12 were magnetic resonance imaging (MRI) studies, and 31 were ultrasound (US) studies. A more detailed breakdown of study types is found in Table 1.
| Type of study | Number of studies |
|---|---|
| XR chest | 68 |
| CTA chest | 18 |
| Echocardiogram | 13 |
| US extremity | 10 |
| CTA head neck | 9 |
| CT head | 9 |
| CT abdomen and pelvis | 6 |
| CT head venogram | 6 |
| CT chest, abdomen, and pelvis | 4 |
| MRI brain | 3 |
| MRI cervical spine | 3 |
| XR shoulder | 2 |
| MRI thoracic spine | 2 |
| MRI total spine | 2 |
| MRV brain | 2 |
| US head/neck | 2 |
| US transvaginal/pelvis | 2 |
| US soft tissue | 2 |
| CT chest | 1 |
| CT head cervical spine | 1 |
| CT humerus | 1 |
| CT neck | 1 |
| CTA chest, abdomen, and pelvis | 1 |
| US appendix | 1 |
| US right upper quadrant | 1 |
| XR hip | 1 |
| XR humerus | 1 |
| XR neck | 1 |
CT: Computed tomography, CTA: CT angiography, XR: X-ray, MRI: Magnetic resonance imaging, MRV: Magnetic resonance venography, US: Ultrasound
Signs and symptoms reported by these patients, detailed in Table 2, were varied, but most commonly included chest pain (27%; 48 reports on 50 studies performed for 47 patients), shortness of breath (17%; 31 studies/reports for 30 patients), headache (12.5%; 23 reports on 25 studies performed for 21 patients), cough (11.9%; 20 studies/reports for 20 patients), and fever (10%; 17 studies/reports for 17 patients). A total of 72 patients (44.7%) presented with more than one sign/symptom.
| Chief complaint | Number of patients |
|---|---|
| Chest pain | 47 |
| Shortness of breath | 30 |
| Headache | 21 |
| Cough | 20 |
| Fever | 17 |
| Dizziness | 9 |
| Emesis | 8 |
| Trauma | 7 |
| Lower extremity swelling | 6 |
| Nausea | 6 |
| Abdominal pain | 5 |
| Lower extremity pain | 5 |
| Upper extremity pain | 5 |
| Altered mental status | 4 |
| Upper extremity swelling | 4 |
| Weakness | 4 |
| Hemiparesis | 3 |
| Paresthesia | 3 |
| Dysuria | 2 |
| Foreign body sensation | 2 |
| Lightheadedness | 2 |
| Lower extremity weakness | 2 |
| Neck swelling | 2 |
| Seizure | 2 |
| Sore throat | 2 |
| Speech deficit | 2 |
| Tachycardia | 2 |
| “Unwell” | 2 |
| Upper extremity redness | 2 |
| AICD firing | 1 |
| Axillary swelling | 1 |
| Body aches | 1 |
| Chills | 1 |
| Congestion | 1 |
| Diarrhea | 1 |
| Dysmenorrhea | 1 |
| Dysphagia | 1 |
| Facial palsy | 1 |
| Fatigue | 1 |
| Flank pain | 1 |
| Hip pain | 1 |
| Lower extremity numbness | 1 |
| Malaise | 1 |
| Myalgias | 1 |
| Near-syncope | 1 |
| Neck pain | 1 |
| Palpitations | 1 |
| Rectal tingling | 1 |
| Saddle anesthesia | 1 |
| Sensory changes | 1 |
| Shoulder pain | 1 |
| Side pain | 1 |
| Slurred speech | 1 |
| Syncope | 1 |
| Upper extremity numbness | 1 |
| Vaginal bleeding | 1 |
| Wheezing | 1 |
AICD: Automated implantable defibrillator
For the resultant 168 reports, 97 (57.7%) demonstrated no evidence of post-vaccine complications. Of the remaining 71 reports (42.3%) identified a positive finding, lung opacities/consolidation (36.6%), followed by cervical and/or axillary lymphadenopathy (35.2%). Other major findings included diverticulitis with abscess (1.4%), saddle embolus (1.4%; Figure 2), septic arthritis (1.4%), small bowel intussusception (1.4%), spinal cord lesion (1.4%), and vertebral artery occlusion (1.4%; Figure 3). A complete list of findings and their instances is reported in Table 3. Two or more findings were described in 16 reports (9.2%). Three patients (1.9%) were found to have both lung opacities/consolidation and adenopathy, and two patients (1.2%) were found to have both cervical and axillary adenopathy. Findings for each of the most commonly reported signs and symptoms are detailed in Table 4.
