Translate this page into:
Congenital Uterine Arteriovenous Malformation Presenting as Postcoital bleeding: A Rare Presentation of a Rare Clinical Condition
Address for correspondence: Dr. Seema Chopra, Department of Obstetrics and Gynecology, Post Graduate Institute of Medical Education and Research, Chandigarh, India. E-mail: drseemachopra@yahoo.com
-
Received: ,
Accepted: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Congenital uterine arteriovenous malformation (AVM) is an extremely rare condition with <100 cases documented in literature. We report multiparous women presenting to us with a history of postcoital bleed. Initial Doppler ultrasonography was consistent with features suggestive of AVM. Subsequently, computed tomography (CT) angiography confirmed the diagnosis. Embolization was chosen as the treatment because of the large extension of AVM and the risk of hemorrhage during hysterectomy. The patient was discharged in a stable condition with a plan of repeat embolization in the next setting. At 6 and 12 weeks of follow-up, she did not experience any further episodes of bleed. The purpose of this case report is to highlight the salient clinical features, diagnosis, and the management options available for this rare clinical condition.
Keywords
Congenital arteriovenous malformation
computed tomography angiography
Doppler ultrasonography
embolization

Introduction
Uterine arteriovenous malformation (AVM) is a rare cause of vaginal bleeding in women of reproductive age group. Also known by other names, such as cirsoid aneurysm, arteriovenous fistula, arteriovenous aneurysm, pulsating angioma and cavernous angioma, it is still a rare clinical entity with only 100 cases reported in the literature.[1] Clinically, it has varied presentation, from being completely asymptomatic to various degree of vaginal bleeding. It can be congenital or acquired. Congenital uterine AVM arises from an abnormality in the embryological development of primitive vascular structures. Acquired uterine AVMs occur following surgical procedures such as dilatation and curettage, uterine surgery, or direct uterine trauma and are less commonly associated with various gynecological malignancies.[2] Color Doppler ultrasound is a useful noninvasive method for initial diagnosis of this rare condition whereas definite diagnosis requires computed tomography (CT) angiography. Conservative management or embolization is a preferable method of treatment to avoid a hysterectomy in patients of childbearing age.[3] We report this case of congenital uterine AVM because of rarity of its clinical presentation, as a postcoital vaginal bleed. The purpose of presenting this case is to highlight the clinical features and management alternatives available for uterine AVM.
Case Report
A 25-year-old Para 1321 female from North India presented to emergency room with sudden bout of heavy postcoital bleed associated with suprapubic cramping since the last 4 h. In her obstetric history, she had four-term vaginal deliveries and two complete abortions. There was no similar history of either postcoital bleeding or excessive bleeding following childbirth or abortion in the past. Her menstrual history was unremarkable. There was no family history of such bleeding or any bleeding disorders. She was hemodynamically stable at admission. On pelvic examination, uterus was normal in size, shape, and was non tender. After history and examination, test for urine and serum human chorionic gonadotropin (HCG) was carried out which were negative. Transabdominal ultrasonography (USG) showed bulky uterus with cystic areas within it [Figure 1]. Color Doppler imaging demonstrated multiple tortuous vascular channels and spaces in intramyometrial and in bilateral parametrial region with flow in both arterial and venous phase [Figure 2]. As USG and color Doppler features were suggestive of AVM, angiography was done to confirm the diagnosis. CT-angiography revealed a large AVM involving the uterus, bilateral parametria, and vagina [Figure 3b]. There were multiple arterial feeders from bilateral internal iliac arteries, aorta including bilateral gonadal arteries, multiple lumbar arteries, and bilateral common femoral arteries [Figure 3a]. Subsequently, the patient was planned for elective embolization for treatment. Embolization was carried out with 3 ml of 50% cyanoacrylate glue in view of large caliber of vascular channels with high flow in it [Figure 4a and b]. During the procedure, the patient developed mild chest discomfort after the injection of glue. On examination, she was tachypneic but maintained saturation of 100% on room air. With the possibility of glue embolism, she was started on low molecular weight heparin (LMWH). Chest X-ray showed cardiomegaly with prominent central pulmonary vasculature and branching radioopacities in bilateral lung fields. Features were suggestive of particulate emboli [Figure 5]. Two-dimensional echocardiography showed right ventricular dysfunction with severe tricuspid regurgitation and severe pulmonary arterial hypertension. Computed tomography pulmonary angiography revealed multiple hyper dense filling defects in segmental branches of the right upper lobe and subsegmental branches of the right and left pulmonary arteries secondary to glue embolism [Figure 6]. LMWH was stopped subsequently as the patient improved clinically. A check pelvic USG done after 1 week of glue embolization showed few vessels in the anterior aspect with varicose dilatation with high-velocity low-resistance waveform. The patient was eventually discharged under stable condition with the plan of repeat embolization in next setting.
