Translate this page into:
Pulmonary Kaposi Sarcoma without Mucocutaneous Involvement: The Role of Sequential Thallium and Gallium Scintigraphy
* Corresponding author: Dr. Nader Kamangar, Department of Medicine, Division of Pulmonary and Critical Care Medicine, UCLA Geffen School of Medicine, Olive View-UCLA Medical Center, 14445 Olive View Drive, 2B-182, Sylmar, California 91342, USA. kamangar@ucla.edu
-
Received: ,
Accepted: ,
Abstract
Kaposi sarcoma (KS) is a vascular-related tumor that has been associated with human immunodeficiency virus (HIV). It commonly involves the skin and lymph nodes, and infrequently involves the lungs. In very rare instances, pulmonary KS can be found in the absence of endobronchial and mucocutaneous involvement. Utilization of sequential thallium and gallium scintigraphy can aid in the diagnosis of pulmonary KS in the absence of mucocutaneous and endobronchial involvement. In this report, we discuss a case of a patient with acquired immunodeficiency syndrome who presented with dyspnea and cough and was found to have subtle pulmonary parenchymal nodular airspace opacities. He underwent negative infectious evaluation, including bronchoscopy. Despite the absence of mucocutaneous findings, sequential positive thallium and negative gallium scintigraphy led to an early diagnosis of pulmonary KS. Pulmonary KS in the absence of mucocutaneous involvement is a rare finding that is exceedingly difficult to diagnose. However, pulmonary KS should be considered in patients with HIV who present with respiratory symptoms even if the typical mucocutaneous manifestations of KS are absent. In such circumstances, sequential thallium and gallium scintigraphy can help differentiate pulmonary KS from other processes such as infections and lymphoma, and assist in establishing an earlier diagnosis.
Keywords
Acquired immunodeficiency syndrome thallium scintigraphy
gallium scintigraphy
human immunodeficiency virus
Kaposi sarcoma

INTRODUCTION
Kaposi sarcoma (KS) is a highly vascular tumor spread by the human herpesvirus-8 (HHV-8) virus that garnered increased recognition as an acquired immunodeficiency syndrome (AIDS)-defining illness in the 1980s following the human immunodeficiency virus (HIV)/AIDS epidemic. It is estimated to have affected 15%–20% of men in that demographic.[1] The disease usually involves lymph nodes and mucocutaneous tissues but on occasion can also affect visceral organs such as the gastrointestinal tract, lungs, liver, and spleen. Pulmonary involvement with KS portends a poor prognosis. Pulmonary KS can involve the lung parenchyma, pleural space, tracheobronchial tree, and mediastinal and hilar lymph nodes. Although a few cases have been reported as isolated visceral manifestations of KS, it is extremely uncommon for visceral involvement to occur without mucocutaneous manifestations. Postmortem evaluation of patients with AIDS and mucocutaneous KS found lung involvement in 47%–75% of cases.[2] The frequency of pulmonary KS without mucocutaneous involvement in patients with AIDS ranges from 0% to 14% but rarely does pulmonary KS present before the presence of mucocutaneous disease.[1] When pulmonary KS is suspected, bronchoscopic visualization of endobronchial lesions is the most sensitive diagnostic modality. There are exceedingly rare instances where pulmonary KS exists in the absence of mucocutaneous and endobronchial disease. In such circumstances, diagnosis can prove difficult, as symptoms and radiographic findings can mimic those of other diseases, particularly opportunistic infections and lymphoma. Herein, we present a case that entails those circumstances: a patient with AIDS, presenting with respiratory symptoms and radiographic findings concerning for pulmonary KS in whom there was no mucocutaneous or endobronchial abnormalities. This case highlights the clinical utility of sequential thallium and gallium scintigraphy in establishing a more timely diagnosis of pulmonary KS.
