Translate this page into:
Venous Intravasation as a Complication and Potential Pitfall During Hysterosalpingography: Re-Emerging Study with a Novel Classification
Address for correspondence: Dr. Abdurrahim Dusak, Department of Radiology, Dicle University, Faculty of Medicine, 21280 Diyarbakir, Turkey. E-mail: adusak@gmail.com
-
Received: ,
Accepted: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Objectives:
Presently, hysterosalpingography (HSG) is used as a means to evaluate women with infertility and repetitive pregnancy loss. Venous intravasation is a complication and potential pitfall during HSG and analogous procedures including hysteroscopy. The aim of our study was to assess the venous intravasation and to obtain critical information for more secure and more accurate procedures. In particular, the primary goal of the present study was to compare HSG without and with intravasation to identify differences seen on HSG and to assess the predisposing factors of intravasation. The secondary goal was to describe clinical- and imaging-based novel classification of intravasation.
Materials and Methods:
This study included a patient cohort of 569 patients who underwent HSG between 2008 and 2011 at our center in the absence (control group) or presence (study group) of intravasation. Intravasation classified from level 0 (no intravasation) to level 3 (severe intravasation) was compared with preprocedural (demographic and clinical) and procedural (HSG) data. Data were analyzed using Statistical Package for Social Sciences (SPSS) statistical software.
Results:
Of the 569 patients undergoing HSG, 528 showed no intravasation and 41 (7.2%) patients showed intravasation when associated with preprocedural (leukocytes, menometrorrhagia, secondary infertility, ectopic pregnancy, abortus, polycystic ovaries, endometriosis, and interventions) and procedural (pain, scheduling, endometrial-uterine nature, and spillage) parameters. Moreover, intravasation was lower in women with smooth endometrium, triangular uterus, and homogeneous peritoneal spillage. No association was found between age, tubal patency, increased pressure, and intravasation.
Conclusions:
Using a novel classification method, intravasation can be observed in women during HSG and associates with preprocedural and procedural predisposing factors in subsumed conditions. This classification method will be useful for improving the efficiency and accuracy of HSG and related procedures by minimization of severe complications caused by intravasation.
Keywords
Complications
hysterosalpingography
novel classification
potential pitfalls venous intravasation
INTRODUCTION

Hysterosalpingography (HSG), also called uterosalpingography, is a fluoroscopic imaging method that uses an iodinated contrast media to investigate endometrial-uterine morphology and fallopian patency in women with infertility and repeated abortions.[12] HSG can identify many lesions, including hyperplasia, polyps, fibroids, scarre-synechiae, and Mullerian anomalies.[345] Fallopian occlusion due to infection, scarring, ectopic pregnancy, diverticula, tubal ligation, closure devices, and reopening interventions can be evaluated by HSG.[678] Peritoneal spillage provides insight concerning peritoneal adhesions, uterine contour, and endometriosis.[910] HSG is easy, safe, useful, and cost-effective with excellent diagnostic and therapeutic outcomes.[5] However, a few complications, including radiation exposure, vasovagal attack, uterine injury, vaginal bleeding, infection, hypersensitivity, and intravasation might be observed during or after the procedure.[211] Hysteroscopy like HSG is a useful screening test for the evaluation of infertility through analysis of the uterine cavity. However, these methods increase the risk of severe complications like intravasation.[512]
Intravasation is the passage of contrast media into the veins due to local or systemic abnormalities. It can be observed with uterophlebography; however, this technique can create reticular patterns and multiple thin lines that ultimately lead to false assumptions in diagnosis.[913] Prevention of intravasation during HSG is critical for procedural safety and may be related to predisposing factors, including endometrial vascularity and permeability.[1415] The prevalence of intravasation has been reported to be 0.4-6.9%.[1617] The variability between clinical and basic research on the determination of intravasation suggest the need for a classification to reduce misdiagnosis.
To the best of our knowledge, the main preprocedural (leukocytes, menometrorrhagia, secondary infertility, ectopic pregnancy, abortus, polycystic ovaries, endometriosis, interventions) and procedural (pain, scheduling, endometrial-uterine nature, spillage) parameters associated with intravasation and classification of intravasation have not yet been evaluated. Our report represents the first classification of intravasation since the work of Rindfleisch in 1910 using bismuth.[1018]
The primary aim of the present study was to compare differences in patients whose HSG scans show no intravasation with patients whose HSG scans show intravasation and to assess the predisposing factors of intravasation. The secondary goal was to describe clinical- and imaging-based novel grading of intravasation. By eliminating predisposing factors, intravasation may be minimized and reduce further severe complications.
