Translate this page into:
Imaging in the Diagnosis of Juvenile Nasopharyngeal Angiofibroma
Address for correspondence: Dr. Satyaranjan Mishra, Prasanti, Kathogola Road, Mangalabag, Cuttack 753 001, Odisha, India. E-mail: drsatyaranjanmds@gmail.com
-
Received: ,
Accepted: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Juvenile nasopharyngeal angiofibroma (JNA) is a rare, benign, highly vascular, and locally aggressive tumor that predominantly occurs in adolescent males. Usually, the presenting symptom is a painless nasal obstruction or epistaxis; however, other symptoms may develop depending on the size and extent of the tumor mass. Owing to the vascularity of the tumor, incisional biopsy is not attempted. The diagnosis is dependent on multiplanar imaging modalities like Computed Tomography (CT), Magnetic Resonance Imaging (MRI), and Angiography. These imaging modalities help in assessing the tumor mass, pre-operative embolization of the feeder vessel, and treatment planning. Usually, patients with JNA are diagnosed by otorhinolaryngologists, but here, we present a rare case of JNA reporting to the dental hospital due to a tender palatal swelling.
Keywords
Adolescent males
aggressive vascular benign tumor
Juvenile nasopharyngeal angiofibroma
multiplanar imaging
INTRODUCTION

Juvenile nasopharyngeal angiofibroma (JNA) is a rare, benign neoplasm that accounts for less than 0.5% of all head and neck tumors; but it is the most common tumor of the nasopharynx.[1] JNA occurs commonly in the nasopharynx of adolescent males. The site of origin of JNA remains controversial. While some authors stress its origin from the superior lip of the sphenopalatine foramen at the junction of the pterygoid process and the sphenoid process of the palatine bone; others believe that it arises from the vidian (pterygoid) canal. JNAs are slow-growing and initially expand intra-nasally into the nasopharynx and nasal cavity and later spread into the pterygomaxillary space. Over time, JNAs will eventually erode bone and invade the infratemporal fossa, orbit, and middle cranial fossa.[2] The diagnosis of JNA is essentially based on a detailed history and clinical examination; confirmed by multiplanar imaging studies like Computed Tomography (CT) and Magnetic Resonance Imaging (MRI). Incisional biopsies are not recommended because of the vascular and hemorrhagic nature of this tumor. Angiography is done to assess the vascular supply in larger tumors and to make it possible to embolize the feeder vessel to reduce intra-operative bleeding. Surgery is the Gold Standard of treatment, but for large expansile lesions radiotherapy, chemotherapy, and hormone therapy are also used.
CASE REPORT
A 14-year-old male child reported to the dental hospital with the complaint of a painful swelling in the palate that had persisted for 1 year. History revealed that the swelling was small in size in the beginning, gradually increasing in size over time. Initially, there was no pain associated with the swelling, but with an increase in size, the patient experienced pain, dysphagia, nasal obstruction, and epistaxis. There was no history of toothache and the patient's medical and dental histories are non-contributory.
On clinical examination, the patient appeared dull and weak with mild facial asymmetry due to a diffuse facial swelling on the right side [Figure 1]. The patient was febrile with a temperature of 38°, with enlarged sub-mandibular lymph-nodes which were soft, mobile, and tender on palpation.
