Translate this page into:
Vascular Trauma in the Head and Neck and Endovascular Neurointerventional Management

*Corresponding author: Jeet Patel, Department of Radiology, University of Florida College of Medicine - Jacksonville, Jacksonville, Florida, United States. jeet.patel@jax.ufl.edu
-
Received: ,
Accepted: ,
How to cite this article: Patel J, Huynh TJ, Rao D, Brzezicki G. Vascular trauma in the head and neck and endovascular neurointerventional management. J Clin Imaging Sci 2020;10:44.
Abstract
Traumatic vascular injuries of the head and neck can pose life-threatening emergencies, and therefore, the detection and accurate characterization of these injuries by the radiologist is essential. Computed tomographic angiography (CTA) is commonly performed as part of the initial imaging work-up of patients who have sustained blunt or penetrating craniocervical injuries and are suspected to have or are at risk for vascular injuries. This pictorial essay reviews the CTA and conventional angiographic imaging appearance of various vascular injuries that can occur from trauma in the head and neck and also explores the neurointerventional management of these types of injuries.
Keywords
Trauma
Vascular
Neurointerventional radiology
Interventional radiology
Computed tomographic angiography
INTRODUCTION
Craniocervical trauma causing vascular injury to the head and neck is categorized as either penetrating or blunt (non-penetrating) depending on the mechanism of injury to the vessel. Common causes of blunt vascular injury include motor vehicle accidents, falls, and direct strikes from assault; in the neck, similar injury could also be sustained by strangulation or might be associated with coinciding whiplash during a traumatic event. Penetrating vascular injuries could be the result of a skull base fracture, gunshot, stabbing, or penetrating debris, with high-velocity objects such as bullets causing the most extensive damage.
Vascular injury may be present in up to 3%–20% of cases of blunt and penetrating craniocervical injury.[1] It is imperative to detect vascular injuries to prevent or mitigate the risk of complications such as hemorrhage, vessel occlusion, and thromboembolic stroke. Although conventional angiography is considered the gold standard for detection and characterization of these injuries, computed tomographic angiography (CTA) is more readily available and also a highly sensitive modality to screen for such vascular injuries.[2] Follow-up vascular imaging may also be helpful in identifying delayed progression of vascular injuries.[3,4] By detecting or excluding vascular injuries, imaging routinely helps direct clinical management whether medical, surgical, or endovascular therapy or a combination is chosen. Endovascular treatment options depend on the location and degree of the injury and include a variety of deconstructive and reconstructive techniques reviewed in this article.
VASCULAR INJURIES OF THE NECK
Blunt traumatic vascular injuries in the neck tend to be caused by rapid deceleration events in which there is stretching of a vessel with resulting dissection injury, and common causes include extreme neck movement (such as extension, flexion, and rotation), a direct blow in the region of the vessel, or a lacerating injury from an adjacent fracture.[5] The leading cause of blunt vascular injury in the neck is motor vehicle accidents, and other common causes include assault with a direct blow or strangulation, falls, and hanging. Cervical carotid artery injuries occur most commonly in the distal portions due to stretching by the lateral masses of the superior cervical vertebrae or from compression between the mandible and the spine, and vertebral artery injuries occur most commonly in the V2 or V3 segments.[5]
A clinical algorithm can be applied to the evaluation for and management of blunt vascular injuries in the emergency setting. For example, the Western Trauma Association (WTA) algorithm for diagnostic evaluation and management of blunt cerebrovascular injuries (BCVIs) incorporates the Denver screening criteria for imaging with recommendation that CTA should be performed emergently in a patient with signs or symptoms of a BCVI, including arterial hemorrhage from the neck, nose, or mouth; an expanding cervical hematoma; a cervical bruit in a patient <50 years old; a focal neurological deficit; a stroke seen on CT or magnetic resonance imaging; or a neurologic deficit inconsistent with the head CT findings.[6] If these signs or symptoms are not present, CTA should also be considered urgently in patients who have important risk factors for BCVI, which, in the WTA algorithm, include cervical vertebral body or transverse foramen fractures, subluxation, or ligamentous injury as well as any fracture at C1-C3; a LeFort II or III fracture pattern; a closed head injury consistent with diffuse axonal injury and Glasgow Coma Scale <6; a basilar skull fracture traversing the carotid canal; near hanging with anoxia; and a clothesline-type injury or seatbelt abrasion with associated swelling, pain, or altered mental status.[6]
The Denver scale is a commonly used grading scale for the classification of blunt vascular injury in the carotid and vertebral arteries [Figure 1]: A Grade 1 injury consists of vessel luminal irregularity or intramural hematoma with <25% luminal narrowing; Grade 2 injuries have the greatest range of appearance and could consist of an intramural hematoma with ≥25% luminal narrowing, a raised intimal flap, or intraluminal thrombus; a Grade 3 injury is a pseudoaneurysm; a Grade 4 injury is traumatic occlusion of the vessel secondary to dissection; and a Grade 5 injury is vessel transection with extravasation.[7] Patients with Grade 5 injuries are rarely seen at cross-sectional imaging, likely due to death in the field from the severity of the injury.