MATERIALS AND METHODS
The present study protocol was planned in accordance with the Declaration of Helsinki and was approved by our institutional ethics board. All subjects provided written informed consent.
Patients
Our study included 569 women (mean age 31.1 ± 6.0 (19-49) years) who underwent HSG for infertility and repeated abortions between 2008 and 2011 in our center. It is a retrospective study of the HSG scans based on the complication-related grouping, the women without intravasation were assigned to the control group (n = 528) and those with intravasation to the study (n = 41) group. Women with increased serum β-human chorionic gonadotropin, vaginal bleeding, and hypersensitivities to the contrast medium were excluded.
Technique
HSG was scheduled between the 3rd and 13th days of the menstrual cycle to ensure that menstruation had ended and the women were not pregnant. Thus, the women were grouped as follows, post-menstrual (P1 : 3rd-5th), mid-follicular (P2 : 6th-10th), and preovulatory (P3 : 11th-13th) periods [Figure 1]. Bowel preparation was recommended the night before the procedure to improve diagnostic quality. HSG was performed by an experienced radiologist (AD) as described in four gradual steps in the supine position.[23] Speculum was inserted to display the cervix and tenaculum was applied after topical lidocaine (10% xylocaine; Astra Zeneca, Mississauga, ON, Canada). Leech Wilkinson cannula was positioned in the cervical canal before obtaining first image as described.[7] Hydrosoluble iodized contrast medium (Omnipaque; Nycomed, Amersham, UK) 15 mL was slowly administered with fluoroscopic guidance.[18] A second image was obtained at the early phase to evaluate contour irregularity or small filling defects in the endometrial cavity. A third image was obtained when the endometrial cavity distended to evaluate uterine morphology and tubal patency. Peritoneal spillage was shown in the last image. Sedoanalgesic premedication was not applied and the procedure was completed within 15 min.
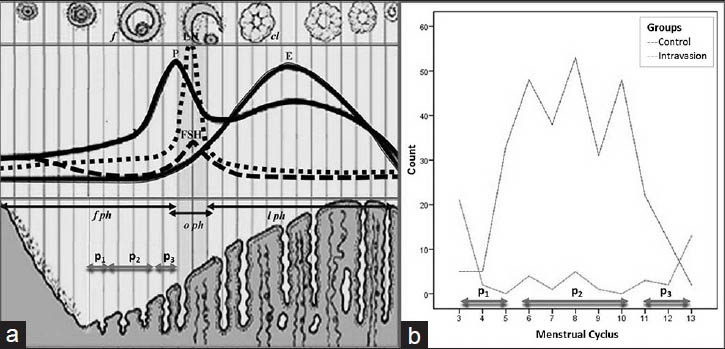
- (a) Schematic view of the schedule of menstrual cycle. (b) Distribution of scheduling of HSG. Intravasation was observed to be higher in the post-menstruation (P1) and preovulation (P3) phases than in the mid-follicular (P2) phase.
Image interpretation
The aim of HSG imaging was to answer the critical clinical questions - the cause of infertility and abortion, prior to the intervention. These questions concerned presence or absence of the venous intravasation and its type (using a novel classification described by authors). All images were reviewed by two radiologists (AD and AB) and two gynecologist (HS and NG), and were grouped by consensus into two (without and with intravasation) groups based on clinical and imaging characteristics.
Intravasation severity score
Intravasation severity score [Table 1], was designed based on qualitative and quantitative parameters, including loss of contrast media, systemic hypersensitivity reactions, misdiagnosis, peritoneal spillage, occurrence, extension of zonal location, and visualized urine bladder.