| Imaging findings | Number of reports |
|---|---|
| None | 97 |
| Lung opacities/consolidation | 25 |
| Axillary adenopathy | 21 |
| Cervical adenopathy | 6 |
| Lung nodule | 3 |
| Pleural effusion | 3 |
| Sinus disease | 3 |
| Abnormal LV function | 2 |
| Ovarian cyst | 2 |
| Pericardial effusion | 2 |
| Possible midsternal fracture | 2 |
| Rib fracture | 2 |
| Abscess | 1 |
| Bowel inflammation | 1 |
| Cellulitis | 1 |
| Complicated diverticulitis with abscess | 1 |
| Duodenitis | 1 |
| Enhancing spinal cord lesion with cord edema and expansion | 1 |
| Enlarged perineural nerve root sleeve cysts | 1 |
| Fundal fibroid with decreased perfusion | 1 |
| Lung hyperinflation | 1 |
| Manubrial fracture | 1 |
| Possible residual thymic tissue | 1 |
| Prominent cardiac phasicity | 1 |
| Prominent CSF in the optic nerve sheath complexes | 1 |
| Saddle embolus | 1 |
| Scalp hematoma | 1 |
| Sellar/suprasellar mass | 1 |
| Septic arthritis | 1 |
| Small bowel intussusception | 1 |
| Thickened endometrial cavity | 1 |
| Vertebral artery occlusion | 1 |
ER: Emergency room, LV: Left ventricle, CSF: Cerebrospinal fluid
| Finding | Number of reports (%) |
|---|---|
| Chest pain (48 reports on 50 studies for 47 patients) | |
| Abnormal LV function | 2 (4.0%) |
| Axillary adenopathy | 10 (20.0%) |
| Cervical adenopathy | 1 (2.0%) |
| Lung consolidation | 1 (2.0%) |
| Lung nodule | 2 (4.0%) |
| Lung opacities | 3 (6.0%) |
| None | 32 (64.0%) |
| Pericardial effusion | 2 (4.0%) |
| Cough (20 reports/studies for 20 patients) | |
| Lung opacities | 5 (25.0%) |
| None | 15 (75.0%) |
| Pleural effusion | 1 (5.0%) |
| Fever (17 reports/studies for 17 patients) | |
| Axillary adenopathy | 1 (5.9%) |
| Cervical adenopathy | 1 (5.9%) |
