Translate this page into:
Gastropleural Fistula with Aortic Intramural Involvement
Address for correspondence: Dr. Akshya Gupta, Department of Imaging Sciences, University of Rochester Medical Center, 601 Elmwood Avenue, Box 648, Rochester, NY 14642, USA. E-mail: akshya_gupta@urmc.rochester.edu
-
Received: ,
Accepted: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Gastropleural fistula is a relatively rare complication that can be seen as a result of traumatic, nontraumatic, benign, and neoplastic etiologies. Most commonly, these are found in patients with diaphragmatic herniation or prior thoracic surgery. Aortoenteric fistulas are rare communications typically between the abdominal aorta and bowel. We present a rare case of an 88-year-old male who developed a gastropleural fistula with erosions into the wall of the descending thoracic aorta. Computed tomography (CT) is a leading modality in evaluation of suspected gastropleural or aortoenteric fistulas given the quick scan time and widespread availability. Prompt diagnosis is essential and requires an understanding of appropriate CT protocols and CT imaging appearance.
Keywords
Aortoenteric fistula
computed tomography
empyema
gastropleural fistula

Introduction
Gastropleural fistulas are rare abnormal communications between the pleural space and stomach.[1] Aortoenteric fistulas are rare communications between the aorta and gastrointestinal (GI) tract.[2] We present the first reported case of gastropleural fistula with intramural extension into the wall of descending thoracic aorta.
Case Report
An 88-year-old male presented to the emergency room with several weeks of cough and fatigue as well as recent onset of melena. He had been treated unsuccessfully as an outpatient with oral antibiotics for a presumed pneumonia. Past medical history was notable for endovascular prior stent-graft placement in a thoracoabdominal aneurysm, 2 years before current presentation [Figure 1]. Initial laboratory evaluation demonstrated mild leukocytosis, and initial chest radiograph demonstrated left lower lobe opacities with pleural effusion. A noncontrast computed tomography (CT) of the chest was obtained, which demonstrated air and soft tissue thickening abutting the descending thoracic aorta, wall thickening of the adjacent stomach, as well as a heterogeneous, thick-walled left pleural air and fluid containing collection with layering intermediate to high-density material [Figure 2]. No intervening fat plane was noted between the stomach and the pleural fluid collection. Diaphragmatic continuity also could not be established. A repeat CT scan of the chest and abdomen was obtained before and after administration of intravenous (IV) contrast and after an 80 s delay. Dilute water-soluble oral contrast was also administered before repeat scan. The pre-IV contrast image confirmed a large gastropleural fistula as the cause for patient's empyema [Figure 3] with a clearly visualized diaphragmatic defect. However, the oral contrast extended to the level of the patient's vascular graft struts, concerning for intramural involvement [Figure 4]. The post-IV contrast images demonstrated the extensive periaortic soft tissue thickening extending to the infrarenal abdominal aorta and displacing it anteriorly [Figure 5]. There was also evidence of pseudoaneurysm formation below the ostium of the right renal artery and below the level of patient's vascular stent [Figure 5] suggestive of an infectious pseudoaneurysm formation.
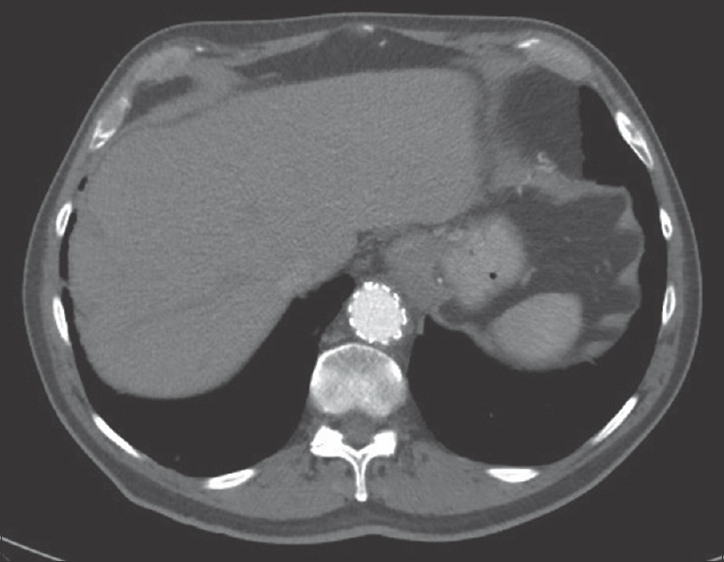
- 88-year-old male presents with several weeks of cough and fatigue. His medical history is notable for endovascular aortic aneurysm repair at an unknown date. An axial postcontrast computed tomography image from 2014 demonstrates normal appearance of the graft.