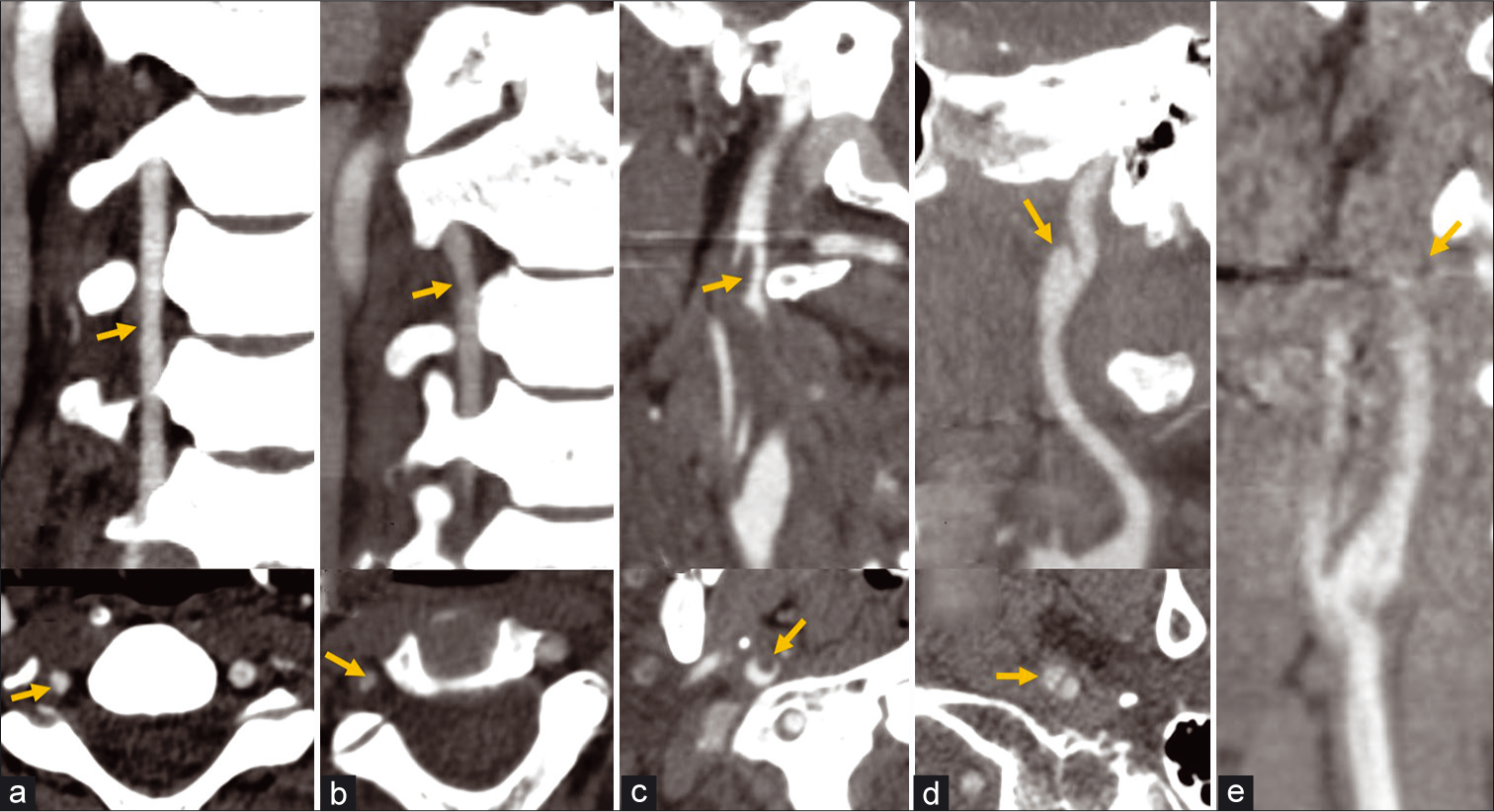
- Denver scale for dissection injuries. (a) CTA coronal (top) and axial images (bottom) of the neck show a Grade 1 dissection injury of the right vertebral artery with <25% luminal narrowing from intramural hematoma (arrows). (b) A Grade 2 dissection of the right vertebral artery with greater than 25% luminal narrowing (arrows). (c) An additional example of a Grade 2 dissection of the right ICA with a raised intimal flap and superimposed intraluminal thrombus (arrows) projecting into the lumen. (d) A Grade 3 dissection with a vascular outpouching from the right ICA consistent with pseudoaneurysm (arrows). (e) A Grade 4 dissection of the left ICA with occlusion.