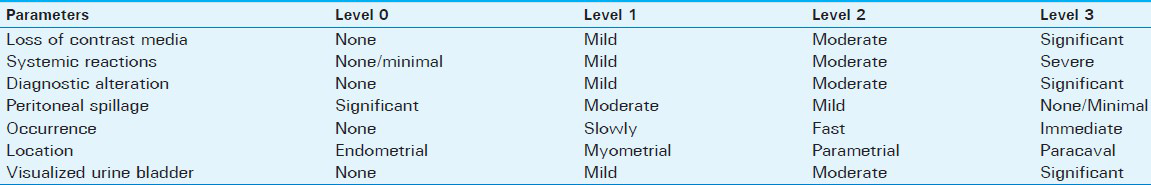
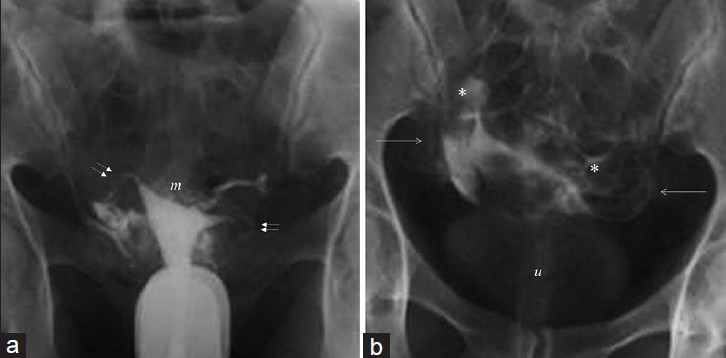
On imaging, intravasation has varied appearance from a reticular pattern to linear pattern seen as multiple thin lines.[9] Intravasation severity score included four levels: Level 0, no intravasation; Level 1, mild intravasation limited to the myometrium;[1920] Level 2, moderate intravasation restricted within the parametrial-adnexial veins occurring slowly;[21] and Level 3, severe intravasation extending from the myometrial-parametrial to the paracaval veins occurring immediately.[2223] To apply this tool, we devised a schema divided into four independent levels based on easily identifiable landmarks as (0) endometrium, (1) myometrium, (2) parametrial, and (3) parailiac veins [Figure 2].

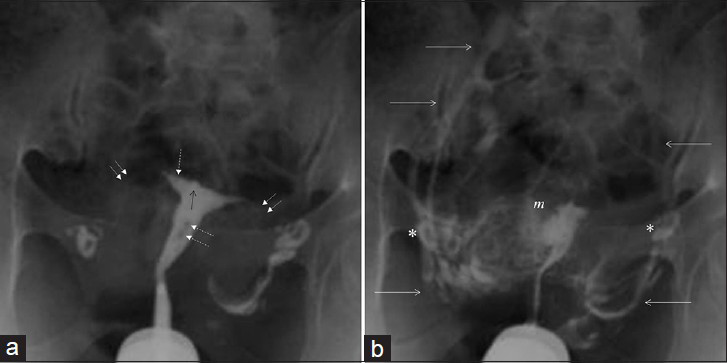
- Schematic view of the intravasation severity score (ISS) based on regional landmarks for intravasations: (a) Level 0: Endometrium (none); Level 1: Myometrium (mild); Level 2: Parametrium (moderate), and Level 3: Parailiac (severe). (b-d) 24-year-old women with arquat uterus. Images show Severe (Level 3) intravasation in internal iliac veins occurring immediately (thin arrows), endometrial bulging (black arrows), myometrial enhancement (m), and patent tubes (double arrows) with loculated peritoneal spillage (*), and notable urine bladder (u) visualization.
Statistics
The Statistical Package for Social Sciences (SPSS) software package for Windows (SPSS version 18.0; Chicago, IL, USA) was used for statistical analysis. Continuous (demographic) data were expressed as the median (range, minimum value − maximum value). Categorical (clinical and procedural) data were expressed as frequencies and percentages. HSG findings were recognized as reference values. Variables (clinical and procedural data) were analyzed using the Chi-squared test and compared using the Mann-Whitney U-test and Student's t-test. A P < 0.05 indicated a statistically significant difference.