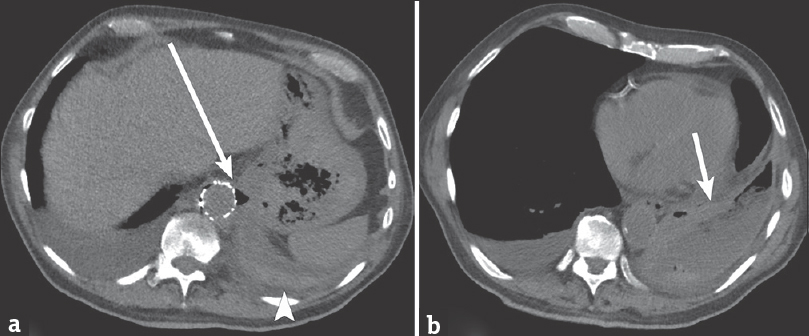
- 88-year-old male presents with several weeks of cough and fatigue. (a) Axial noncontrast computed tomography image of the chest demonstrates air and soft tissue thickening (arrow) at the level of the aorta, in proximity to the adjacent stomach. There appears to be continuity between the gastric fundus and pleural space, with no intervening diaphragm. There is high-attenuation material layering in the pleural space (arrowhead). (b) Axial computed tomography image demonstrates a heterogeneous left pleural collection (arrow) with thick-wall, air, fluid, and high-attenuation material.
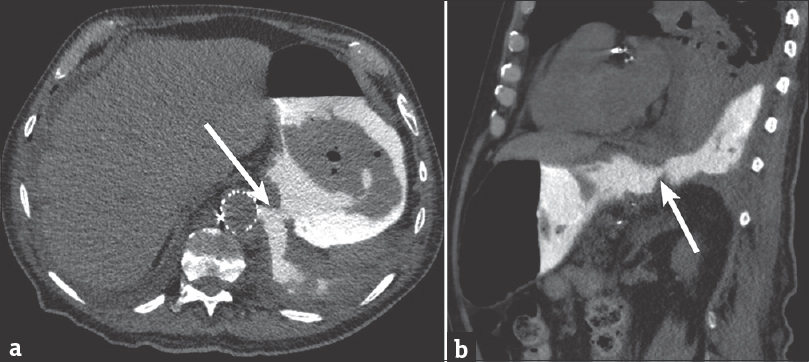
- 88-year-old male presents with several weeks of cough and fatigue. (a) Axial computed tomography image of the chest and abdomen after administration of dilute water-soluble oral contrast demonstrating clear extravasation of oral contrast from the stomach into the left pleural space through a large defect in the gastric wall and diaphragm (arrow). (b) Sagittal computed tomography image demonstrating similar finding (arrow).
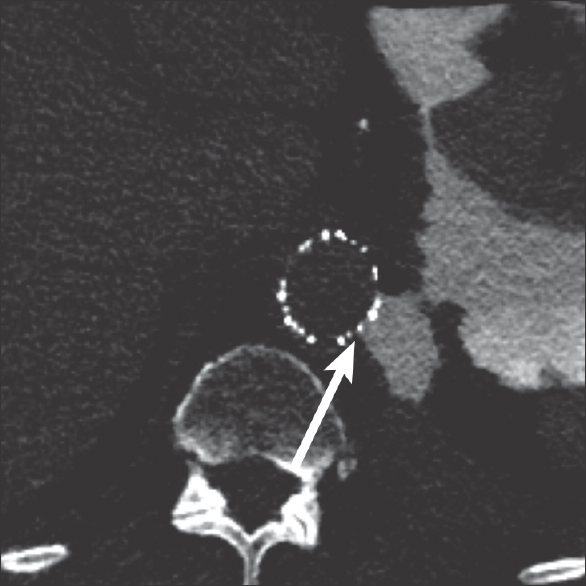
- 88-year-old male presents with several weeks of cough and fatigue. Axial computed tomography image of the chest after administration of dilute water-soluble oral contrast demonstrating extension of the contrast from the stomach, through the pleural space, and between the struts of the aortic vascular stent (arrow).
