Translate this page into:
Virilizing Adrenal Oncocytoma
Address for correspondence: Dr. Dinesh Sharma, Department of Radiodiagnosis, Indira Gandhi Medical College, Shimla- 171 001, India. E-mail: dineshss108@gmail.com
-
Received: ,
Accepted: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Adrenal oncocytoma is a rare adrenal neoplasm with only 57 cases reported in literature. Adrenal oncocytomas can achieve large sizes and are usually nonfunctioning. They are detected accidentally during abdominal scans. Most of these adrenal neoplasms are benign. A functioning adrenal oncocytoma manifested with virilization in a 16-year-old female child. There seems to be little benefit in biopsying these tumors and surgery remains the optimum management.
Keywords
Adrenal tumor
adrenal oncocytoma
oncocytoma
virilization
INTRODUCTION

The term “oncocyte,” first used by Hamperl in 1950, describes large, highly eosinophilic, granular cells associated with a Hurthle cell tumor of the thyroid gland.[1] Oncocytoma of the adrenal gland is defined as a neoplasm composed exclusively or predominantly of oncocytes. These oncocytes are large polygonal cells with eosinophilic cytoplasm that result from the abnormal accumulation of mitochondria.[2] Oncocytomas can arise in several organs including the kidney, thyroid, salivary glands, parathyroid, lung, pituitary gland, and ovary.[3] Most of these adrenal neoplasms are benign, usually nonfunctional, and hence, accidentally detected.[2] Only 10 cases of functioning adrenal oncocytomas are reported so far in literature and only three cases manifesting with virilization were seen in children.[34] We present a rare functioning adrenal oncocytoma manifesting as a virilizing entity.
CASE REPORT
A 16-year-old girl presented with a 6-month history of primary amenorrhea and pain in the abdomen. She also noticed excessive facial hair growth during the 6-month period. The weight of the patient was 55 kg. The height was normal for her age. She had normal secondary sexual development. There was male pattern of hair distribution [Figure 1]. Breast development was Tanner Stage IV. Pubic hair development was Tanner Stage V. Clitomegaly was present. Uterus and adenexa were normal on per rectal examination. Her blood pressure was normal and there was no diabetes mellitus. The total testosterone level was raised and value was 435 ng/dl (normal range, 14-76 ng/dl). Dehydroepiandrosteronesulfate (DHEA-S) level was >1500 μg/dl (normal range, 61-493 μg/dl). Vanillylmandelic acid (VMA) level was 10.10 mg/24 h (normal range, <15.0 mg/24 h). Rest of the blood parameters were normal.

- Photograph of the female patient shows male pattern of facial hair growth.
Imaging findings
Computed tomography (CT) scan of the abdomen was performed on a 64-slice multidetector MDCT scanner (GE light speed VCT XTe whole body 64 slice CT system from Wipro GE Healthcare Private Limited). On noncontrast CT scan, there was a well-defined hypodense mass of size 11.6 × 10.7 × 12 cm, with scattered areas of calcifications. Center of the mass showed a stellate shaped noticeably hypodense area. Plain CT attenuation was around 59 HU. Arterial phase scan (acquired at 30 s) showed a tangle of serpiginous, thin intratumoral vessels throughout the mass except in the central stellate nonenhancing area [Figure 2]. These vessels gave a can-of-worms like appearance on volume rendered images [Figure 3]. The periphery of the mass was supplied by dilated, thick, tortuous vessels arising from phrenic and renal vessels [Figure 2]. The average postcontrast attenuation of the mass was around 70HU. It had a thin intensely enhancing capsule. In the venous phase scan (acquired at 60 s), the mass showed homogenous enhancement except for the central nonenhancing area [Figure 4]. The values were in the range of around 103 HU. The right kidney was pushed inferomedially by this mass. The fat planes with liver were maintained. There was no lymphadenopathy or ascites. Big vessels were normal.