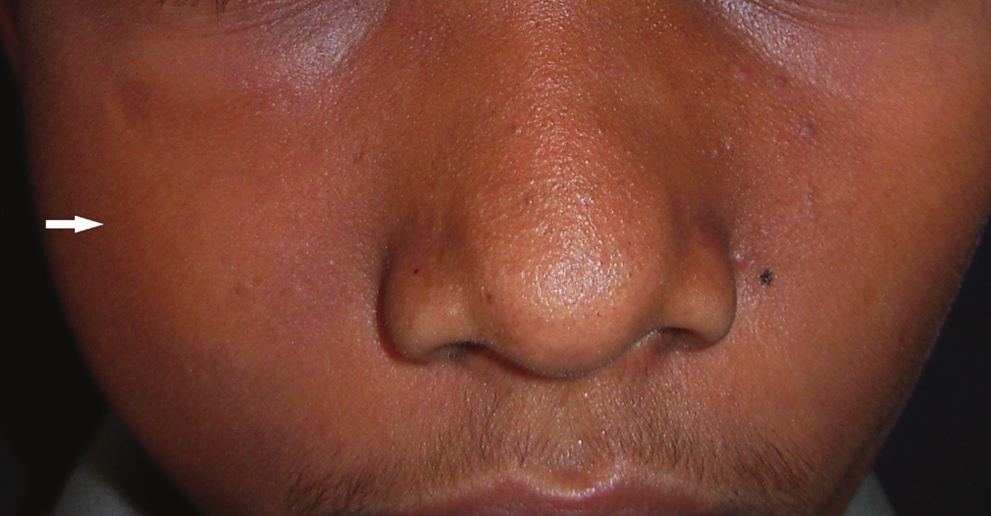
- Diffuse swelling (arrow) is seen in the molar region on the right side of the face.
Intra-orally, a well-circumscribed swelling was seen confined to the soft palate in the midline. The swelling was roughly spherical in shape, with a color of the mucosa, smooth-surfaced, sessile, soft in consistency, and without any secondary changes like ulceration or discharge [Figure 2]. The soft palate movements were reduced. Fine-needle aspiration cytology of the palatal swelling revealed no fluid.
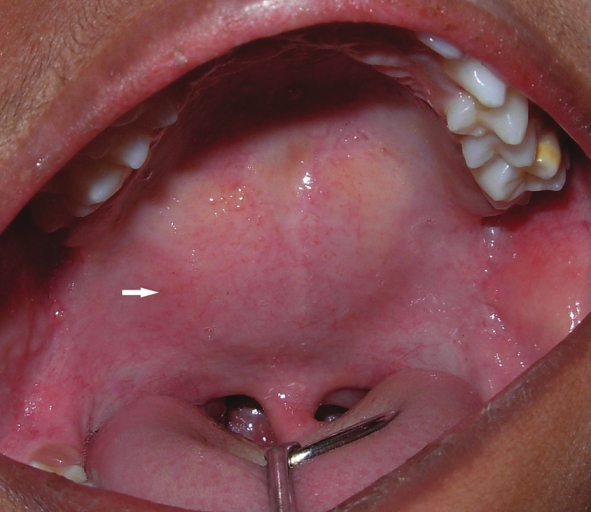
- Well-circumscribed, ovoid swelling (arrow) is seen in the midline of the soft palate.
As the swelling had been present for 1 year, increasing in size, accompanied by constitutional symptoms like fever, dyspnea, dysphagia, fatigue, lymphadenopathy, and epistaxis, a provisional clinical diagnosis of aggressive soft-tissue neoplasm was made. Conventional radiographic and advanced multiplanar imaging were performed to arrive at a definitive diagnosis.
Periapical, maxillary occlusal, and panoramic radiographs did not reveal any bone destruction but the right maxillary sinus appeared radio-opaque, obliterating the antrum. Postero-anterior projection of the skull revealed diffuse opacification of the right antrum, with deviation of the nasal septum toward the left side. No bony destruction was, however, observed [Figure 3].
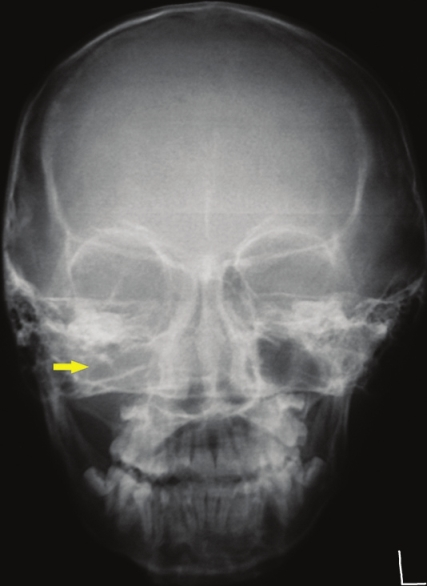
- Postero-Anterior view of the skull radiograph shows diffuse opacification of the right maxillary antrum (yellow arrow).