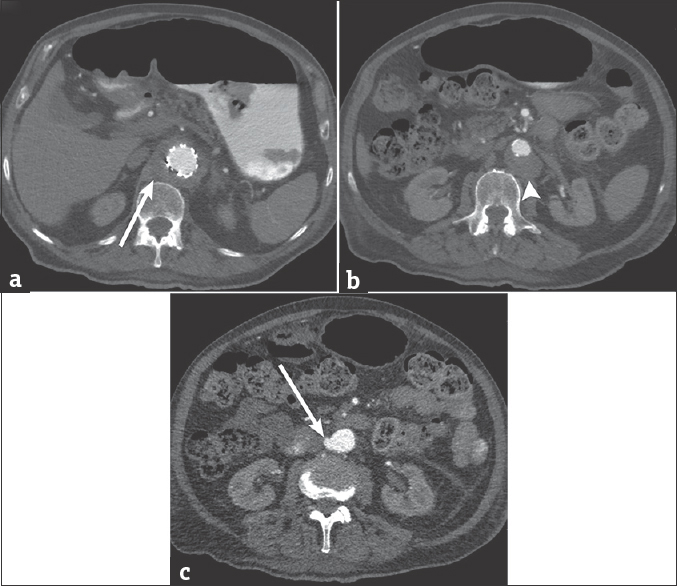
- 88-year-old male presents with several weeks of cough and fatigue. (a) Axial computed tomography image of the chest and abdomen after the administration of intravenous contrast demonstrates extension of the periaortic soft tissue thickening into the abdomen (arrow). (b) Axial computed tomography image of the abdomen demonstrating anterior displacement of the aorta (arrowhead). (c) Axial computed tomography image of the abdomen demonstrating the contained extravasation of contrast (arrow) from the right lateral wall of the abdominal aorta.
Discussion
Gastropleural fistulas are rare conditions that are typically seen in the setting of trauma and diaphragmatic injury or gastric perforation secondary to peptic ulcer disease and malignancy.[3] Patients who have a prior history of thoracic or upper abdominal surgery, as well as local radiation therapy, are also at risk.[45] Our patient had no known risk factors for fistula formation. However, initial noncontrast imaging demonstrated wall thickening of the gastric fundus in close approximation to a left pleural empyema, raising suspicion for fistula formation. Diagnosis of a gastropleural fistula can be made with an upper GI examination, preferably with water-soluble contrast. The fluoroscopic images demonstrate contrast extravasation into the pleural space. Analysis of the pleural fluid can demonstrate gastric contents or bile, which would also confirm the diagnosis.[4] Alternatively, rapid diagnosis in the acute setting can be obtained with CT after administration of dilute water-soluble oral contrast. Pleural air and fluid alone can suggest the diagnosis in the appropriate clinical scenario; these findings are less specific and are seen in the setting of an isolated empyema although in this scenario, the diaphragm is intact and the perigastric fat planes are preserved. Additional imaging findings that can be found in the presence of a gastropleural fistula include hydropneumothorax and tension pneumothorax.[3]
Our patient's initial noncontrast CT demonstrated extensive air and soft tissue thickening surrounding the distal descending thoracic aorta with loss of fat planes and subtle thinning of the aortic wall. These findings are concerning for but not diagnostic of aortoenteric communication and may be found in patients who have aortitis or infection without fistula formation.[2] Diagnosis requires a high index of suspicion. These cases can be separated into primary aortoenteric fistulas versus secondary aortoenteric fistulas, which occur in patients who have undergone aortic repair and have underlying perigraft infection.[2] This blurs the imaging distinction between infections with associated fistula versus infections without fistula.