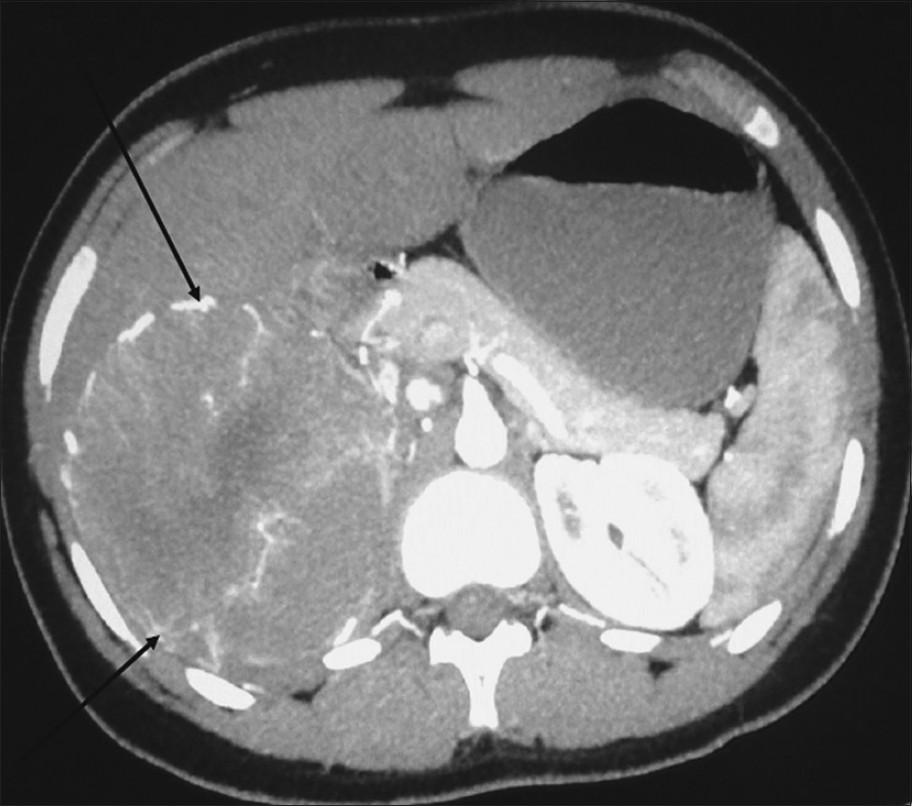
- Arterial phase axial multidetector computed tomography (MDCT) scan of the abdomen shows a hypervascular heterogeneously enhancing mass with radially arranged vessels (arrows) in the right adrenal region.
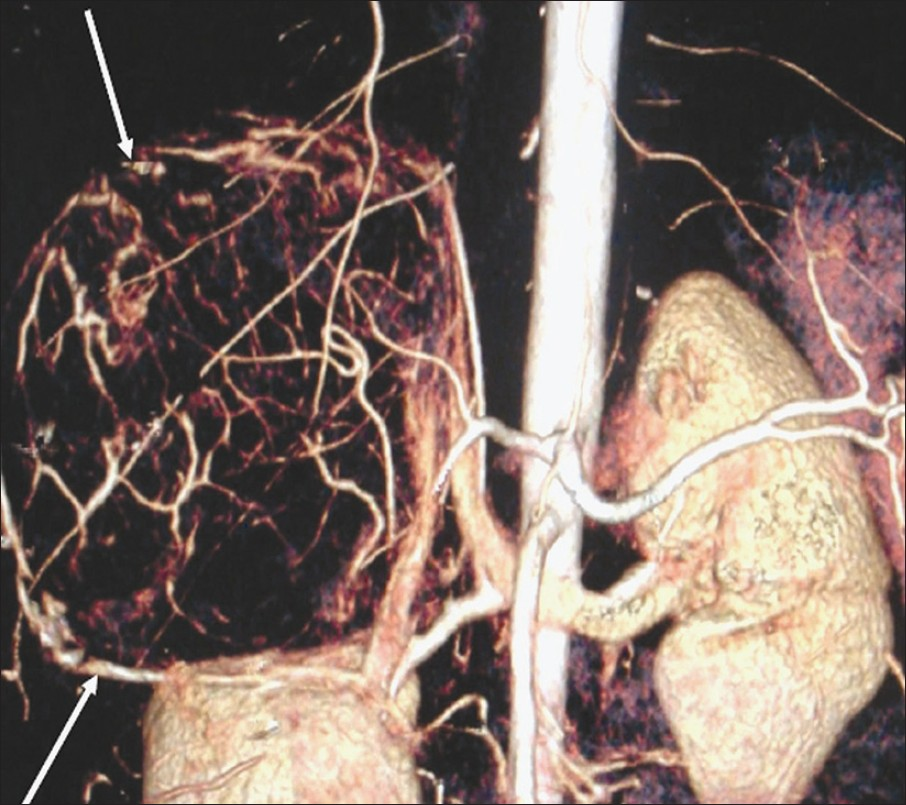
- Volume rendered image of the abdomen depicts the hypervascular mass, in the right adrenal area, giving a can-of-worms appearance (arrows).