Penetrating neck trauma is defined as a skin breaching injury that violates the platysma muscle.[8] An algorithmic approach can also be used in the management of penetrating injuries of the neck. In the WTA algorithm for the management of penetrating neck injury, if there are any “hard signs” indicating a major vascular or aerodigestive tract injury, then a patient should immediately proceed to the operating room after securing an airway; examples of these hard signs include active hemorrhage, an expanding or pulsatile hematoma, a bruit from arteriovenous fistula (AVF), a pulse deficit, an acute neurologic deficit, hematemesis, massive subcutaneous emphysema, or airway compromise.[8] If there are no such hard signs, but injury is still suspected, then patients should be managed according to the zone of injury in the neck [Figure 2]. In the modern zonal division of the neck, Zone 1 extends from the clavicles and sternum superiorly to the cricoid cartilage, Zone 2 extends from the cricoid cartilage to the angle of the mandible, and Zone 3 extends from the angle of the mandible to the base of the skull. As a further brief summary of the WTA algorithm from the imaging perspective, if there is no hard sign of a major vascular injury or hemodynamic instability that would necessitate immediate operative exploration, then a patient with a Zone 1 or Zone 3 injury should proceed to CTA first to screen for a vascular injury. However, a patient with a Zone 2 injury who does not have a hard sign of vascular injury but who does have any physical signs or symptoms suspicious for an aerodigestive tract or vascular injury should still proceed first to the operating room; if there are no such physical symptoms associated with the Zone 2 injury, then a CTA should be performed for screening.[8]
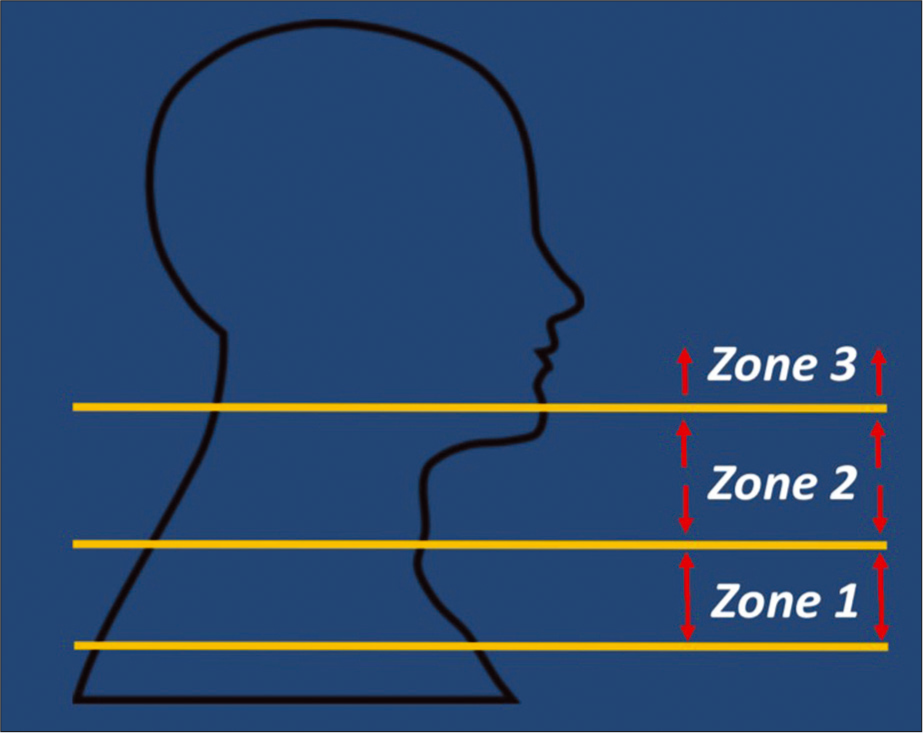
- Modified three zone classification for penetrating trauma. Zone 1: Clavicles/sternum to the cricoid cartilage. Zone 2: Cricoid cartilage to the angle of the mandible. Zone 3: Angle of the mandible to the skull base.