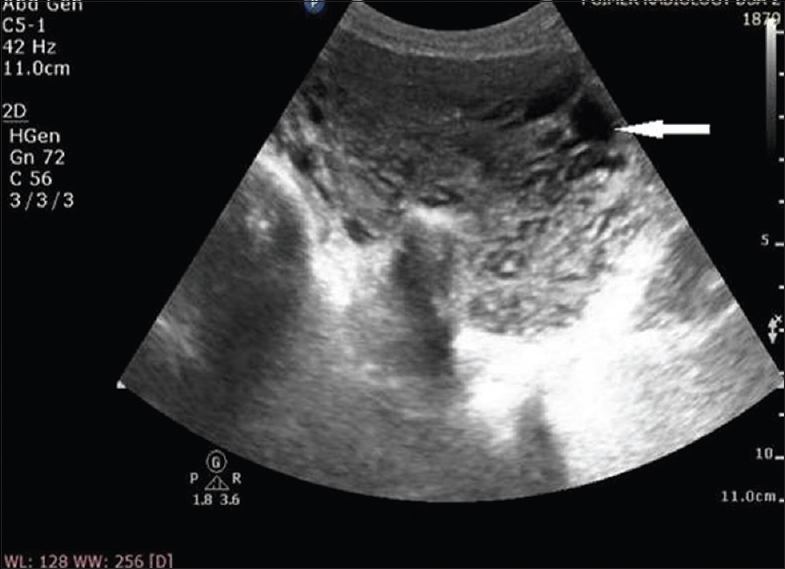
- A 25-year-old female with uterine arteriovenous malformation who presented with postcoital vaginal bleeding. Transabdominal gray-scale ultrasound image of uterus showing myometrial heterogeneity with multiple anechoic cystic structures (arrow).
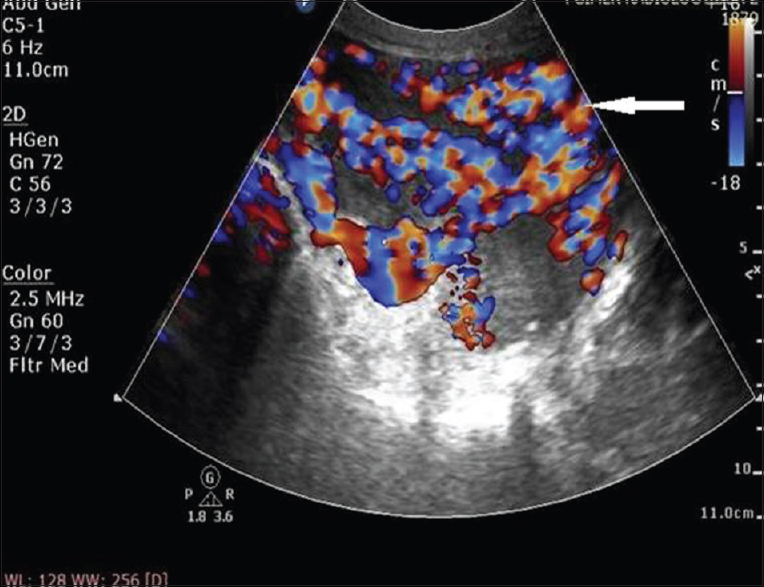
- A 25-year-old female with uterine arteriovenous malformation who presented with postcoital vaginal bleeding. Doppler ultrasound image of uterus shows typical mosaic pattern signals (arrow) with the spectral analysis revealing a high-velocity flow with low-resistance index suggesting arteriovenous malformation
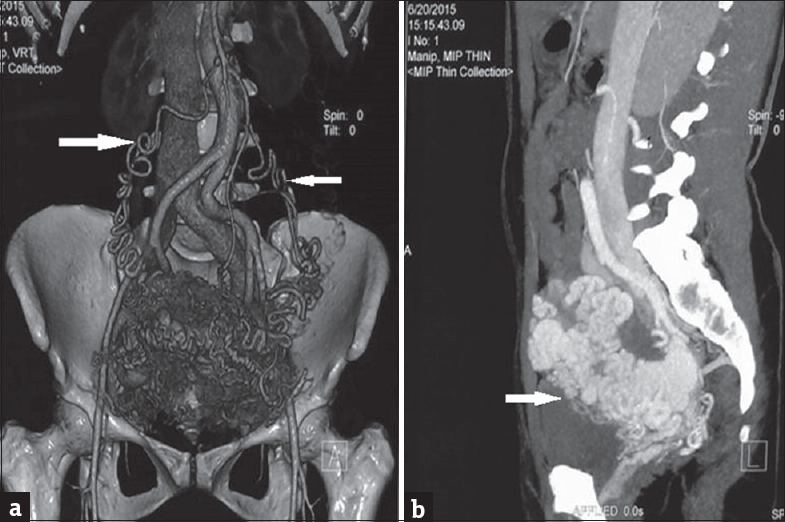
- (a) Contrast-enhanced computed tomography scan of pelvic cavity in a woman with uterine arteriovenous malformation. The coronal volume rendered image shows multiple serpiginous vascular channels in the uterus and parametrium with multiple feeders from bilateral gonadal arteries (arrow) and internal iliac arteries suggesting arteriovenous malformation. (b) Contrast-enhanced computed tomography scan of pelvic cavity in a woman with uterine arteriovenous malformation. The sagittal maximum intensity projection shows multiple serpiginous enhancing vascular channels (arrow) within the myometrium and parametrium suggesting arteriovenous malformations.