- Axial multidetector computed tomography scan scan of the abdomen in the portal venous phase shows the mass in right adrenal region with early wash-out of contrast and a central nonenhancing area (arrow).
Open right adrenalectomy was performed. The tumor was round in shape and well encapsulated [Figure 5]. It was highly vascular and had blood supply from the aorta, renal, and phrenic vessels. The histopathological examination showed oncocytic cells with eosinophilic cytoplasm and small round nuclei. There was no mitosis. Necrosis was seen in the smears [Figure 6]. The final diagnosis was testosterone secreting adrenocortical oncocytoma. The recovery was uneventful and features of virilization receded in follow-up done after 3 months.
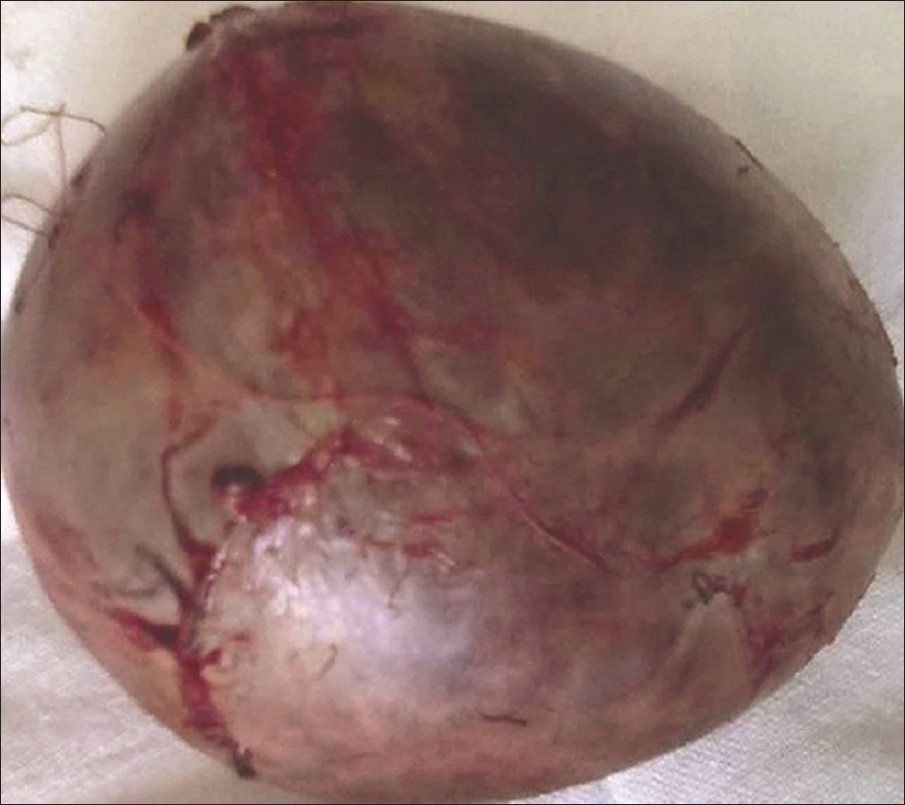
- Image of the well-encapsulated mass measuring 16 × 12 cm removed by surgery.