CT scan revealed a large heterodense destructive soft tissue lesion showing intense contrast enhancement [Figure 4]. (+25 ±35 HU pre-contrast and +60 ±120 HU post-contrast) The tumor mass was seen in the superior postero-lateral wall of the right nasal cavity, extending into the nasopharynx and adjacent pterygopalatine fossa and the right pre-maxillary space causing bowing of the posterior antral wall with erosion/destruction of adjacent bones. The infra-temporal fossa, orbit, and middle cranial fossa on the right side were also encroached by the lesion [Figure 5].
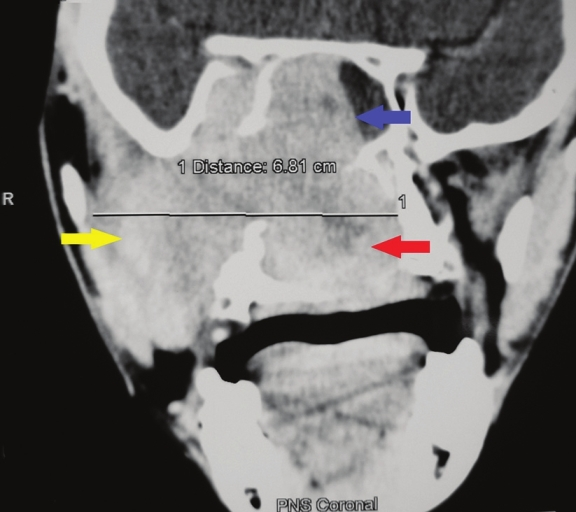
- Coronal section, contrast-enhanced computed tomography scan shows a large heterodense destructive soft tissue lesion with intense contrast enhancement on the right maxillary antrum (yellow arrow), crossing the midline and displacing the nasal septum to the left (red arrow) and superiorly into the nasal cavity (blue arrow).
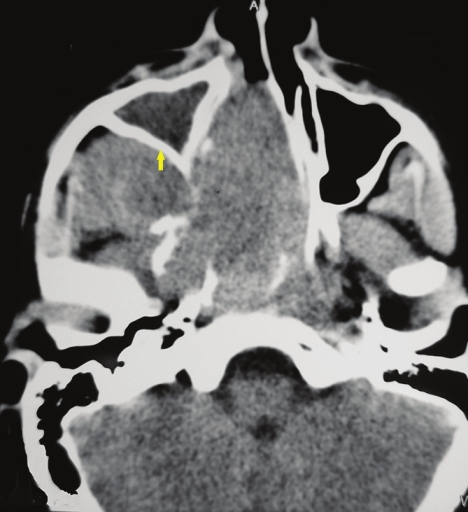
- Axial section, contrast-enhanced computed tomography scan shows a large heterodense destructive soft tissue lesion seen in the superior postero-lateral wall of the right nasal cavity, extending into the nasopharynx and adjacent pterygopalatine fossa, right pre-maxillary space causing bowing of the posterior antral wall: The characteristic Holman Miller sign (yellow arrow) with erosion/ destruction of adjacent bones.
MRI showed a large, well-defined mass in the region of the pterygo-maxillary fissure and sphenopalatine foramen on the right side with a heterogeneous intensity in both T1-weighted [Figure 6] and T2-weighted images [Figure 7]. Tiny flow voids were noted within the lesion consistent with hypervascularity [Figure 8]. The mass encroached into the masticator, buccal, and parapharyngeal spaces on the right side, right nasal cavity, right sphenoid sinus, floor of the middle cranial fossa, and the right cavernous sinus [Figure 9].