CASE REPORT
A 28-year-old male patient with a history of HIV/AIDS complicated by numerous prior opportunistic infections, presented with progressive dyspnea on exertion and chronic, nonproductive cough. He also endorsed night sweats, chills, and nonbloody diarrhea, as well as weight loss over a period of a few months. He had been off antiretroviral therapy (ART) for the past 2 years. He had a history of intravenous drug use and resided at a rehabilitation facility.
On presentation, he was afebrile with unremarkable vital signs and normal oxygen saturation on room air. Physical examination was only notable for oral thrush, shotty right anterior cervical and submandibular lymphadenopathy, and faint inspiratory crackles on chest auscultation. No abnormal lesions were noted on a thorough skin examination.
Laboratory analysis was notable for a white blood count of 3 × 103/mm3and hemoglobin of 9.6 g/dL. CD4 count was 3 cells/mm3and viral load was 2,990,000 copies/mL. Renal, hepatic, and thyroid function, as well as electrolytes, were all within normal limits.
Initial chest radiograph was unremarkable. Computerized tomography (CT) of the chest showed multiple bilateral, right greater than left-sided, ill-defined peribronchovascular ground-glass nodules [Figure 1]. CT scan of the abdomen and pelvis with contrast showed periportal, celiac, mesenteric, and retroperitoneal lymphadenopathy, as well as several low-density lesions in the liver.
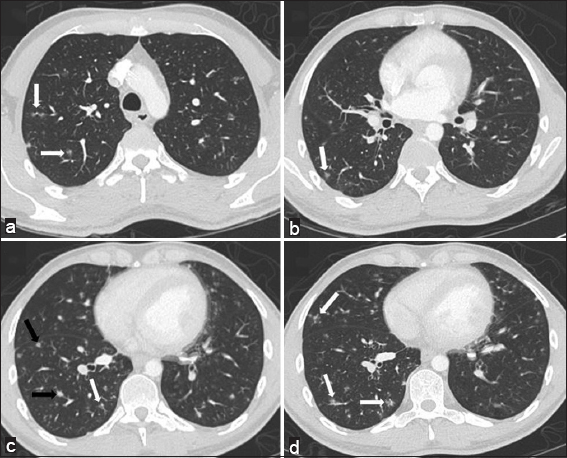
- 28-year-old man with a history of human immunodeficiency virus/acquired immunodeficiency syndrome complicated by numerous prior opportunistic infections, presented with progressive dyspnea and chronic, nonproductive cough, diagnosed with pulmonary Kaposi sarcoma. Computerized tomography chest, lung window. Panels a-d demonstrating multiple, right greater than left-sided, ill-defined ground glass (white arrows) and solid (black arrows) peribronchovascular nodules.
An extensive infectious investigation, including blood, sputum, and fungal cultures, and viral and fungal serologies, was negative. Initial bronchoscopy was remarkable for the absence of oral, laryngeal or endobronchial lesions, and bronchoalveolar lavage was negative. Bone marrow biopsy showed myeloid maturation arrest consistent with AIDS with no evidence of lymphoma or KS.
Sequential thallium 201 and gallium 67 scintigraphy was notable for abnormal delayed thallium uptake in and the absence of gallium uptake, raising concern for pulmonary KS over lymphoproliferative disorders or opportunistic infections [Figure 2]. Based on these findings, the patient underwent an ultrasound-guided core biopsy of a sub-centimeter submandibular lymph node which revealed central sarcomatoid proliferation with positive immunohistochemical staining for CD31, CD34, and HHV-8, confirming the diagnosis of KS [Figure 3].
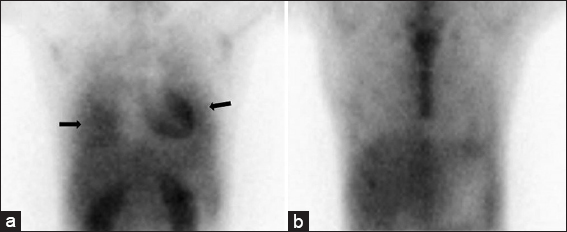
- 28-year-old man with a history of human immunodeficiency virus/acquired immunodeficiency syndrome complicated by numerous prior opportunistic infections, presented with progressive dyspnea and chronic, nonproductive cough, diagnosed with pulmonary Kaposi sarcoma. Scintigraphy demonstrating right greater than left lung delayed thallium uptake (panel a; black arrows) without corresponding gallium uptake (panel b).
