Translate this page into:
Visualization of a Small Ventricular Septal Defect at First-pass Contrast-enhanced Cardiac Magnetic Resonance Imaging
Address for correspondence: Dr. Francesco Secchi, Radiology Unit, IRCCS Policlinico San Donato, Piazza Malan, San Donato Milanese, Milano, Italy. E-mail: francesco.secchi@grupposandonato.it
-
Received: ,
Accepted: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Ventricular septal defect (VSD) is a congenital heart disease that accounts for up to 40% of all congenital cardiac malformations. VSD is a connection between right and left ventricle, through the ventricular septum. Echocardiography and magnetic resonance imaging (MRI) help identify this entity. This case presents a 12-year-old male diagnosed with a small muscular apical VSD of 3 mm in diameter, at echocardiography. Cardiac MRI using first-pass perfusion sequence, combining the right plane of acquisition with a short bolus of contrast material, clearly confirmed the presence of VSD.
Keywords
Cardiac magnetic resonance
first-pass perfusion
ventricular septal defect
INTRODUCTION

Ventricular septal defect (VSD) is one of the most common congenital heart lesions. This case report shows a particular cardiac magnetic resonance imaging (MRI) technique to detect a small VSD associated with myocardium hypertrabeculation.
CASE REPORT
Here we present a case of a 12-year-old male (height 144 cm, weight 32 kg), born at term by spontaneous delivery, who had been previously diagnosed with a muscular VSD and an atrial septal defect (ASD). The ASD was closed by surgical intervention. In 2006, non-compaction of left ventricle (LV) posterior wall was suspected at echocardiography. In 2010, an echocardiographic examination was performed at our institution. The report was as follows: Situs solitus, normal atrio-ventricular and ventricular-arterial connections, no ASDs; small muscular apical VSD of 3 mm in diameter [Figure 1] and patchy appearance of the posterior wall of the LV. The patient was recommended a cardiac MRI exam.
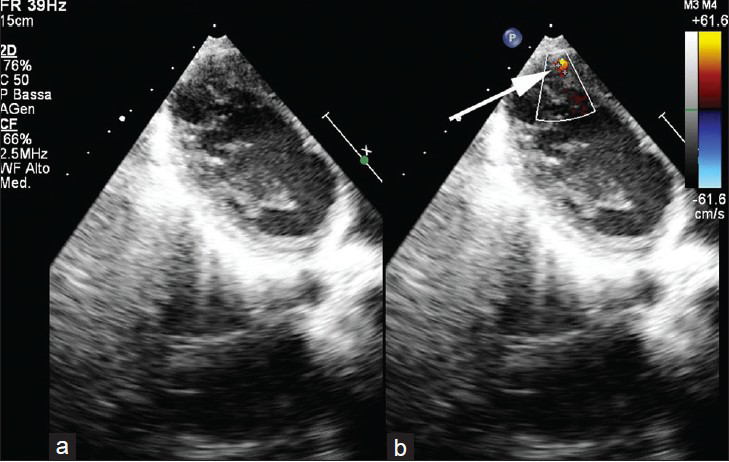
- 12-year-old male with a small muscular apical ventricular septal defect ventricular septal defect. Ecocardiography in (a) a long-axis four-chamber plane b) color Doppler image shows the presence of a small apical muscular ventricular septal defect (arrow in b).
Cardiac MR was performed using a 1.5-T scanner (Sonata, Siemens, Erlangen, Germany), with a 4-element phased-array cardiac coil. Cine steady-state free precession (SSFP) sequences were acquired on long-axis two-chamber, four-chamber, and short-axis views to cover the whole LV. A retrospective electrocardiogram-gating was used. A turbo-gradient-echo first-pass perfusion with echoplanar readouts sequence was acquired for three long-axis four-chamber slices. Contrast material (0.1 mmol/kg of gadoterate meglumine for a total of 7 ml), followed by 20 ml of saline solution were intravenously injected, through a 22 G plastic needle in an antecubital vein of the left arm, at a rate 2 ml/min. Cine true-fast imaging with steady-state precession images were segmented semiautomatically (Syngo-Argus, version VE32B, Siemens Medical Solutions, Erlangen, Germany) to obtain ejection fraction (EF), end-diastolic volume (EDV), normalized to body surface area (EDV index [EDVI]), and end-systolic volume index (ESVI) of the LV and right ventricle (RV).
The cine SSFP sequences showed a hypertrabeculation of the mid-lateral and inferior wall and of the apical septum with a ratio between normal and hypertrabeculated myocardium of about two[12] [Figure 2 and Video 1]. No septal defect was initially detected on cine evaluation. During the first-pass perfusion, a jet of highly concentrated contrast material was clearly visible from the apical VSD throughout the RV [Figure 3 and Video 2]. We calculated following LV and RV volumes and EF: EDVI 91 ml/m2 and ESVI 29 ml/m2 and EF 68% for LV, EDVI 49 ml/m2, ESVI 24 ml/m2 and EF 52% for RV. The following diagnosis was reported: Apical VSD and hypertrabeculation of the mid-lateral and inferior wall and of the apical septum.