| Lung consolidation | 1 (5.9%) |
| Lung opacities | 6 (35.3%) |
| None | 10 (58.8%) |
| Pleural effusion | 2 (11.8%) |
| Headache (23 reports on 25 studies for 21 patients) | |
| Axillary adenopathy | 3 (12.0%) |
| Cervical adenopathy | 1 (4.0%) |
| Lung opacities | 1 (4.0%) |
| None | 14 (56.0%) |
| Prominent CSF in the optic nerve sheath complexes | 1 (4.0%) |
| Scalp hematoma | 1 (4.0%) |
| Sellar/suprasellar mass | 1 (4.0%) |
| Sinus disease | 2 (8.0%) |
| Vertebral artery occlusion | 1 (4.0%) |
| Extremity pain/redness/swelling (17 reports/studies for 17 patients) | |
| Abscess | 1 (5.9%) |
| Axillary adenopathy | 1 (5.9%) |
| Cellulitis | 1 (5.9%) |
| None | 13 (76.5%) |
| Septic arthritis | 1 (5.9%) |
| Prominent cardiac phasicity | 1 (5.9%) |
| Shortness of breath (31 reports/studies for 30 patients) | |
| Abnormal LV function | 1 (3.2%) |
| Axillary adenopathy | 6 (19.4%) |
| Lung hyperinflation | 1 (3.2%) |
| Lung nodule | 2 (6.5%) |
| Lung opacities | 6 (22.6%) |
| None | 16 (51.6%) |
| Saddle embolus | 1 (3.2%) |
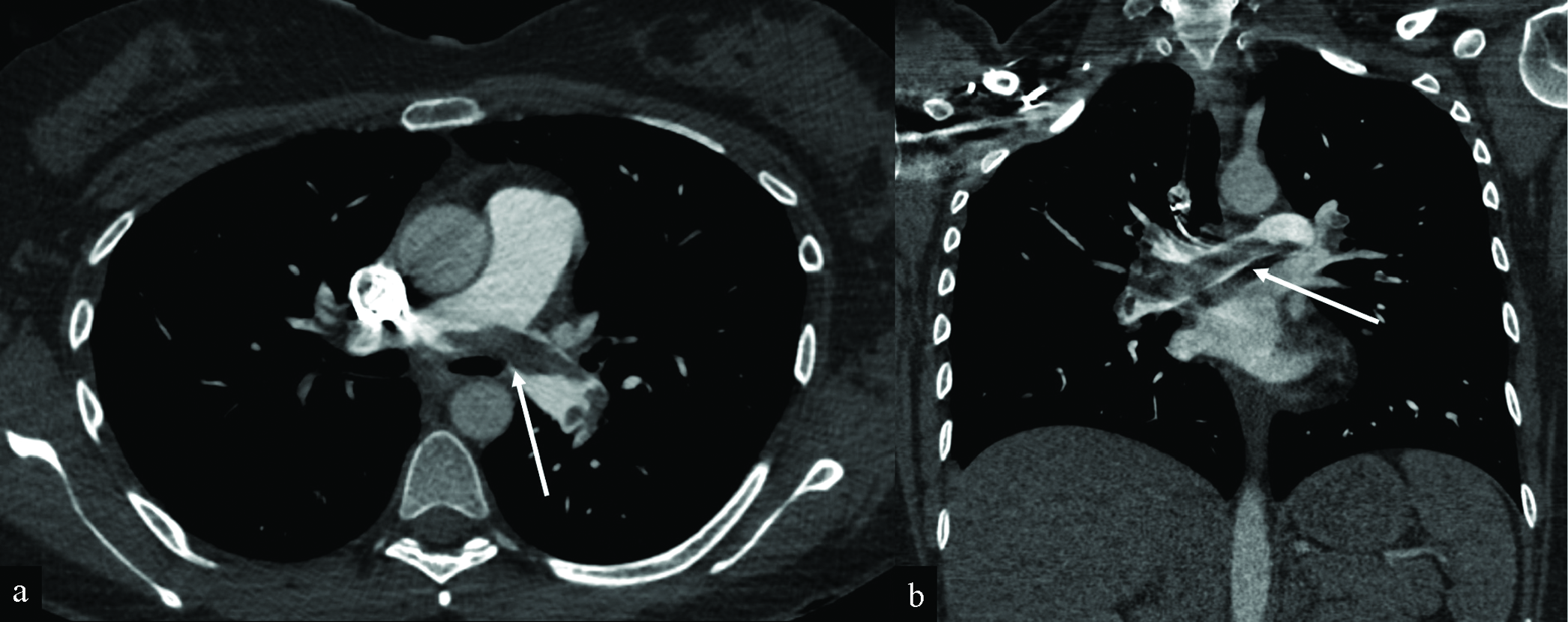
- Saddle pulmonary embolus in a 30-year-old woman presenting with shortness of breath and chest pain 4 days after receiving her first Moderna COVID-19 vaccination. Axial (a) and coronal (b) CT angiography of the chest images demonstrate saddle pulmonary embolus (white arrows) with extensive bilateral clot burden and associated CT findings of right heart strain (not shown). The RV/LV ratio measures greater than 1. There is a trace reflux of contrast into the IVC (not shown). The patient underwent pulmonary artery thrombectomy, with a significant clot burden aspirated from the right lower lobe. After thrombectomy, the patient’s symptoms improved. Heparin drip was continued.