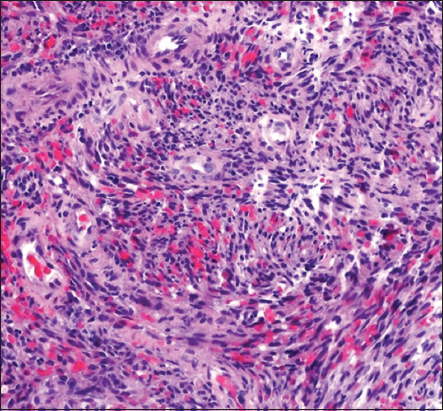
- 28-year-old man with a history of human immunodeficiency virus/acquired immunodeficiency syndrome complicated by numerous prior opportunistic infections, presented with progressive dyspnea and chronic, nonproductive cough, diagnosed with pulmonary Kaposi sarcoma. Submandibular lymph node biopsy showing central sarcomatoid proliferation (hematoxylin and eosin, original magnification ×100).
Treatment with doxorubicin was initiated, and ART was resumed. On completion of two cycles of chemotherapy, the patient developed worsening pancytopenia and neutropenic fever. Repeat thoracic imaging was notable for significant progression of the ill-defined peribronchovascular infiltrates, interlobular septal thickening, and mediastinal and hilar lymphadenopathy [Figure 4]. The patient’s course was complicated by bland alveolar hemorrhage due to progressive pulmonary KS and severe thrombocytopenia. Repeat bronchoscopy revealed characteristic red to slightly violaceous macular lesions in the endobronchial tree, not seen on examination 2 months prior [Figure 5]. The patient was deemed too ill to tolerate further chemotherapy or radiation for treatment of KS. His symptoms worsened, and he expired shortly thereafter.
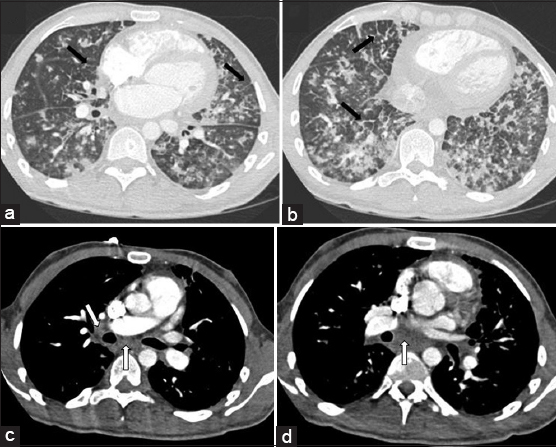
- 28-year-old man with a history of human immunodeficiency virus/acquired immunodeficiency syndrome complicated by numerous prior opportunistic infections, presented with progressive dyspnea and chronic, nonproductive cough, diagnosed with pulmonary Kaposi sarcoma. Computerized tomography chest, lung window. Panels a and b demonstrating progressive bilateral ill-defined ground glass and solid peribronchovascular nodules, and interlobular septal thickening (black arrows). Computerized tomography chest with IV contrast, soft-tissue window. Panels c and d demonstrating mediastinal and hilar lymphadenopathy (white arrows).