We present a series of cases of traumatic arterial injury in the neck from both blunt and penetrating trauma and with depiction of the intervention used for each of these patients.
Case 1
A 31-year-old female involved in a motor vehicle collision sustained a left C7 transverse process fracture with a left cervical ICA Grade 2 dissection and left proximal vertebral artery Grade 2 dissection, which were managed conservatively with dual antiplatelet therapy [Figure 3]. Follow-up CTA 6 weeks later demonstrated resolution of the carotid dissection and progression of the left vertebral artery injury to Grade 3 with the development of a pseudoaneurysm, necessitating endovascular treatment with a carotid stent. Zone I vascular injuries are usually treated using endovascular techniques as open surgery may be challenging given the need for difficult proximal exposure and need for proximal control. Usually, bare metal stents are sufficient to obliterate a dissection with pseudoaneurysm overtime by tacking down the dissection flap and slowing inflow within the pseudoaneurysm to promote thrombosis.[9] Most vascular dissections in the neck can be treated with non-covered stents while in more tortuous anatomy, intracranial stents or even flow-diverting stents can be considered.[10] Large pseudoaneurysms originating form a large tear in the intima can be coiled by a jailed microcatheter or a covered stent can be used. Covered stents are a good alternative if side branches can be covered safely and there is a need for more immediate vessel reconstruction like in direct penetrating injuries [Figure 4] or high-flow fistulas [Figure 5].