In patients with suspected aortic fistulas, precontrast and arterial phase imaging should be obtained. A 70–80 s postcontrast delayed scan should also be obtained to evaluate for endoleak, in patients with prior aneurysm repair. Oral contrast is not routinely administered given the potential for obscuration of IV contrast extravasation into the bowel lumen.[2] In our patient, high-attenuation material within the pleural space on the initial noncontrast CT may have represented blood products or gastric contents. However, performing an additional scan with oral contrast clearly identified this to be gastric material. Ideally, when the diagnosis is uncertain but an aortoenteric fistula is suspected, a triphasic CT should be obtained: initially noncontrast, followed by arterial phase IV contrast and then a late phase after administering oral contrast.
Imaging features that more strongly correlate with the presence of an aortoenteric fistula include extravasation of contrast into the bowel lumen, ectopic perigraft gas, and focal disruption of the aortic contour. In this case, there was soft tissue thickening and air surrounding the aorta. In addition, oral contrast could be seen extending between the struts of the aortic stent confirming the intramural involvement. Other imaging findings such as perigraft fluid, loss of the intervening fat plane between bowel and aorta, and pseudoaneurysm formation can be seen in cases of infectious aortitis or perigraft infection without fistula formation.[2] Our patient did have infection and inflammation extending along the aorta, with pseudoaneurysm formation at a location distal to the fistula. This overlap in CT findings highlights the importance of the clinical context. The presence of these abnormalities in the setting of GI bleeding should raise suspicion for a possible aortoenteric fistula.
While there have been reported cases of gastropleural and aortoenteric or aortogastric fistulas, to our knowledge, this is the first reported case of a gastropleural fistula with extension into the aortic wall. The discontinuous posterior gastric wall suggested a perforated gastric ulcer leading to gastropleural fistula formation. This was not diagnosed in the early stages, with infection and inflammation consequently spreading into the mediastinum and aortic wall at the level of the patient's aortic graft. This unique case demonstrates that corrosive contents of a gastric perforation can erode through an intact diaphragm with spillage into the pleural space.[4] If left untreated, these corrosive gastric contents in the pleural space can further erode into adjacent mediastinal structures such as the descending aorta, as in this case. This stresses the importance of prompt diagnosis and treatment in these life-threatening cases. On initial imaging, the fact that the fistula potentially involved three separate compartments – vascular, pleural, and GI – complicated the subsequent CT protocol that was utilized. To correctly identify such fistulas and their associated complications, we recommend an initial noncontrast CT to identify the suspected fistula. The initial contrast-enhanced phase can be determined by the suspected source of the fistula on the noncontrast imaging: if enteropleural, then oral contrast should be administered first while if aortopleural or aortoenteric, then IV contrast without oral contrast should be administered [Table 1].

Conclusion
Gastropleural and aortoenteric fistulas are rare complications that require prompt diagnosis and clinical intervention. Understanding the CT imaging features of both of these entities is essential to pursue the appropriate CT protocol based on the patient's prior imaging findings or presenting clinical scenario.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Available FREE in open access from: http://www.clinicalimagingscience.org/text.asp?2017/7/1/7/200571
References
- Acquired gastrointestinal fistulas: Classification, etiologies, and imaging evaluation. Radiology. 2002;224:9-23.
- [Google Scholar]
- Aortoenteric fistulas: CT features and potential mimics. Radiographics. 2009;29:197-209.
- [Google Scholar]
- Gastropleural fistula in metastatic ovarian cancer. J Surg Case Rep 2014 2014 pii: Rju033
- [Google Scholar]
- Gastropleural fistula: Rare entity with unusual etiology. Ann Thorac Med. 2007;2:64-5.
- [Google Scholar]
- Gastropleural fistula: A rare complication of Ewing sarcoma. Korean J Thorac Cardiovasc Surg. 2013;46:293-4.
- [Google Scholar]