RESULTS
Demographic and clinical data
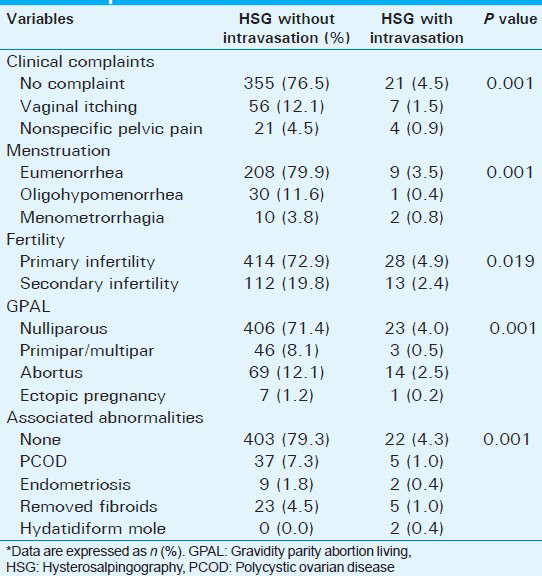
HSG was successfully carried out in 569 women. Intravasation was classified as Level 0 (n = 528; 92.8%), Level 1 (n = 12; 2.1%), Level 2 (n = 18; 3.2%), and Level 3 (n = 11; 1.9%). All patients were divided into two groups: Those without intravasation (Level 0: n = 528, 92.8%) and with intravasation (from Level 1 to Level 3; n = 41, 7.2%). Intravasation was evaluated using the demographic data and clinical data noted prior to HSG procedure. No significant difference was observed between groups regarding age (30.9 ± 6.0 years vs. 32.0 ± 6.6 years, P = 0.182). Intravasation was associated with an increased leukocyte count (6.8 ± 2.4 vs. 8.2 ± 2.5, P < 0.02), painful procedure (P < 0.04), women with vaginal itching and nonspecific pelvic pain using visual analog pain scale (VAS) score during HSG (3.8 ± 1.8 vs. 7.3 ± 2.7, P < 0.04), menometrorrhagia (P < 0.001), secondary infertility (P = 0.019), ectopic pregnancy and abortus (P < 0.001), and polycystic ovarian disease, endometriosis, recently removed fibroids, and hydatidiform mole (P < 0.001) [Figure 3 and Table 2].
- (a and b) 26-year-old women with recently operated hydatidiform mole. Images show Mild (Level 2) intravasation with myometrial reticular enhancement (m), parametrial veins (arrows), patent tubes (double arrows), loculated peritoneal spillage (*), and visible urine bladder (u).
HSG imaging data
Intravasation was evaluated using the HSG imaging data in Table 3. Intravasation was higher during post-menstrual (P1) and preovulatory (P3) than middle follicular (P2) periods (P < 0.001), women with endometrial notch and synechia or bulging (P < 0.001) [Figure 4], Mullerian anomalies (P < 0.001), and loculated peritoneal spillage (P < 0.001). Mullerian anomalies consist of hypoplasia/agenesis (1.2% vs. 0.0%), arcuate (16.5% vs. 2.1%), septate (4.6% vs. 0.5%), bicornuate (6.1% vs. 0.7%) [Figure 5], unicornuate (0.9% vs. 0.4%) [Figure 6], and didelphus (0.2% vs. 0.2%) uterus without and with intravasation during HSG, respectively were detected according to the American Fertility Society (AFS) classification.[242526] No statistically significant difference was detected between the control and intravasation groups regarding the tubal patency due to increased pressure (P = 0.172).


- (a and b) 39-year-old women with recent operated myoma, endometrial notch and synechiae (arrows). Images show Severe (Level 3) intravasation with endometrial bulging (arrow), involving myometrium (m), parametrial and paracaval veins (arrows), patent tubes (double arrows), and minimal peritoneal spillage (*).
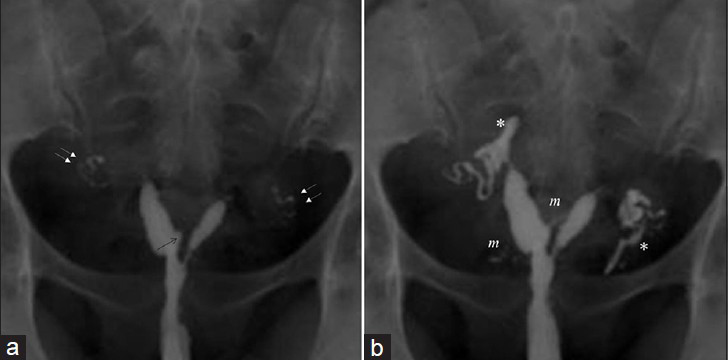
- (a and b) 36-year-old women with communicating unicornuate uterus (American Fertility Society (AFS) IIa. Images show Mild (Level 1) intravasation endometrial bulging (arrow), myometrial enhancement (m), patent tubes (double arrows), and minimal peritoneal spillage (*).