[11] The benefits of immediate reconstruction should balance a typically higher rate of restenosis/occlusion of covered stents compared to uncovered stents.[12] If dual- antiplatelet therapy is contraindicated or if there is evidence of active extravasation and need for urgent embolization, vessel sacrifice with coils or liquid embolic agents also remains an alternative treatment option.[13] A follow-up 6 month CTA for the patient in this case demonstrated patency of the left vertebral artery origin stent and complete obliteration of the aneurysm; clopidogrel was stopped after 6 months, and she remains on aspirin monotherapy.
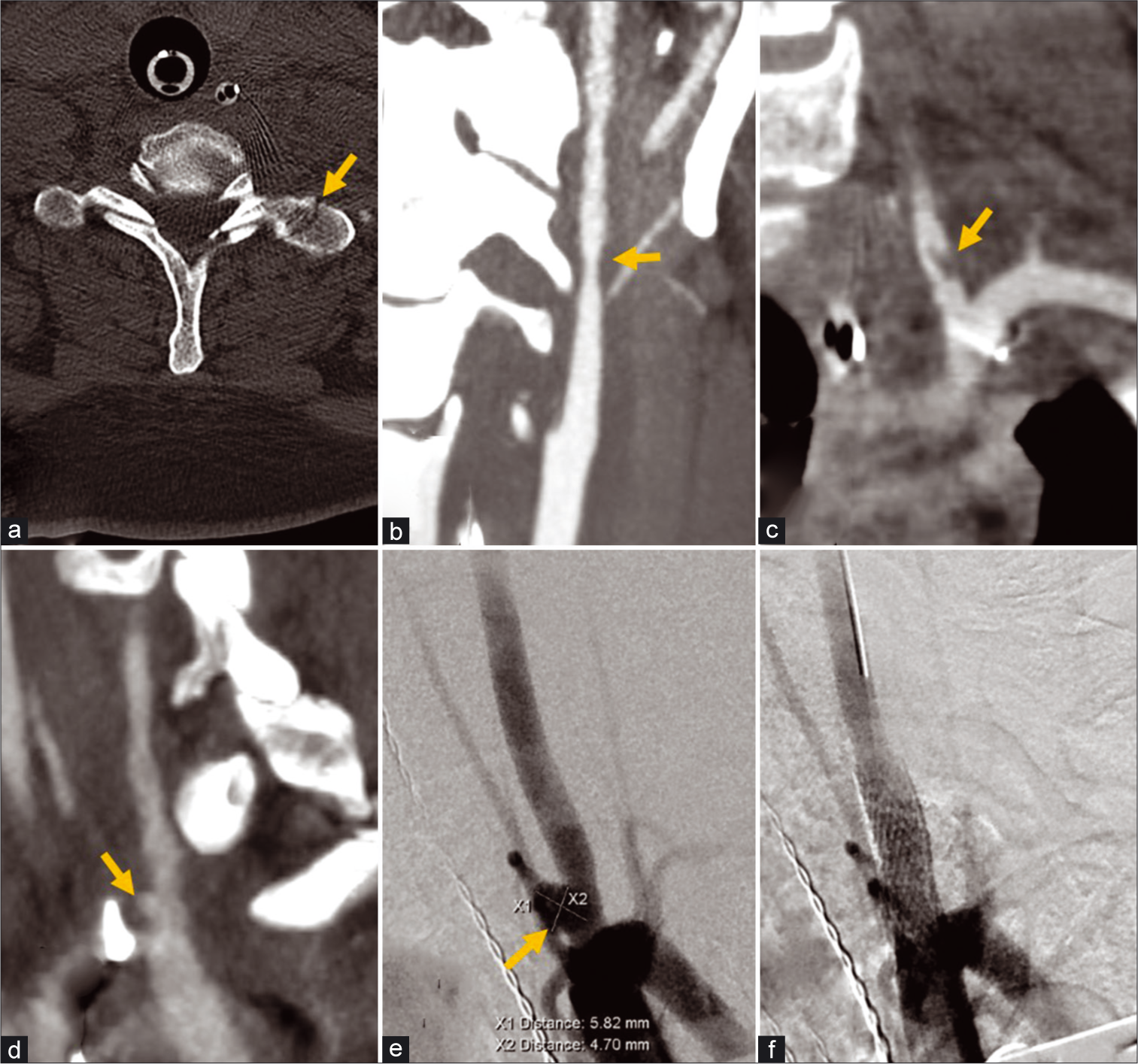
- A 31-year-old female with cervical spine fracture from motor vehicle collision. (a) Non-contrast axial CT image of the cervical spine demonstrates a non-displaced fracture of the left C7 transverse process (arrow). (b) CTA coronal MIP images of the neck demonstrates Grade 2 dissection with >25% stenosis in the mid let cervical ICA (arrow), and (c) Grade 2 dissection with raised intimal flap in the V1 segment of the left vertebral artery (arrow). Follow-up CTA of the neck demonstrated healing resolution of the left ICA dissection, but the left vertebral artery dissection (d) progressed to a pseudoaneurysm/Grade 3 injury (arrow). (e) Conventional angiography of the neck demonstrated further increase in the size of the pseudoaneurysm (arrow). (f) A 6 × 20 mm Precise Pro Carotid Stent (Cordis, Santa Clara, California, USA) was deployed across the dissection with subsequent injection demonstrating successful embolization of the pseudoaneurysm.