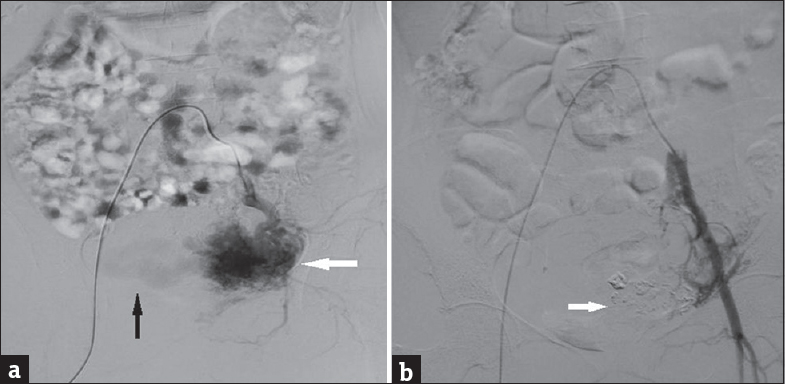
- (a) Digital subtraction angiography image of uterus in a woman with uterine arteriovenous malformation. Preembolization image with selective catheterization of the left internal iliac artery and run shows contrast blush (white arrow) within arteriovenous malformation and early draining veins (black arrow) suggesting abnormal communicating blood vessels. (b) Digital subtraction angiography image of uterus in a woman with uterine arteriovenous malformation. This post embolization image with run shows glue cast (arrow) and no contrast blush suggesting sealing arteriovenous malformation by glue.
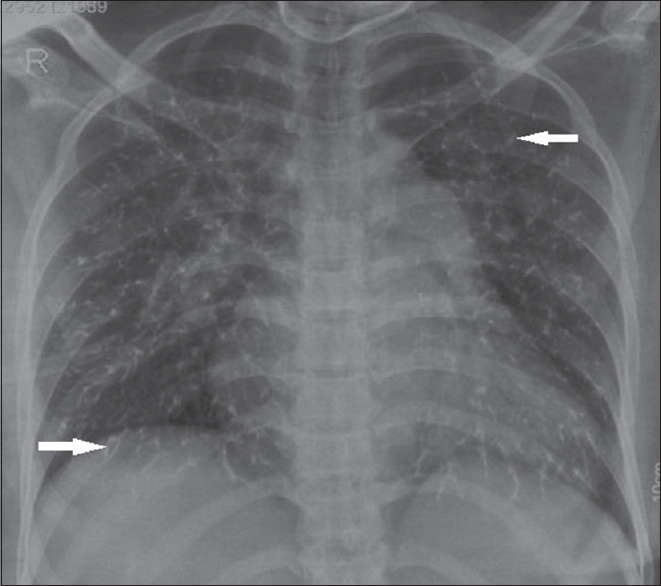
- Chest X-ray (posteroanterior view) of a woman with uterine arteriovenous malformation. She had undergone glue embolization and developed shortness of breath immediately after the procedure. Chest X-ray shows multiple branching patterns of linear radio densities (arrow) scattered in the bilateral lungs and prominent pulmonary bay suggesting glue embolization.