- (a and b) 32-year-old women with uterus bicornis. Images show Moderate (Level 2) intravasation endometrial bulging (black arrow), myometrial enhancement depicting a fundal lobulation (f), patent tubes (double arrows), and peritoneal spillage (*).
DISCUSSION
In the present study, we found that intravasation can be observed during HSG in women with certain clinical symptoms (preprocedural) like increased leukocytes, vaginal itching, nonspecific pelvic pain, menometrorrhagia, secondary infertility, ectopic pregnancy and abortus, polycystic ovarian disease, endometriosis, recently removed fibroids, hydatidiform mole, and subclinical urinary infections. Intravasation was seen more frequently in women who experienced pain during HSG procedure, who were in post-menstrual and preovulatory phase and also in women with predisposing factors such as endometrial notch and synechiae or bulging, Mullerian anomalies, and loculated peritoneal spillage. No association was found between tubal occlusion (increased pressure) and intravasation. To avoid and minimize complications as well as potential pitfalls, this novel classification (particularly in subsumed conditions) may be useful for more secure and more accurate HSG and related procedures.
Venous intravasation is a well described phenomenon in HSG. The contrast transits from the uterine cavity directly to myometrial vessels with subsequent draining to the pelvic veins.[25] Overall, complications of HSG are not so infrequent. In addition, complications may be accompanied by intravasation, which may involve hypersensitivity, bleeding, and infection.[18] Venous intravasation, passage of contrast media, or fluid into the veins from the endometrium can cause pulmonary embolism along with systemic side effects.[232223]
The prevalence of intravasation was reported to be 0.4-6.9%.[1617] This variability (misdiagnosis) might be due to the fact that the staging of intravasation has not been done before. To the best of our knowledge, our report represents the first classification of intravasation since the work of Rindfleisch in 1910.[18] We defined a novel classification system for intravasation with four levels: Level 0, no intravasation; Level 1, mild intravasation limited to the myometrium (leading to false assumptions in diagnosis and confused with adenomyosis);[1920] Level 2, moderate intravasation restricted within the parametrial-adnexial veins and occurring slowly;[21] and Level 3 severe intravasation extending from the myometrial-parametrial to the paracaval veins and occurring immediately.[2223]
Endometrial histologic dating is related to endometrial maturation, which is assessed by luteinizing hormone, follicle-stimulating hormone, and estradiol levels during menstrual cycles.[272829] Endometrium is thin in the early proliferative phase and is an advantage that helps facilitate imaging.[330] Studies have documented that HSG appearance and endometrial characteristics change with the menstrual cycle.[2131] HSG should be scheduled between the cessation of menstruation and before ovulation, yet early enough so that sufficient time exists to clear blood and menses-related residue.[732] Moreover, performing HSG during the first 10 days of menstruation is not reliable for unsuspected pregnancy in women with irregular menstruation.[33] A histopathological study showed that endometrial dating was related to vascular features and permeability. Microvascular blood flow increases in the early follicular and luteal phases, which reflect preparation for menstrual bleeding, and vascular permeability increases during menstruation.[3435] In another study, HSG was observed to double endometrial contour during late secretory phase.[2136] We found an increased association between intravasation and scheduling of HSG when it is done during the early postmenstrual and the late preovulatory period.
HSG with intravasation generally causes pain during the procedure.[23] Discomfort and a painful procedure may be related to spasms caused by cervical fixation and contrast application during HSG.[183237] Cervical cannulation can be traumatic and cause intravasation.[711] Prostaglandin inhibitors can be used to reduce pain and pseudoimages.[38] Pelvic discomfort and unusual lingering pain during HSG might be related to intravasation and may require prompt intervention.[3739] Although intravasation was historically associated with an increased risk of venous embolus due to the used contrast agents, negative side effects have been reduced since HSGs are now performed with hydrosoluble contrast media.[23] Hydrosoluble contrast media are associated with less complications and good radiographic quality as compared to the liposoluble contrast media.[18] For this reason, the hydrosoluble media achieved popularity for use with HSG.[917] We did not report systemic effects caused by intravasation due to the use of hydrosoluble contrast media. We also excluded from the study patients who were hypersensitive.