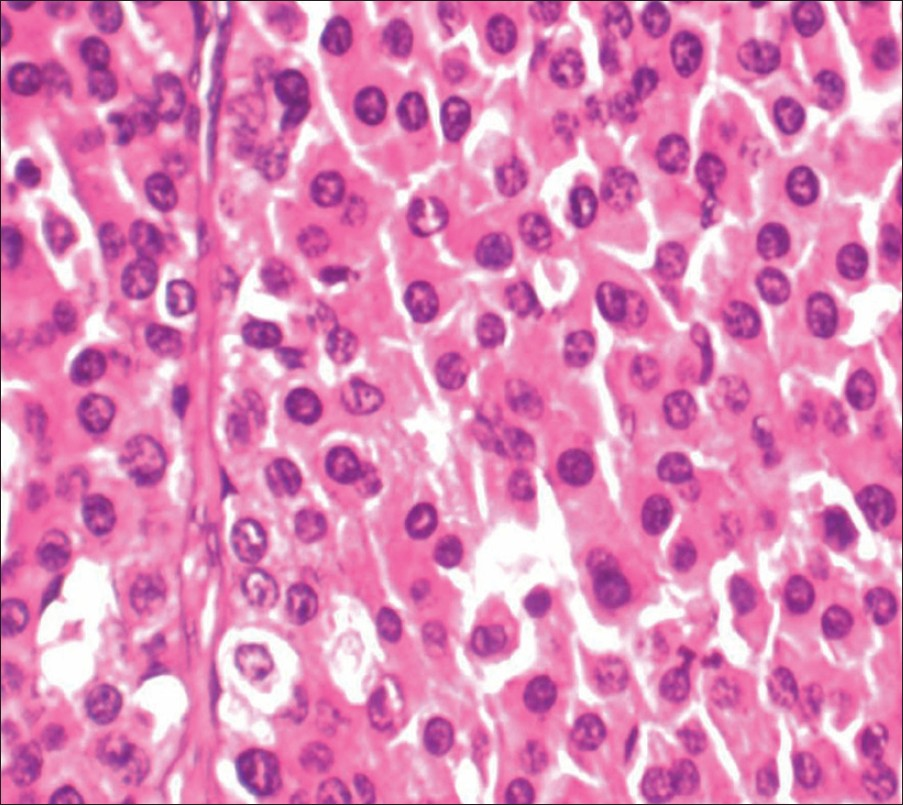
- Hematoxylin and eosin stain at 400× of the smear shows sheets of oncocytic cells with uniform round nuclei and abundant eosinophilic cytoplasm.
DISCUSSION
Accidentally detected adrenal masses are found in 1-2% of abdominal CT scans.[5] Such adrenal masses are also known as “incidentalomas” and can be cortical adenomas, cysts, myelolipomas, ganglioneuromas, pheochromocytomas, adrenocortical carcinomas, and adrenal metastases. In a patient without a known primary malignancy, the most common adrenal “incidentalomas” are nonfunctioning cortical adenomas.[6] Oncocytomas can occur in various organs, especially the kidneys where oncocytomas represent around 3-7% of all renal neoplasms.[7] The classic central radiating scar that has been described in renal oncocytomas is not necessarily present in adrenal oncocytomas. They are much rarer in the endocrine, respiratory, and gastro-intestinal tissues. They are predominantly benign tumors, which may reach considerable size, if unresected.[78]
Oncocytomas of the adrenal gland are particularly rare, although exact overall incidence is unknown. They have been reported in patients across a wide range of ages (6 - 77 years). They occur more frequently in females (2.5:1) and are more common on the left side (3.5:1). There are no identified environmental or genetic risk factors. The tumors described have varied in size from 3.0 to 17.0 cm, but they are usually larger than 6 cm.[478] Our patient was a 16-year-old girl and the tumor was 12 cm in size. Functioning adrenocortical oncocytomas have been reported so far in only 10 cases, of whom seven patients were 40-60 years of age and three patients were 6, 12, and 14 years of age. Virilization in an adolescent girl can manifest as clitoral enlargement, increased muscle strength, acne, hirsutism, frontal hair thinning, deepening of the voice, and amenorrhea/menstrual disruption. Some of the possible causes of virilization in women are polycystic ovary syndrome, androgen-producing tumors (of the ovaries, adrenal glands and pituitary gland), hypothyroidism, anabolic steroid exposure, congenital adrenal hyperplasia due to 21-hydroxylase deficiency (late-onset), and hormone replacement therapy (female-to-male).[4] In three reported cases, children presented with virilization and pseudopuberty. Our patient also presented with virilization showing high serum testosterone and DHEA-S levels.