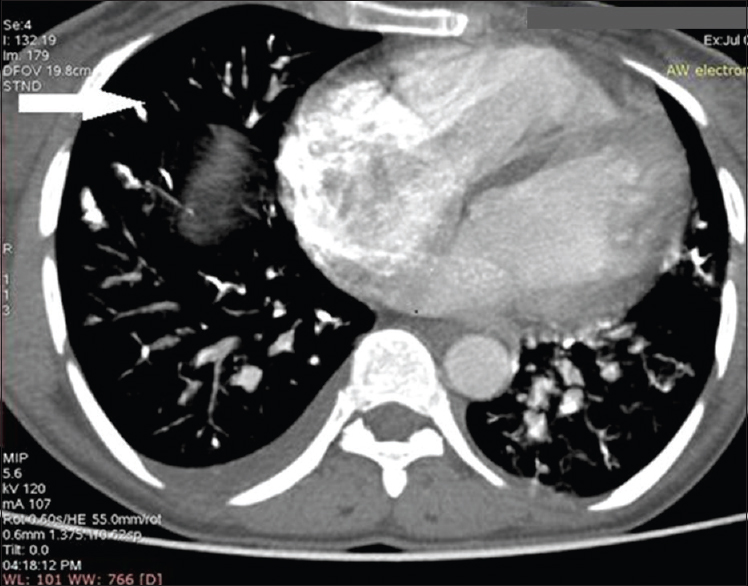
- Contrast-enhanced computed tomography scan of chest in a woman with uterine arteriovenous malformation and glue embolization. The axial image of computed tomography chest shows the presence of linear hyperdensities within the segmental branches of bilateral pulmonary artery (arrow) suggesting glue embolization in the pulmonary artery.
On follow-up after 6 and 12 weeks, the patient was doing well, she has not had excessive vaginal bleeding ever since the time of discharge.
Discussion
Uterine AVM is a rare and unusual cause of vaginal bleeding with fewer than 100 cases reported in literature.[1] It is potentially a life-threatening disorder, in which patients present with vaginal bleeding that may be intermittent or profuse and can cause hemodynamic instability. O’Brien et al. have predicted a rough incidence of uterine AVM to be 4.5% in their study.[4] First reported by Dubreuil and Loubat in 1926, uterine AVM consists of an abnormal growth and connection between arteries and veins without a capillary bed, creating areas of high and low flow, which are fragile and prone to bleeding.[5] Uterine AVM may be congenital or acquired.[56] Congenital AVMs are believed to arise from arrested vascular embryologic development resulting in anomalous differentiation in the capillaries and abnormal communication between arteries and veins. On the other hand, acquired AVM most commonly occur as a complication of uterine surgery or curettage, gestational trophoblastic disease, choriocarcinoma, and infection.[7] Surgical procedure, especially curettage is considered as the most common predisposing factor leading to acquired uterine AVM formation.[8]
In our case, it is possible that a congenital form of uterine AVM was present in the patient given the absence of the previous history of surgery, uterine trauma, curettage, and infections. However, if it was truly congenital rather than acquired, it is unclear why the patient did not have vaginal bleeding during her previous four vaginal deliveries or two abortions. Further, she never had any previous episode of postcoital bleeding. However, it is possible that during this time the AVM was not large or superficial enough for the vessels to be exposed and subsequently bleed.