Pelvic inflammatory disease is a contraindication for HSG.[340] Analysis of acute-phase reactants can be useful to exclude active inflammation.[1538] Endometrial and tubal tuberculosis can cause infertility as a consequence of the immunosuppression of the endemic areas.[49] HSG has been reported to demonstrate tubal irregularity, multiple small diverticula in the isthmic portion of the tube wall as salpingitis isthmica nodosa often associated with tubal contraction, hydrosalpinx, synechiae, distortion, peritubal adhesions, and intravasation.[1341] A recent paper reported that the treatment of the suspected inflammation beforehand is better than undertreatment to reduce complications of HSG.[2] We excluded all women with pelvic inflammatory disease.
Uterine malformations are related to secondary infertility, repetitive abortion, endometrial injury, and complicated delivery.[44243] In a population-based study, the prevalence of Mullerian anomalies was reported to be 3%.[24] Moreover, Mullerian anomalies prevalence was reported as 5-10% and 25% in patients with recurrent first- and second-trimester abortus, respectively.[26] The higher incidence of abortus risk among patients with Mullerian anomalies was demonstrated as well.[24] Although intravasation can occur in patients during HSG, there are some predisposing factors such as uterine anomalies.[21] We found an association between Mullerian anomalies and intravasation as a result of increased predisposing factors.
Intravasation can mimic tubal occlusion.[25] If the contrast medium is in the uterine tubes, intravasation tends to persist. If not, it tends to be washed out. Intravasation may extend along the venous route.[44] Most of the studies reported that tubal occlusion might be associated with intravasation due to increasing intrauterine pressure.[1912141744] However, recent studies of the effectiveness of tubal closure devices reported no intravasation during HSG.[345] Although a relatively rare event, an awareness of uterine intravasation can prevent potential misinterpretation of HSG. This is a complication and potential pitfall during HSG procedure as the intravasation can mimic intraperitoneal spillage in the occluded tube.[25] We did not observe intravasation in all occluded tubes or as a result of increased pressure. If associated predisposing factors were present, then intravasation might occur. Our conclusion was that increased pressure was necessary but not sufficient for intravasation.
Periprocedural complications reported anecdotally during hysteroscopy including venous intravasation, possible anaphylactic or hypertonic reaction for irrigation solution, pulmonary edema from fluid overload, and air embolism, are similar to those seen with HSG.[512]
Recent uterine and endometrial interventions, repetitive curettage due to placental remnants, and missed or medical abortion might be related to intravasation.[46] The prevalence of Asherman's syndrome, related to secondary amenorrhea following abortion and curettage, was reported to be 1.5-43%.[5224748] Endometrial synechie/notch associated filling defects and asymmetrical disturbance of pressure are facilitating factors for venous intravasation.[332. In accordance with the literature, we hypothesize an association between recent uterine intervention and intravasation as a result of increased permeability.
Hysteroscopy and related interventions carry a risk for intravasation and fluid overload due to increased permeability, opened vessels, and distention/irrigation; all of which require increased pressure.[14] Transcervical endometrial resection is a widely used treatment method for menometrorrhagia. This method uses a glycine solution to irrigate and distend the endometrial cavity which carries a dilutional hyponatremia risk as a result of the fluid intravasation.[4950] Additionally, a study reported that the endometrial laser ablation influenced fluid or gas intravasation.[12] Administration of a warm isotonic solution with a pressure below 70 mmHg was shown to minimize intravasation.[1351] Furthermore, the possibility of intravasation and the hazards of cooling of laser heads has been recognized. With increasing experience, proponents of the HSG procedure appear to be achieving its potential as a less invasive and safer alternative to hysterectomy.[12] Venous intravasation, a well-described complication during HSG, is a prototype of hysteroscopic interventions whereby contrast and fluids transit from the endometrial cavity through the myometrial, pelvic, and paracaval veins. This is an important complication and potential pitfall in uterine interpretation.[1444]
Limitations
Some limitations of our study have to be considered. First, the present study was a hospital-based, cross-sectional study with a limited number of cases. Second, we used Leech Wilkinson cannulation (not a balloon catheter) and compared them because the study was retrospective. Third, we could not evaluate control HSG for intravasation group.