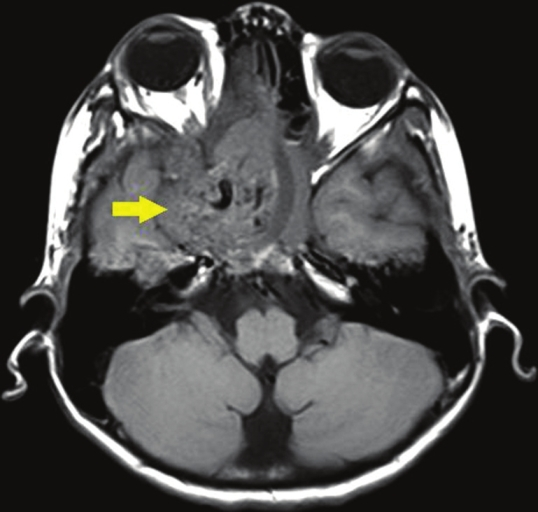
- Axial section, T1-weighted magnetic resonance image shows a large, well-defined mass (arrow) in the region of the pterygo-maxillary fissure and sphenopalatine foramen on the right side with a heterogenous intensity.
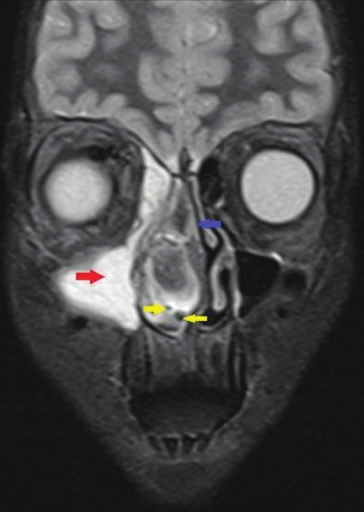
- Coronal section, T2-weighted magnetic resonance image demonstrates a large, well-defined hyperintense mass in the right maxillary antrum (red arrow), displacement of the nasal septum (blue arrow) by the heterointense tumor mass to the left side and tiny flow voids are noted within the lesion consistent with hypervascularity (yellow arrows)
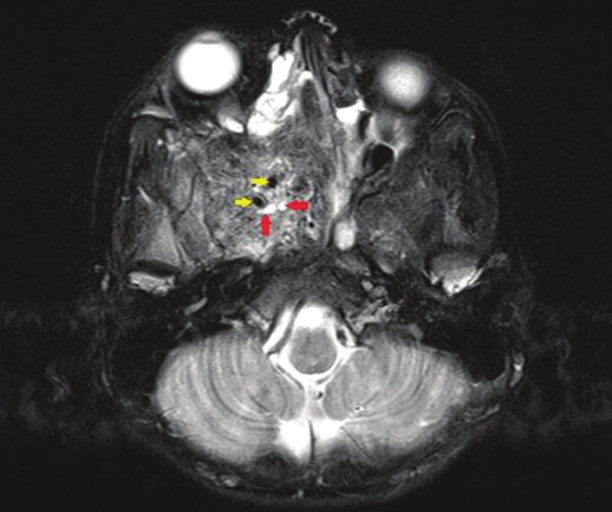
- Axial section, T2-weighted magnetic resonance image reveals a large, well-defined mass in the region of the pterygo-maxillary fissure and spheno-palatine foramen on the right side with heterogenous intensity. Avid enhancement of the mass (red arrows) and tiny flow voids are noted within the lesion (yellow arrows) consistent with hypervascularity.
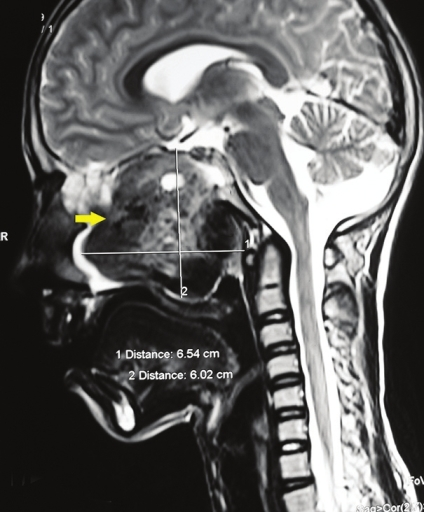
- Sagittal section, T2-weighted magnetic resonance image shows a large, well-defined tumor mass (arrow) with a heterogenous intensity measuring 6.54 cm × 6.02 cm.
Right external carotid artery angiogram [Figure 10] revealed the feeding internal maxillary artery and the hypervascular lesion.