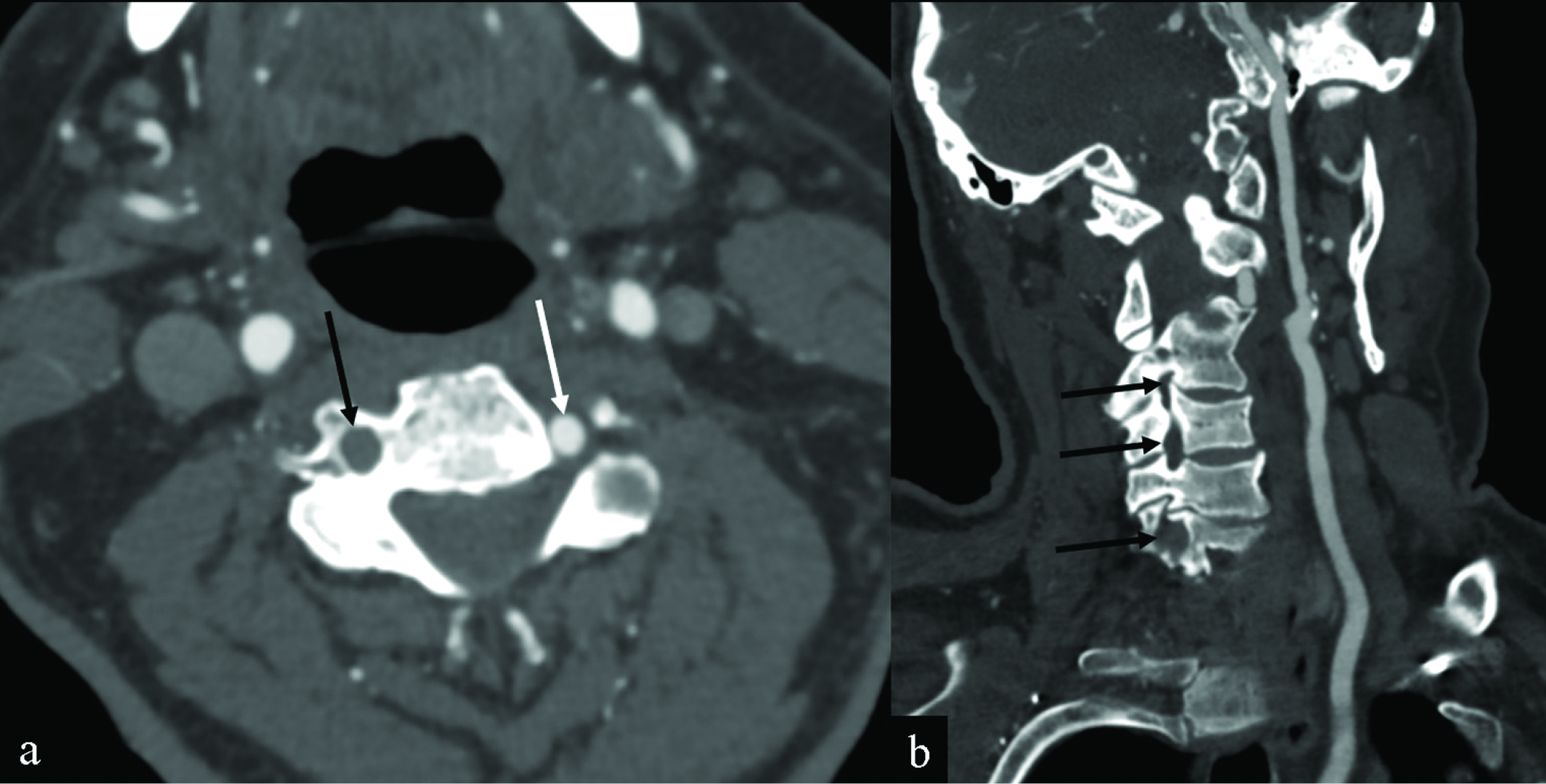
- Vertebral artery thrombosis in an 82-year-old man presenting with altered mental status, headaches, and myalgias after receiving his COVID-19 vaccination the day before. Axial (a) and coronal (b) CT angiography MIPs of the neck images demonstrate non-visualization of the V1 and V2 segments of the right vertebral artery (black arrows). Note that the patient had no significant atherosclerotic disease. The left vertebral artery is patent (white arrow).