CONCLUSION
In conclusion, we found that intravasation might be related to certain variables, including preprocedural or procedural predisposing factors, which include menometrorrhagia, secondary infertility, abortus, endometriosis, Mullerian anomalies, recent uterine interventions, and painful procedure. Scheduling of HSG during the middle follicular period, eliminating of predisposing factors, and using of hydrosoluble contrast media was shown to minimize or prevent intravasation. Radiologists and gynecologists should be familiar with the technique, interpretation, and intravasation for safer HSG or related procedures. Clarification of the mechanism of intravasation might refine current HSG techniques and facilitate future studies focusing on the prevention and management of intravasation.
ACKNOWLEDGEMENT
We thank Gursel Savci, MD, Gokhan Gokalp, MD, Department of Radiology, Uludag University, for their excellent contribution. In addition, we thank Guven Ozkaya, PhD, from the Department of Statistics, Uludag University, for statistical analyses. We also thank, Shweta Bhatt, MD, Vikram Singh Dogra, MD, Department of Imaging Science, University of Rochester, for their valuable support in successful completion of the study.
Available FREE in open access from: http://www.clinicalimagingscience.org/text.asp?2013/3/1/67/124105
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- Structural findings at hysterosalpingography in patients with infertility at two private clinics in Kampala, Uganda. Afr Health Sci. 2004;4:178-81.
- [Google Scholar]
- Diagnostic value of hysterosalpingography in the detection of intrauterine abnormalities: A comparison with hysteroscopy. AJR Am J Roentgenol. 2004;183:1405-9.
- [Google Scholar]
- Hysteroscopic sterilization using a micro-insert device: Results of a multicentre Phase II study. Hum Reprod. 2003;18:1223-30.
- [Google Scholar]
- Hysterosalpingography and sonohysterography: Lessons in technique. AJR Am J Roentgenol. 2006;186:24-9.
- [Google Scholar]
- Re-evaluation of the indication for and limitation of laparoscopic salpingotomy for tubal pregnancy. Eur J Obstet Gynecol Reprod Biol. 2008;137:210-6.
- [Google Scholar]
- Hysterosalpingography with a balloon catheter versus a metal cannula: A prospective, randomized, blinded comparative study. Hum Reprod. 1998;13:75-7.
- [Google Scholar]
- Sorbitol 2.5% mannitol 0.54% irrigation solution for hysteroscopic endometrial ablation surgery. Can J Anaesth. 1997;44:473-8.
- [Google Scholar]
- Salpingitis isthmica nodosa: Radiologic and clinical correlates. Radiology. 1985;154:597-600.
- [Google Scholar]
- Complications in hysteroscopy: Prevention, treatment and legal risk. Curr Opin Obstet Gynecol. 2002;14:409-15.
- [Google Scholar]
- Selective injection of contrast media: Inflammatory effects on rabbit fallopian tubes. Radiology. 1991;180:97-9.
- [Google Scholar]
- Pericaval intravasation of contrast media in the changed indication for hysterosalpingography. Rontgenblatter. 1984;37:26-8.
- [Google Scholar]
- Intravasation during hystero-salpingography using using oil-base contrast medium: A second look. Obstet Gynecol. 1987;70:309-12.
- [Google Scholar]
- Diagnostic quality and complications of hysterosalpingography: Oil versus water-soluble contrast media: A randomized prospective study. Radiology. 1991;179:69-74.
- [Google Scholar]
- Echogenic foci mimicking adenomyosis presumably due to air intravasation into the myometrium during sonohysterography. Diagn Interv Radiol. 2007;13:26-9.
- [Google Scholar]
- Adenomyosis: From the sign to the diagnosis. Imaging, diagnostic pitfalls and differential diagnosis: A pictorial review. Radiol Med. 2011;116:1267-87.
- [Google Scholar]
- Hysterosalpingography: Spectrum of normal variants and nonpathologic findings. AJR Am J Roentgenol. 2011;177:131-5.
- [Google Scholar]
- Pulmonary and cerebral oil embolism after hysterosalpingography with oil soluble contrast medium. Respirology. 2004;9:134-6.
- [Google Scholar]
- Trial of labor and vaginal birth aft er cesarean section in patients with uterine Mülleriananomalies: A population-based study. Am J Obstet Gynecol. 2007;196:537.e11.
- [Google Scholar]
- Fertility after the demonstration of intravasation during hysterosalpingography. Int J Gynaecol Obstet. 1986;24:431-3.
- [Google Scholar]
- The unicornuate uterus and its variants: Clinical presentation, imaging findings, and associated complications. J Ultrasound Med. 2012;31:319-31.