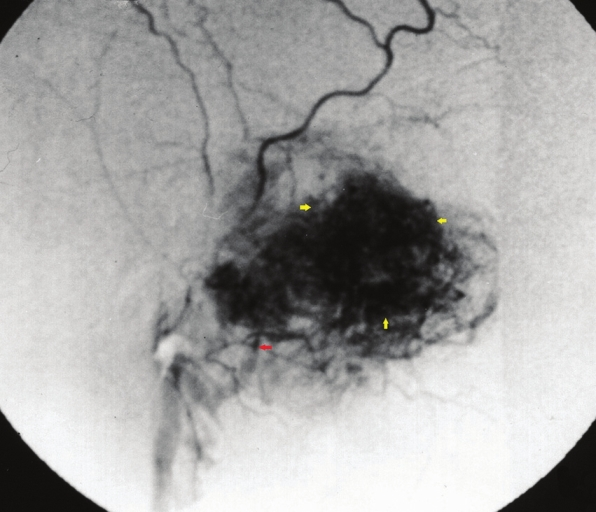
- Right external carotid artery angiogram reveals the feeding internal maxillary artery (red arrow) and the hypervascular lesion (yellow arrows).
Correlating the clinical and imaging findings, a final diagnosis of JNA was made. Biopsy was not done owing to the vascular nature and the site of the tumor mass. The patient was referred to an Otorhinolaryngologist for management.
DISCUSSION
Hippocrates first described the tumor in the 5th century BC, but Friedberg first used the term juvenile angiofibroma in 1940. JNA is an uncommon, highly vascular, locally invasive, unencapsulated tumor with a distinct predilection for origin in the nasopharynx of adolescent males. Genetic studies have shown a close relation between these angiomas and androgen receptor expression, indicating that this tumor is possibly androgen-dependent. This could explain the high prevalence rate of JNA in males.[3]
JNA is supposed to originate from a vascular nidus in the posterolateral wall of nasal cavity near the superior margin of the sphenopalatine foramen. JNA, as the name suggests, is a neoplasm of the young. The average age of occurrence is between 14 and 18 years.
In most cases, the clinical presentation of the angiofibroma comprises of the triad: Nasal blockage, epistaxis, and a nasopharyngeal mass.[1] Conductive hearing loss, dacrocystits, nasal twang of voice, hard and soft palate deformity as seen in our case, and loss or alteration of smell may also be encountered. Advanced lesions may cause facial swelling, proptosis, neuropathy of the affected cranial nerves, and massive hemorrhage.[4] In its extreme form, JNA can cause gross clinical changes producing the characteristic “frog-face” deformity.
The patient should be examined using nasal endoscopy, which usually shows a large, lobulated mass behind the middle turbinate filling the choana, smooth-surfaced with signs of hypervascularity.[5]
The diagnosis of JNA is essentially based on patient history, clinical examination including nasal endoscopy supplemented by imaging studies using CT, MRI, and Angiography for confirmation.[6] JNA presents characteristic imaging signs, many of which allow diagnosis and accurate estimation of extent without recourse to the dangers of biopsy.
On CT images, a JNA appears as a heterodense mass that is centered within the sphenopalatine foramen. On contrast-enhanced CT images, the mass shows avid enhancement.[7] At the time of diagnosis, the mass classically involves the pterygopalatine fossa; in this location, it produces a bowing of the posterior wall of the maxillary antrum, widening of the pterygopalatine fossa, and inferior orbital and pterygomaxillary fissures. Bony erosion of the nasal cavity, hard palate, and pterygoid plates are also common. Anterior bowing of the posterior maxillary wall, due to tumor in the pterygomaxillary space on axial CT, known as the Holman-Miller sign, is one of the characteristic findings of JNA.[1] CT provides good hard tissue imaging to show invasion of the cancellous bone of the sphenoid. The deeper the extension, the greater is the chance of tumor remnants being left behind after surgery, resulting in recurrence. Coronal CT images are used to evaluate the staging of the tumor, showing the relationship between the tumor and surrounding structures, so that the choice of operative approach, prognosis, and other treatment options can be planned accordingly.