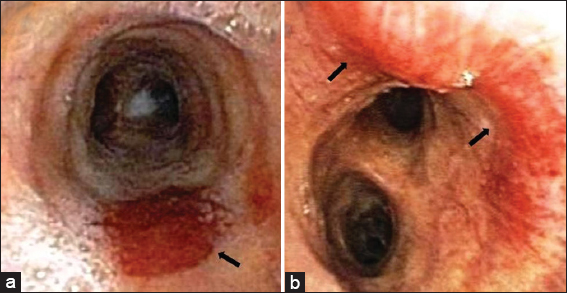
- 28-year-old man with a history of human immunodeficiency virus/acquired immunodeficiency syndrome complicated by numerous prior opportunistic infections, presented with progressive dyspnea and chronic, nonproductive cough, diagnosed with pulmonary Kaposi sarcoma. Bronchoscopy images. Panel a and b showing characteristic red to slightly violaceous macular lesions in the mid and distal trachea (black arrows).
DISCUSSION
The diagnosis of KS in the HIV/AIDS patient without mucocutaneous involvement is often complicated by the common co-prevalence of opportunistic infections or other malignancies. The absence of characteristic lesions should not exclude the diagnosis in the appropriate clinical scenario. As illustrated here, although somewhat archaic, sequential thallium-gallium scintigraphy is nonetheless a valuable diagnostic tool.
Thoracic imaging can suggest but is not specific for pulmonary KS. Chest radiographs are most commonly notable for parenchymal nodules and reticular markings.[2] On CT imaging, pulmonary KS is often associated with peribronchovascular interstitial prominence and interlobular septal thickening with irregular, poorly-defined nodules and consolidations in a peribronchovascular distribution.[3] In the appropriate clinical scenario, these findings raise suspicion for KS but do not exclude the presence of other disease processes such as infection or lymphoma.
On bronchoscopy, characteristic endobronchial lesions have a macular cherry red or violaceous appearance and are commonly located at airway bifurcations.[1] Diagnosis of pulmonary KS may be made presumptively if the infection has been excluded and characteristic endobronchial lesions are present along with typical radiologic features. Although endobronchial KS may be a marker for parenchymal involvement by the tumor, endobronchial lesions are only present in 45%–73% of cases of pulmonary KS.[4] In prior studies, bronchoscopy was nondiagnostic in up to 30% of cases which were later confirmed on open lung biopsy or autopsy.[5] Indeed, bronchoscopy can be nondiagnostic in the following settings: distal lesions that are not reached by the bronchoscope; lesions that do not involve the bronchi or adjacent tissues; microscopic interstitial involvement; and lesions that do not erupt through the submucosa.[3]
Transbronchial and endobronchial biopsies are of limited utility if characteristic lesions are present. The estimated diagnostic yield for KS via transbronchial and endobronchial biopsy is variably low between 20% and 60%.[6,7] Histologically, the subtle appearance of abnormal spindle cells and vasculature may easily be distorted by crush artifact or mistaken for fibrotic tissue. The highly vascular nature of KS lesions may also increase the risk for harm. In one case series, biopsy resulted in significant hemorrhage in 30% of cases.[8] In view the low yield and the hemorrhagic risk associated with transbronchial biopsy for the diagnosis of KS, the decision was made not to pursue this diagnostic modality in our patient.
Although bronchoscopy is the most sensitive modality for the assessment of pulmonary KS, it is more difficult to avail an accurate diagnosis in the absence of characteristic endobronchial lesions. As illustrated in this case, when the clinical suspicion for pulmonary KS is high – despite the absence of endobronchial and mucocutaneous lesions – sequential thallium and gallium scintigraphy can be valuable in confirming the diagnosis as pulmonary KS demonstrates delayed uptake on thallium scintigraphy and no abnormal uptake on gallium scintigraphy.
Thallium is a biochemical analog of potassium and is thus regulated by sodium-potassium pumps. Active uptake is increased in malignant cells (e.g., KS, lymphoma, and other neoplasms) due to increased blood flow, cell density, and high adenosine triphosphate-related energy consumption.[5,9] Due to active transport, uptake persists on delayed imaging. Conversely, in infectious and inflammatory etiologies, delayed imaging shows significant washout of uptake due to passive diffusion back into the intravascular space. Thallium scintigraphy is particularly effective in evaluating pulmonary pathology since normal pulmonary parenchyma has minimal background uptake compared to other visceral organs. Delayed thallium-avidity can thus help distinguish malignant pulmonary lesions from infectious lesions.