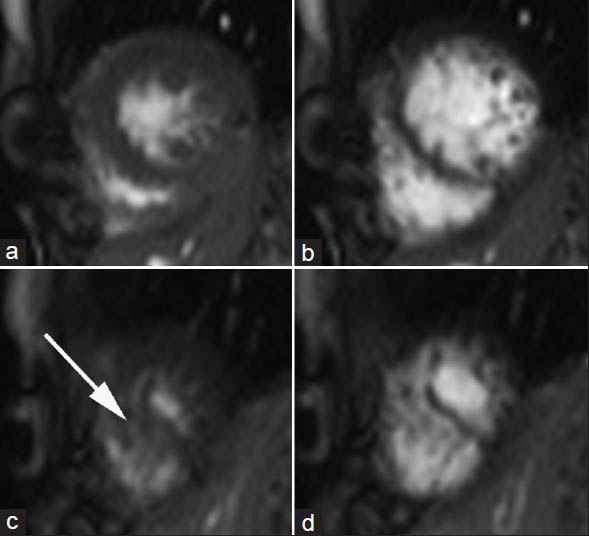
- 12-year-old male with a small muscular apical ventricular septal defect. Cine true-fast imaging with steady-state precession short-axis in the apical portions in (a and c) systole and (b and d) diastole show hypertrabeculation of the myocardium of the left ventricle (arrow).
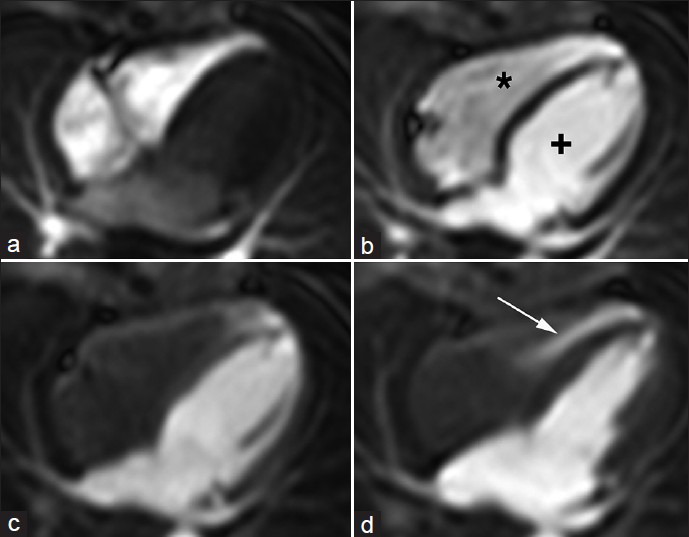
- 12-year-old male with a small muscular apical ventricular septal defect (VSD). First Pass Perfusion long-axis four-chambers shows the passage of the contrast material through a) the right chambers (*), then b) through the left (+) chambers. (c, d), When the right chambers are empty and clear the image shows the passage of the contrast material (arrow) from left to right ventricle through the small apical muscular ventricular septal defect
DISCUSSION
The cine SSFP sequences, acquired in the cardiac planes in different phases from systole to diastole, allow a clear assessment of myocardium hypertrabeculation and of big septal defects that appear as void signal jets. Small VSDs are not detectable at these sequences because of their not significant hemodynamic appearance and the low spatial resolution of MRI. Using the first-pass perfusion sequence, which has low signal-to-noise ratio but is sensitive to changes in T1, combining the right plane of acquisition with a short bolus of contrast material and a high heart rate, small VSDs can also be shown. In our case, a short bolus of contrast agent was used. When it filled the left chambers, the RV was empty and this allowed viewing of the presence of a high signal-intensity jet from the LV into the RV through a small apical VSD [Figure 3].
Although echocardiography remains the gold standard in the diagnosis of VSDs, MRI using perfusion sequences acquired under appropriate planes and an appropriate dose of contrast material (a small dose so as to see at the same time the LV full and the RV empty), can detect the presence of even a small VSD that occurs frequently in many conditions. This technique could be used in all congenital patients who require a confirmation of the presence/absence of small VSD or ASD. Thus, a small septal defect, usually not visible either at black-blood or at cine-SSFP sequences, can be detected by the use of a first-pass perfusion sequence MRI.
CONCLUSION
This report attempts to address cardiac MRI using first-pass perfusion sequences, combining the right plane of acquisition with a short bolus of contrast material, which in this case clearly confirmed the presence of VSD. This technique should be used to confirm the presence of atrial or ventricular defects.
Video available on www.clinicalimagingscience.org
Available FREE in open access from: http://www.clinicalimagingscience.org/text.asp?2013/3/1/59/124083
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- Contemporary definitions and classification of the cardiomyopathies: An American Heart Association Scientific Statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation. 2006;113:1807-16.
- [Google Scholar]
- High prevalence of muscular ventricular septal defect in neonates. J Am Coll Cardiol. 1995;26:1545-8.
- [Google Scholar]