Traditionally, uterine AVMs used to be diagnosed by laparotomy or during the examination of uterus after hysterectomy.[6] Availability of bedside USG now aids in the initial diagnosis of uterine AVMs. Gray-scale sonography shows multiple heterogeneously hypoechoic or “spongy” anechoic tortuous spaces in the myometrium in a case of AVM.[9] Color and spectral Doppler USG reveals a meshwork of vessels producing a “color mosaic” pattern with high and low velocity flows in different directions throughout the systole and diastole.[410] In our case, as there was no history of preceding amenorrhea with β-hCG being negative and furthermore classical Doppler findings lead us to the possibility of AVM as our initial diagnosis. Among other diagnostic modalities, magnetic resonance imaging provides accurate anatomical details as well as relation with surrounding structures. However, it has obvious disadvantages, i.e., limited availability and being expensive. Both of these disadvantages are a major constraint in its widespread use, especially in the developing countries. Angiography is the gold standard in the diagnostics of AVM, depicting complex vascular connections supplied by hypertrophied feeding arteries, and early drainage through enlarged, tortuous, and hypertrophic veins.[7]
Management of uterine AVM depends on the hemodynamic status, degree of bleeding, patient age, and desire for future fertility. Emergency treatment involves stabilizing the patient's hemodynamic status and halting the blood loss.[3] As practiced in past, hysterectomy remains the definitive treatment, especially in a symptomatic patient without desired fertility.
A minimally invasive approach through angiographic embolization of the AVM is currently the preferred treatment for uterine AVM. There are both permanent and temporary methods of embolic agents that can be used as an embolic agent. We did not use temporary agent such as gel foam as permanent occlusion of the vessels was required. We used n-butyl 2 cyanoacrylate glue for embolization. It was initially approved for embolization of cerebral AVM results in complete and permanent embolization of the AVM, but it requires expertise.
Various embolic materials have been used, including polyvinyl alcohol, histoacryl (glue), stainless steel coils, detachable balloons, and hemostatic gelatine. However, uterine artery embolization may not always be successful and multiple sessions may be required for recurrent episodes.[6] Possible complications of angiographic embolization include infection, perianal skin sloughing, uterovaginal and rectovaginal fistulas, and neurological deficit in the lower limb.[4] Glue embolization, which our patient had, occurring as a complication of angioembolization of uterine AVM has not yet been described in literature.
Fertility preservation was not an issue in our patient. However, we preferred embolization over hysterectomy as the AVM was extending into the parametria and upper vagina. Furthermore, the discussion with interventional radiologist raised the high possibility of massive hemorrhage during the surgery. Unfortunately, our patient had features of glue embolism during the procedure as glue was used for embolizing the nidus of AVM.
In our case, a young fertile female with congenital uterine AVM presented with postcoital bleeding. It is a very rare presentation of uterine AVM and has not been reported yet. A point-of-care ultrasound, followed by a color Doppler helped to establish the diagnosis which was confirmed by CT angiography. Our patient underwent a successful angiographic embolization. However, she had glue embolization as a complication of angiographic embolization which was managed with LMWH. This case report highlights the possible clinical presentation of uterine AVM in women of childbearing age. It also culminates the utility of diagnostic modalities such as ultrasound, color Doppler, and CT angiography together with therapeutic utility of angiographic embolization, its possible complications and its management.
Conclusion
Congenital uterine AVM is a very rare cause of vaginal bleeding. It has varied clinical presentation. However, high index of clinical suspicion together with utilizing the diagnostic modalities such as bedside ultrasound, color Doppler, and CT angiography help in establishing the diagnosis. Therapeutic angiographic embolization is the preferred treatment of uterine AVM, and it has almost abolished the need of hysterectomy in patient with uterine AVM. However, angiographic embolization is also associated with some rare complications which when properly identified can be managed successfully.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Available FREE in open access from: http://www.clinicalimagingscience.org/text.asp?2017/7/1/11/201054
References
- Clinical implications of disturbances of uterine vascular morphology and function. Baillieres Best Pract Res Clin Obstet Gynaecol. 2000;14:937-51.
- [Google Scholar]
- Ultrasound diagnosis of uterine arteriovenous fistula associated with placental site trophoblastic tumor. Ultrasound Obstet Gynecol. 2003;21:606-8.
- [Google Scholar]
- Uterine arteriovenous malformations: From diagnosis to treatment. J Ultrasound Med. 2006;25:1387-92.
- [Google Scholar]
- Uterine arteriovenous malformations: A review of the current literature. Obstet Gynecol Surv. 2005;60:761-7.
- [Google Scholar]
- Arteriovenous malformations of the uterus: An uncommon cause of vaginal bleeding. Obstet Gynecol Surv. 1997;52:736-40.
- [Google Scholar]
- Uterine arteriovenous malformations: Gray-scale and Doppler US features with MR imaging correlation. Radiology. 1998;206:115-23.
- [Google Scholar]
- Color Doppler US in the evaluation of uterine vascular abnormalities. Radiographics. 2002;22:47-53.
- [Google Scholar]