Several systems have been developed for classifying adrenocortical neoplasms as benign or malignant. Bisceglia proposed major and minor criteria as part of a malignancy scoring system based on histology. The three major criteria are: High mitotic rate (>5 mitoses per 50 high power fields), atypical mitoses, or venous invasion. The four minor criteria are: Tumor size >10cm or weight >200g, tumor necrosis, capsular or sinusoidal invasion. Benign lesions would lack all of these features. The presence of any one or more major criteria categorizes the lesion as a malignant oncocytoma. The presence of at least one minor criterion yields a diagnosis of uncertain malignant potential or borderline malignancy.[9]
Adrenal oncocytomas are heterogeneous in appearance on imaging studies and the hypoechoic areas seen on sonography and hypodense areas on CT scans represent necrosis detected on pathological examination.[810] A characteristic finding for adrenal oncocytomas is well-defined fibrous capsule and this is found in both benign and malignant tumors. This capsule could be successfully detected by both ultrasound (US) and CT. Given the large size, heterogeneous appearance and the presence of necrosis in the tumor, adrenocortical carcinoma was obviously the most likely preoperative diagnostic consideration in our case. CT appearance of adrenocortical carcinoma can be similar to oncocytoma with heterogeneous enhancement and central areas of necrosis. Thus unfortunately, the imaging features of adrenocortical oncocytoma does not allow its differentiation from adrenocortical carcinoma.[8] Surgical resection remains the only tool for definitive diagnosis.
CONCLUSION
Adrenal oncocytoma can be included in the differential diagnosis of especially large, well-defined, and nonfunctioning adrenal tumors. However, nonspecific imaging features do not warrant a definitive preoperative diagnosis and unfortunately do not change the patient`s management.[8] A biochemical evaluation should be performed to differentiate nonfunctional from functional adrenal masses. All adrenal masses greater than 6 cm should be surgically removed.[62]
ACKNOWLEDGMENT
Sincere thanks to Dr. Kavita Mardi, Associate Professor Department of Pathology IGMC Shimla, for her help in reaching the final diagnosis. We also thank Dr. Shruti Thakur, Dr. Ashwani Tomar, Dr. Vijay Thakur, Dr. Neeti Aggarwal, Dr. Sushma Makhaik, and Dr. Chidanand Chavan of Department of Radio diagnosis IGMC, Shimla for their assistance.
Available FREE in open access from: http://www.clinicalimagingscience.org/text.asp?2012/2/1/76/104309
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- Functioning adrenocortical oncocytoma: A case report and review of the literature. Ann Diagn Pathol. 2005;9:295-7.
- [Google Scholar]
- Oncocytic adrenocortical neoplasms: A report of seven cases and review of the literature. Am J Surg Pathol. 1998;22:603-14.
- [Google Scholar]
- Virilizing adrenocortical oncocytoma in a child: A case report. J Korean Med Sci. 2010;25:1077-9.
- [Google Scholar]
- Hormonal evaluation of the patient with an incidentally discovered adrenal mass. N Engl J Med. 1990;323:1401-5.
- [Google Scholar]
- Renal oncocytoma: Multifocality, bilateralism, metachronous tumor development and coexistent renal cell carcinoma. J Urol. 1999;162:40-2.
- [Google Scholar]
- Adrenocortical oncocytic tumors: Report of 10 cases and review of the literature. Int J Surg Pathol. 2004;12:231-43.
- [Google Scholar]