Twenty-five chest radiographs and chest CTs with reports describing lung opacities were performed on 24 patients. As one patient returned to the ER and received a repeat chest radiograph only a day following the initial presentation, only the initial presentation was included in the analysis. The medical records for these 24 patients were then reviewed for relevant clinical and laboratory data from the visits in question. The date of the most recent COVID vaccination leading up to ER presentation was reported in the medical records of 23 patients. The average time period between COVID vaccination and ER presentation was 20 days (minimum 1 day, maximum 77 days). Of the 24 patients demonstrating lung opacities/consolidations, 18 were tested for COVID, and four of these patients were found to be COVID-positive (16.7%). Ten of the 24 patients were admitted to the hospital (41.7%), including three of the four COVID-positive patients. Of the 20 patients with either a negative or no COVID test, nine were empirically treated for pneumonia (37.5%), four were diagnosed with and treated for pulmonary edema (16.7%), four were presumed to demonstrate atelectasis (16.7%), two were thought to demonstrate pneumonitis (8.3%), and one was thought to be presented with a nonspecific vaccine reaction (4.2%). For the four COVID-positive patients, the average time period since vaccination was 20 days.
DISCUSSION
COVID-19 vaccines were developed to stimulate the production of antibodies and provide immunity against COVID-19 disease, as well as mitigate the severity of the disease in case of reinfection. Although serious reactions to these vaccines are very rare, some side effects have been experienced by the recipients, which have resulted in ER presentations.[13]
Through July 12, 2021, at our institution recent vaccination was referenced in the imaging reports of 161 patients presenting to the system ERs. Of the 168 reports, more than half (57.7%) demonstrated no evidence of post-vaccine complications, and this remained true for studies ordered to address the most common presenting symptoms (chest pain, cough, fever, headache, extremity pain/redness/swelling, and shortness of breath).
Of the 161 patients who were imaged, two-thirds were female, but patient age varied widely. Chest pain (47 patients) and shortness of breath (30 patients) were among the most common presenting symptoms in ER patients following COVID-19 vaccination, consistent with the most commonly ordered studies being chest radiographs (68 studies) and CT angiography (CTA) of the chest for pulmonary embolism (18 studies).
While lung opacities/consolidations were the most common imaging findings, it is important to note that “lung opacities/consolidation” are nonspecific findings that may reflect atelectasis, aspiration, edema, infection, and more. While a more detailed analysis of clinical and laboratory data from patients who demonstrated lung opacities/consolidation on imaging studies confirmed their nonspecific nature, it also revealed various types of pulmonary infection/inflammation (including COVID pneumonia) in more than 60% of patients. Given an average post-vaccine interval of 20 days in this group of patients, a potential association between lung infection/inflammation and recent COVID vaccination is important to consider.
