Translate this page into:
Radiologic-Pathologic Correlation: Acellular Dermal Matrix (Alloderm®) Used in Breast Reconstructive Surgery
Address for correspondence: Dr. Christine U Lee, Department of Radiology, Mayo Clinic, 200 First Street SW, Rochester, MN 55905, USA. E-mail: lee.christine@mayo.edu
-
Received: ,
Accepted: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Acellular dermal matrix (ADM) such as Alloderm® is sometimes used in tissue reconstruction in primary and reconstructive breast surgeries. As ADM is incorporated into the native tissues, the evolving imaging findings that would correlate with varying degrees of host migration and neoangiogenesis into the matrix can be challenging to recognize. In the setting of a palpable or clinical area of concern after breast reconstructive surgery following breast cancer, confident diagnosis of a mass representing ADM rather than recurring or developing disease can be challenging. Such diagnostic imaging uncertainties generally result in short-term imaging and clinical follow-up, but occasionally, biopsy is performed for histopathological confirmation of benignity. A case of biopsy-proven Alloderm® is described. To the best of our knowledge, this is the first radiologic-pathologic correlation of ADM in the literature.
Keywords
Acellular dermal matrix
Alloderm®
biopsy
pathology
ultrasound

Introduction
Immunologically inert acellular dermal matrix (ADM) sometimes used as a scaffold in tissue reconstruction in primary and reconstructive breast surgeries[12] has more recently been described as having “revolutionized implant-based breast reconstruction.[3]” Illustrations of how ADM is used at the time of tissue expander placement or around the implant have been described.[13] Various ADMs are available[34567] including those from cadaveric human tissue (Alloderm®, AlloMax™, FlexHD®, DermACELL®), porcine tissue (Strattice™, Permacol™, Braxon®), bovine tissue (SurgiMend®), and bovine pericardium tissue (Veritas®).
Within the past decade at the authors’ institution, ADM has increasingly become a differential consideration in diagnostic imaging, following reconstructive breast surgeries. When such patients present with a palpable area of concern, the challenges encountered in differentiating imaging features of ADM from recurrent or developing disease are similar to those faced with fat necrosis or suture granulomas. The literature on imaging features of ADM remains limited.[18910] Generally, imaging diagnosis of ADM remains largely based on clinical and surgical history and short-term follow-up. To the best of our knowledge, there has been no histopathological description of ADM reported in the literature. This manuscript describes a case of biopsy-proven ADM, specifically Alloderm® (LifeCell Corp.; Branchburg, NJ, USA) and its imaging and histopathological features. We hope that this case will add to the limited literature on imaging features of ADM and help improve confidence in diagnosis.
A 42-year-old woman with a high risk of developing breast cancer elected to have bilateral prophylactic nipple-sparing mastectomies with implant reconstruction about 1 year ago. During reconstruction, Alloderm® Tissue Matrix Ready to Use Contour was used. At her annual routine follow-up, the patient described a several-month stable, palpable cord-like area below her left nipple and extending into the lateral aspect of the breast. She denied any associated pain, redness, swelling, or discoloration. Clinical impression suspected Alloderm®, and diagnostic imaging was ordered.
Imaging Features
Targeted ultrasound with simultaneous palpation showed probably benign imaging features [Figure 1], suggestive of fat necrosis or possibly Alloderm®; the patient desired biopsy [Figure 2].
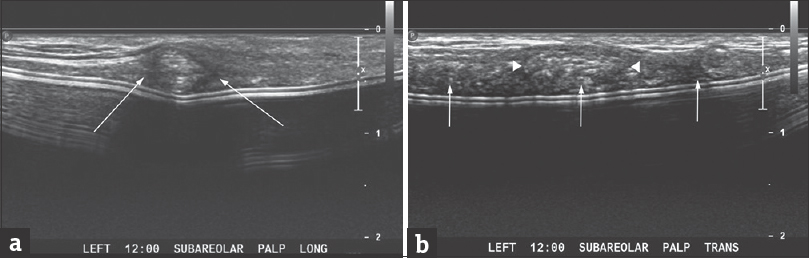
- A 42-year-old woman with a palpable area of concern in the reconstructed left breast. (a) Targeted ultrasonographic longitudinal image of the palpable area of concern showing a heterogeneous, but predominantly hypoechoic mass with indistinct margins (arrows). (b) Targeted ultrasonographic transverse image also showing a heterogeneous, but predominantly hypoechoic mass with indistinct margins (arrowheads) corresponding to the palpable area of concern. Also noted is a relatively heterogeneous hypoechoic area with somewhat nodular areas (arrows) immediately adjacent to the implant capsule.