Gallium scintigraphy was historically used for lymphoma staging, but more recently has been replaced by fludeoxyglucose-positron emission tomography. Nonetheless, to further differentiate malignant lesions from KS, Gallium can still be utilized. The proposed mechanism is via transferrin-mediated endocytosis, similar to iron.[10] While there is significant uptake in lymphoma, malignancy, and other infectious or inflammatory lesions, by contrast, KS is not gallium-avid. A thallium-positive, gallium-negative pattern is highly specific for the diagnosis of pulmonary KS. However, its sensitivity is markedly reduced in the presence of opportunistic infections. In a retrospective study of 83 patients with AIDS and confirmed KS, the sequential thallium-gallium scan had a sensitivity of 89% and specificity of 96% for pulmonary KS. However, this sensitivity was reduced to 37% in the presence of opportunistic infections.[9]
This case highlights the challenge of diagnosing pulmonary parenchymal KS in the absence of mucocutaneous involvement in patients with AIDS. Early identification can be confounded by concomitant opportunistic infections or other malignancies. As seen in this case, in the appropriate clinical scenario, the absence of characteristic mucocutaneous or endobronchial lesions should not exclude the possibility of pulmonary KS. In such cases, sequential thallium-gallium scintigraphy can help differentiate between infection, lymphoma, and KS [Table 1]. Considering the poor life expectancy of patients with pulmonary KS, it is imperative to establish a timely diagnosis in order for treatment to be initiated.
| Thallium | Gallium | |
|---|---|---|
| Kaposi Sarcoma | Positive | Negative |
| Lymphoma | Positive | Positive |
| Infection | Negative | Positive |
CONCLUSIONS
Pulmonary KS without mucocutaneous involvement is exceedingly rare. As this case highlights, it is especially challenging to establish a diagnosis of pulmonary KS in the absence of characteristic mucocutaneous lesions. In such circumstances, particularly when there is high clinical and radiographic suspicion for pulmonary KS, sequential thallium and gallium scintigraphy can be helpful in differentiating between infection, lymphoma, and KS.
Consent
Written informed consent was obtained from the patient’s next of kin for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient has given his consent for his images and other clinical information to be reported in the journal. The patient understand that name and initials will not be published and due efforts will be made to conceal identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- The epidemiologic, pathologic, and clinical features of AIDS-associated pulmonary Kaposi's sarcoma. Chest. 2000;117:1128-45.
- [Google Scholar]
- Intrathoracic Kaposi sarcoma in AIDS patients:Radiographic-pathologic correlation. Radiology. 1987;163:495-500.
- [Google Scholar]
- Pulmonary involvement in Kaposi sarcoma:Correlation between imaging and pathology. Orphanet J Rare Dis. 2009;4:18.
- [Google Scholar]
- Pulmonary Kaposi sarcoma in patients with AIDS:Scintigraphic diagnosis with sequential thallium and gallium scanning. Radiology. 1991;180:409-12.
- [Google Scholar]
- Pulmonary Kaposi's sarcoma in patients with acquired immune deficiency syndrome:A clinicopathological study. Thorax. 1987;42:262-8.
- [Google Scholar]
- Pulmonary Kaposi's sarcoma in the acquired immune deficiency syndrome. Clinical, radiographic, and pathologic manifestations. Am J Med. 1986;81:11-8.
- [Google Scholar]
- Evaluation of sequential thallium and gallium scans of the chest in AIDS patients. J Nucl Med. 1996;37:1662-7.
- [Google Scholar]
- The mechanism of 67Ga localization in malignant disease. Nucl Med Biol. 1996;23:745-51.
- [Google Scholar]