Cervical/axillary lymphadenopathy, seen in 36.6% of positive studies, may represent a common side effect of vaccination, a finding concordant with other previously published studies reporting lymphadenopathy (including FDG-avid lymph nodes) following COVID-19 vaccination.[12,14] A few studies demonstrated more serious/critical findings (i.e., complicated diverticulitis with abscess, pulmonary embolus, septic arthritis, spinal cord lesion, and vertebral artery occlusion). Vascular post-vaccine complications are considered the most dangerous complications. With 2.8% of reports demonstrating positive vascular findings, concern for vascular complications should initiate appropriate imaging to ensure prompt diagnosis and management.
An acknowledged limitation of this study is reliance on reference to recent vaccination in the clinical indication of the imaging report in order to identify relevant studies, as other patients may have been identified as presenting with potential vaccine side effects without this being referenced in the indication by the ordering clinician. This study is also limited by the lack of a control group given the difficulties of matching patients by age, presenting symptoms, time of control presentation, underlying health issues, and gender. Variabilities in the type of vaccine received and the time between vaccination and patient presentation are also limiting factors. Additionally, evaluation was limited to patients presenting to this institution, so the data cannot be extrapolated to estimate the number of side effects relative to the vaccinated population of the state. Despite this limitation, a direct reference to COVID-19 vaccination in the imaging reports suggests heightened clinical suspicion by ordering clinicians that either the patient’s presentation or imaging findings were related to recent COVID-19 vaccination, thereby supporting the assertion that these studies be the focus for analysis in this study.
Declaration of patient consent
Institutional Review Board (IRB) permission obtained for the study.
Financial support and sponsorship
Nil.
Conflict of interest
Author Dr. Margarita V Revzin is Section Editor of the journal.
References
- Multisystem imaging manifestations of COVID-19, part 1: Viral pathogenesis and pulmonary and vascular system complications. RadioGraphics. 2020;40:1574-99.
- [CrossRef] [PubMed] [Google Scholar]
- Coronavirus disease 2019 (covid-19) situation report - 77, World Health Organization
- Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846-8.
- [CrossRef] [PubMed] [Google Scholar]
- Mortality analyses. Coronavirus Resource Center 9/25/2021 [cited 2021 September 26]; Available from: https://coronavirus.jhu.edu/data/mortality
- Asymptomatic patients as a source of COVID-19 infections: A systematic review and meta-analysis. Int J Infect Dis. 2020;98:180-6.
- [CrossRef] [PubMed] [Google Scholar]
- Gastrointestinal and liver manifestations of COVID-19. Saudi J Gastroenterol. 2020;26:226-32.
- [CrossRef] [PubMed] [Google Scholar]
- Gastrointestinal, hepatobiliary, and pancreatic manifestations of COVID-19. J Clin Virol. 2020;128:104386.
- [CrossRef] [PubMed] [Google Scholar]
- Assessment and characterisation of post-COVID-19 manifestations. Int J Clin Pract. 2021;75:e13746.
- [CrossRef] [PubMed] [Google Scholar]
- [cited 2021 June 18]; Available from: https://ourworldindata.org/covid-vaccinations?country=USA
- Side effects after COVID-19 vaccinations among residents of Poland. Eur Rev Med Pharmacol Sci. 2021;25:4418-21.
- [CrossRef] [PubMed] [Google Scholar]
- Axillary adenopathy following COVID-19 vaccination: A single institution case series. Clin Imaging. 2021;80:111-6.
- [CrossRef] [PubMed] [Google Scholar]
- COVID-19 vaccination attitudes, perceptions, and side effect experiences in malaysia: Do age, gender, and vaccine type matter? Vaccines (Basel). 2021;9:1156.
- [CrossRef] [PubMed] [Google Scholar]
- [18F]FDG uptake of axillary lymph nodes after COVID-19 vaccination in oncological PET/CT: Frequency, intensity, and potential clinical impact. Eur Radiol. 2021;32:508-16.
- [CrossRef] [PubMed] [Google Scholar]