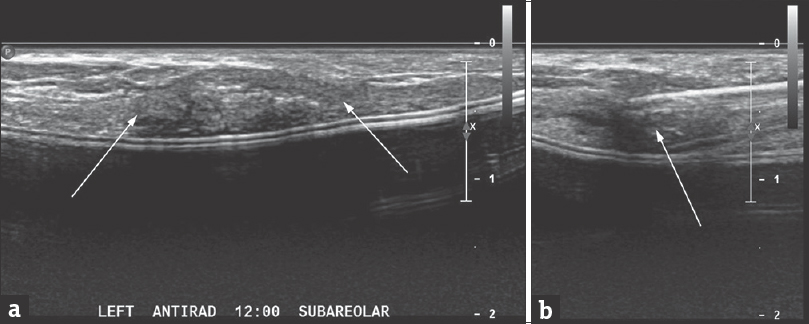
- A 42-year-old woman undergoing biopsy of a mass corresponding to a palpable area of concern in the reconstructed left breast. (a) Targeted ultrasonographic antiradial image showing the mass targeted for biopsy (arrows). (b) Ultrasonographically guided core needle biopsy of the mass was performed using a spring-loaded 18-gauge biopsy device (BioPince™, Argon Medical Devices, Plano, TX, USA); the needle is seen to traverse the mass (arrow). Four core specimens were obtained.
Pathologic Features
The needle core biopsy grossly consisted of four fragments. Microscopically, most of the tissue was presented by dense fibrous tissue, but a very small focus of benign breast parenchyma was also identified. The dense fibrous tissue of the specimen had two separate areas. One area [Figure 3] had disorganized haphazardly oriented collagen fibers; it was acellular and avascular, and its pattern resembled that of decellularized matrix before transplantation.[11] The other area [Figure 4] comprised collagen fibers arranged in thick bundles and oriented in a similar direction. Fibroblasts were present between collagen bundles, and there were many small vessels (capillaries). The pattern of this area was highly suggestive of mature dense connective tissue and similar to Alloderm® that had undergone tissue remodeling and revascularization.[11]
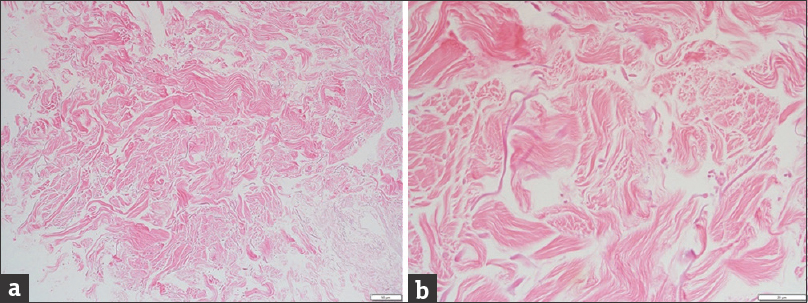
- Histopathology of one of the tissue core specimens (a) at low magnification (×10, white bar in the lower right corner = 50 μm) and (b) at high magnification (×40, white bar in the lower right corner = 20 μm) shows disorganized pink collagen fibers. Note the absence of cells or vessels – an acellular area – consistent with acellular dermal matrix.
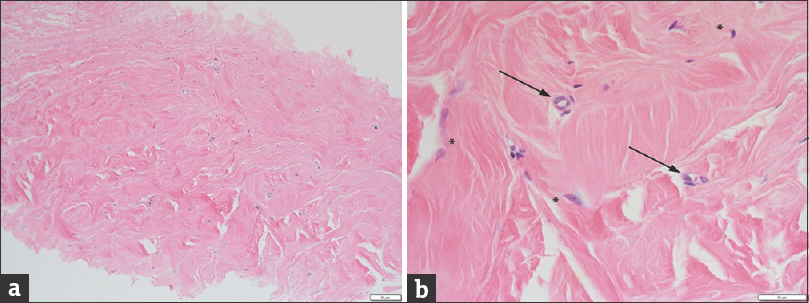
- Histopathology of another of the tissue core specimens (a) at low magnification (×10, white bar in the lower right corner = 50 μm) and (b) at high magnification (×40, white bar in the lower right corner = 20 μm) shows features of an organized dermis with oriented pink collagen fibers, rare fibroblasts (asterisks), and neovascularization (arrows). These features are consistent with reorganized dermal matrix 1 year after surgery.