- [Google Scholar]
- Endometrial and subendometrial blood flow measured during early luteal phase by three-dimensional power Doppler ultrasound in excessive ovarian responders. Hum Reprod. 2004;19:924-31.
- [Google Scholar]
- The feasibility of a less invasive method to assess endometrial maturation: Comparison of simultaneously obtained uterine secretion and tissue biopsy. BJOG. 2009;116:304-12.
- [Google Scholar]
- The effect of intra-uterine device on the endometrial pattern. Asia Oceania J Obstet Gynaecol. 1983;9:155-8.
- [Google Scholar]
- NICHD National Cooperative Reproductive Medicine Network. Histological dating of timed endometrial biopsy tissue is not related to fertility status. Fertil Steril. 2004;82:1264-72.
- [Google Scholar]
- Hysterographic appearance and uterine histology at different stages of the reproductive cycle and after progestagen treatment in the domestic cat. Theriogenology. 2005;64:12-29.
- [Google Scholar]
- Therapeutic efficacy of hysterosalpingography with special reference to application of hydrostatic pressure during the procedure. Int J Health Sci (Qassim). 2007;1:223-7.
- [Google Scholar]
- Glycodelin in endometrial flushing fluid and endometrial biopsies from infertile and fertile women. Eur J Obstet Gynecol Reprod Biol. 2011;156:60-6.
- [Google Scholar]
- Grading a developmental continuum: Elegy on the rise and fall of the endometrial biopsy. Fertil Steril. 2004;82:1286-92.
- [Google Scholar]
- An unexpected role for anticoagulant heparan sulfate proteoglycans in reproduction. Swiss Med Wkly. 2006;136:583-90.
- [Google Scholar]
- Unsuspected pregnancy at hysterosalpingography: A report of three cases with different outcomes. Hum Reprod. 2003;18:2608-9.
- [Google Scholar]
- Organic vs functional obstruction of the fallopian tubes: Differentiation with prostaglandin antagonist- and/ı2-agonist-mediated hysterosalpingography and selective ostial salpingography. AJR Am J Roentgenol. 1991;157:77-80.
- [Google Scholar]
- The effect of flurbiprofen as prophylactic analgesic before hysterosalpingography. J Int Med Res. 2010;38:1780-4.
- [Google Scholar]
- Classical and nerve-sparing radical hysterectomy: An evaluation of the risk of injury to the autonomous pelvic nerves. Surg Radiol Anat. 2003;25:200-6.
- [Google Scholar]
- Investigation of the infertile couple: Should diagnostic laparoscopy be performed after normal hysterosalpingography in treating infertility suspected to be of unknown origin? Hum Reprod. 2002;17:1-3.
- [Google Scholar]
- Enhanced fertility after diagnostic hysterosalpingography using oil-based contrast agents may be attributable to immunomodulation. AJR Am J Roentgenol. 2004;183:1725-7.
- [Google Scholar]
- Distribution of causes of infertility in patients attending primary fertility clinics in Israel. Isr Med Assoc J. 2011;13:51-4.
- [Google Scholar]
- Venous intravasation: A potential pitfall of confirmatory hysterosalpingogram following essure hysteroscopic sterilization. J Radiol Case Rep. 2012;6:18-22.
- [Google Scholar]
- Contemporary hysteroscopic methods for female sterilization. Int J Gynaecol Obstet. 2010;108:79-84.
- [Google Scholar]
- Endometrial resection/ablation techniques for heavy menstrual bleeding. Cochrane Database Syst Rev 2009:CD001501.
- [Google Scholar]
- Intra-uterine adhesions and fertility outcome: How to optimize success? Curr Opin Obstet Gynecol. 2007;19:207-14.
- [Google Scholar]
- Clinical management of the uterine factor in infertility. Clin Obstet Gynecol. 2011;54:696-709.
- [Google Scholar]
- Absorption of glycine irrigating solution during transcervical resection of endometrium. BMJ. 1990;300:304-5.
- [Google Scholar]
- A cost-utility analysis of hysterectomy, endometrial resection and ablation and medical therapy for menorrhagia. Hum Reprod. 2006;21:1878-83.
- [Google Scholar]
- Warming echovist contrast medium for hysterocontrastsonography and the effect on the incidence of pelvic pain. A randomized controlled study. Hum Reprod. 2006;21:1052-4.
- [Google Scholar]