MRI is superior to CT for detecting the intracranial extension of the tumor into soft tissues of the skull base.[6] The lesion characteristically shows low signal intensity on T1-weighted images. On T2-weighted images, heterogeneous intermediate signal intensity is seen in the tumor mass and in contrast-enhanced MR images, avid enhancement with flow voids are observed. These MR imaging features, together with the patient's age, can help in differentiating a JNA from other nasopharyngeal lesions.[7] MR imaging is even more important post-operatively to show any residual or recurrent tumor and monitor the effects of radiotherapy.
The differential diagnosis for large nasal masses includes sinonasal polyp; neurofibroma; hypertrophy of adenoids; and malignant neoplasms like nasopharyngeal carcinoma, lymphoma, or rhabdomyosarcoma. Multiplanar imaging of nasopharyngeal carcinoma shows a nonhomogeneous mass arising from the nasopharyngeal mucosa or submucosal space with erosion of the skull base or intracranial extension. Lymphoma may be associated with lymphadenopathy and tumor mass can be seen in the nasopharynx or Waldeyer's ring. Imaging of rhabdomyosarcoma reveals a soft-tissue mass eroding the bone with mild enhancement on CT and marked enhancement on MR images.[8]
Identification of the feeder vessels and their pre-operative embolization to reduce intra-operative hemorrhage is done, following angiography. The size and site of the lesion as well as the size and location of the feeding vessel are demonstrated in angiography.[9] Commonly, the vascular supply to JNAs is primarily from distal Internal Maxillary Artery (IMA) branches, particularly the sphenopalatine, descending palatine, and posterior superior alveolar branches. JNAs may also be supplied by the ascending pharyngeal artery.
Histologically, JNAs originate from myofibroblasts. The tumor is unencapsulated and usually spreads submucosally. It is composed of endothelial cell-lined vascular spaces or channels, in a fibrous connective stroma, surrounded by a collagenous tissue network without a complete muscular layer.[15]
Surgery is the mainstay of treatment of JNA. Pre-operative embolization and newer surgical approaches result in less hemorrhage and complete resection of the tumor. Aggressive re-resection should be done for resectable tumor recurrences. Radiotherapy must be reserved for unresectable, recurrent/residual disease.[10]
CONCLUSION
JNA is an uncommon neoplasm of adolescent males frequently presenting with nasal obstruction and epistaxis. Although patients with JNA may be rarely encountered by dental surgeons as in the present case, with a sound knowledge of the anatomy and help of advanced imaging techniques, the lesion can be diagnosed. It is of prime importance that the lesion be diagnosed early. The stage of the tumor and the age of the patient are predictors of recurrence post surgical intervention. Hence, early diagnosis not only helps in better management, but also prevents recurrence of JNA.
Available FREE in open access from: http://www.clinicalimagingscience.org/text.asp?2013/3/2/1/109469
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- A seven-year experience with patients with juvenile nasopharyngeal angiofibroma. Braz J Otorhinolaryngol. 2010;76:245-50.
- [Google Scholar]
- Numerical sex chromosome aberrations in juvenile angiofibromas: Genetic evidence for an androgen-dependent tumor? Oncol Rep. 2003;10:1251-5.
- [Google Scholar]
- Nasopharyngeal angiofibroma: A reappraisal of clinical features and treatment at National Taiwan University Hospital. J Formos Med Assoc. 1998;97:845-9.
- [Google Scholar]
- Juvenile angiofibroma: Modern imaging and its influence on the surgical treatment of juvenile angiofibroma. J Laryngol Otol. 1999;113:43-4.
- [Google Scholar]
- Diagnostic imaging in nontraumatic pediatric head and neck emergencies. Radiographics. 2010;30:781-99.
- [Google Scholar]
- Tumors and tumor-like conditions. In: Som PM, Cutin HD, eds. Head and Neck Imaging (4th ed). St. Louis MO: Mosby; 2003. p. :261-373.
- [Google Scholar]
- Imaging of chronic and exotic sinonasal disease: Review. AJR Am J Roentgenol. 2007;189:S35-45.
- [Google Scholar]
- Juvenile nasopharyngeal angiofibroma: A single institution study. Indian J Cancer. 2005;42:35-9.
- [Google Scholar]