The presence of the two distinct areas on the biopsy is well correlated with the process of ADM matrix reorganization that includes vascularization and population of the collagen fibers by fibroblasts.[11] [Figures 3 and 4] clearly demonstrate co-localization of fibroblasts and new vessels, which are needed for their supply. The remodeled area is very similar to the original dense fibrous tissue of the breast, but there are some differences. The remodeled area has a higher density of collagen fibers and a lower density of fibroblasts as compared to the native breast or dermal dense fibrous tissue. Furthermore, in native dense fibrous tissue, fibroblasts are located outside as well as within collagen bundles, while in the remodeled area, fibroblasts are found only outside of the collagen bundles [Figure 4].
In this particular case, the presence of two distinct morphologic areas likely represents unfinished remodeling of the ADM. At the same time, considering the time passed since the surgery (1 year), the failure to remodel the remaining portion of the ADM due to trauma, physical disruption, or any other reason cannot be excluded.
Discussion
The histopathology shows a spectrum of both a disorganized scaffold of collagen fibers as well as an expected transformation of the acellular matrix to the more mature dense fibrous tissue through the process of remodeling with incorporation of fibroblasts and neovascularization. With time, more of the ADM matrix is presumably remodeled to the dense fibrous tissue. Ultrasonographic features of Alloderm® could correlate with the histopathological spectrum. The more centrally located echogenic foci of the biopsied mass might reflect the still disorganized, less tissue-dense components of the Alloderm®.
Benign differential diagnoses based on imaging include fat necrosis and suture granulomas. Given the cord-like palpable nature of this case, superficial thrombophlebitis could be another consideration but would be unlikely if imaging could not confirm a thrombosed superficial vein. Imaging characteristics of fat necrosis are quite broad and varied and can include features, such as spiculation and posterior acoustic shadowing, which overlap with those of a highly suspicious mass. The diagnostic imaging challenges and angst that can sometimes come with excluding a malignant process from fat necrosis are quite similar to that experienced in excluding malignancy from ADM. In such cases, clinical correlation helps increase confidence in imaging diagnosis. Other imaging techniques, such as contrast-enhanced ultrasound for evaluating vascular ingrowth of ADM, are preliminarily hopeful.[9]
Conclusion
This case describes the ultrasonographic and histopathologic findings of Alloderm® presenting as a palpable area of concern 1 year after breast reconstructive surgery. The imaging and histopathology features of Alloderm® are nonspecific evolving over a spectrum of features and remain diagnostically challenging for both radiologists and pathologists. Diagnosis relies heavily on clinical correlation.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Available FREE in open access from: http://www.clinicalimagingscience.org/text.asp?2017/7/1/13/203164
References
- Imaging features of AlloDerm(®) used in postmastectomy breast reconstructions. J Clin Imaging Sci. 2014;4:19.
- [Google Scholar]
- A preliminary report on the clinical experience with AlloDerm in breast reconstruction and its radiologic appearance. Am Surg. 2010;76:1123-6.
- [Google Scholar]
- A guide to prepectoral breast reconstruction: A new dimension to implant-based breast reconstruction. Clin Breast Cancer 2017 pii: S1526-820930350-0
- [Google Scholar]
- Bioprosthetics: Changing the landscape for breast reconstruction? Eur J Surg Oncol. 2013;39:24-5.
- [Google Scholar]
- Acellular dermal matrix in reconstructive breast surgery: Survey of current practice among plastic surgeons. Plast Reconstr Surg Glob Open. 2015;3:e381.
- [Google Scholar]
- Acellular dermal matrices: Economic considerations in reconstructive and aesthetic breast surgery. Clin Plast Surg. 2012;39:187-216.
- [Google Scholar]
- Comparison of different ADM materials in breast surgery. Clin Plast Surg. 2012;39:167-75.
- [Google Scholar]
- Diagnostic dilemma: Acellular dermis mimicking a breast mass after immediate tissue expander breast reconstruction. Plast Reconstr Surg. 2009;124:174e-6e.
- [Google Scholar]
- First experience using contrast-enhanced ultrasound to evaluate vascularisation of acellular dermal matrices after implant-based breast reconstruction. Breast J. 2014;20:461-7.
- [Google Scholar]
- Ultrasonography findings of AlloDerm® used in postmastectomy alloplastic breast reconstruction: A case report and literature review. Iran J Radiol. 2016;13:e38252.
- [Google Scholar]
- Histologic characterization of acellular dermal matrices in a porcine model of tissue expander breast reconstruction. Tissue Eng Part A. 2015;21:35-44.
- [Google Scholar]