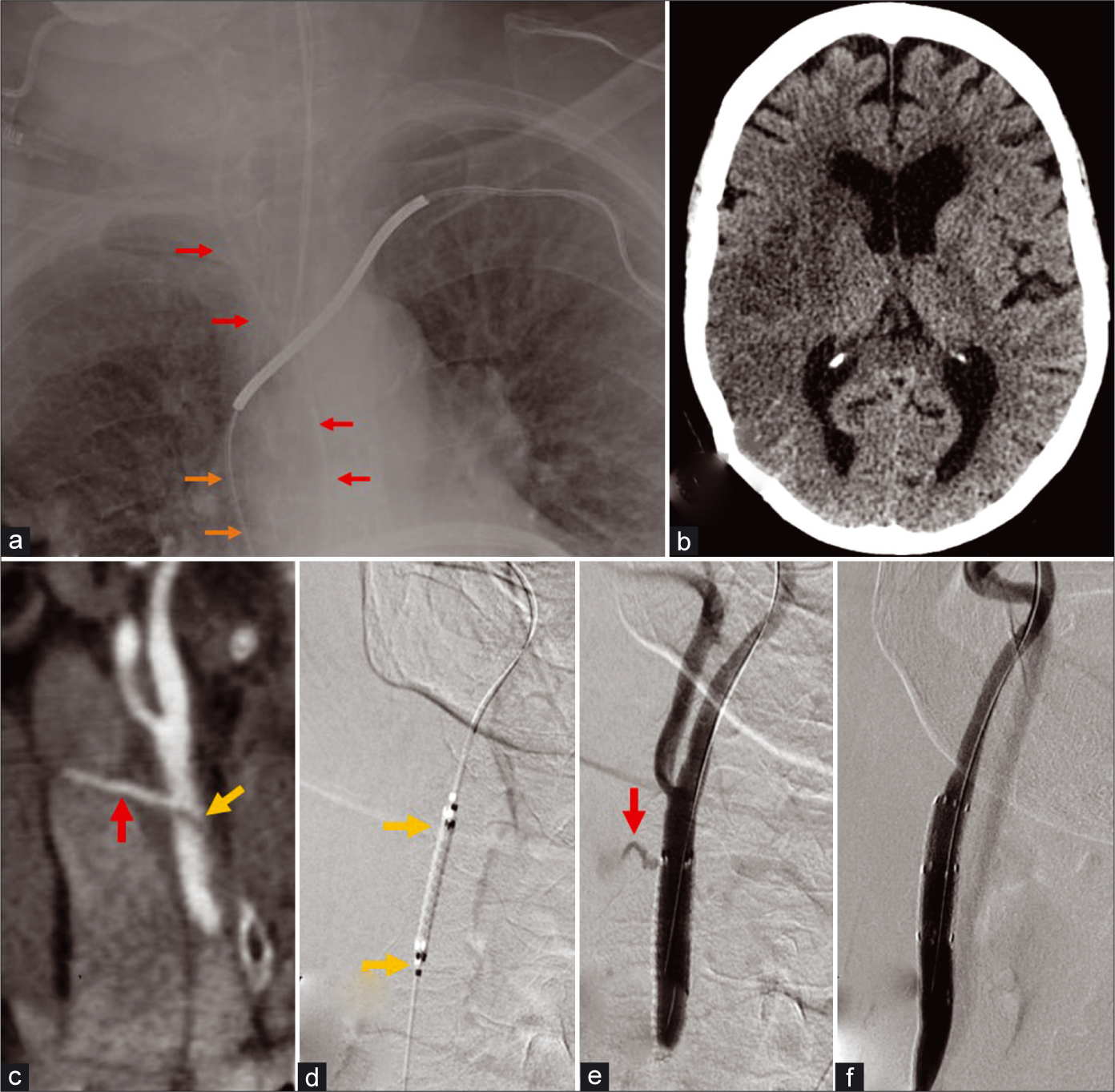
- A 66-year-old female transferred from an outside hospital with arterial placement of right central venous line and left side weakness. (a) AP chest radiograph demonstrates incorrect arterial placement of an intended right internal jugular catheter (red arrows); the left subclavian approach pacemaker lead reveals the true course of the superior vena cava (orange arrows). (b) Non-contrast head CT demonstrates an evolving acute infarct in the right MCA territory from embolic occlusion of the right MCA M1 segment. (c) CTA coronal MIP image of the neck demonstrates a dissection flap in the right common carotid artery (yellow arrow) and discrete tract (yellow arrow) from the removed catheter connecting the right CCA to the right internal jugular vein consistent with an arteriovenous fistula. (d) Conventional angiography pre-contrast image demonstrates initial pre-deployment position of a 6 × 25 mm Viabahn covered stent (yellow arrows) in the right CCA across the site of fistula. (e) Right CCA injection image after angioplasty with 7 × 20 mm Aviator balloon demonstrates proximal migration of the stent and persistent fistula with filling of the right IJV (red arrow). (f) A second Viabahn stent was placed more distally in the CCA and injection image after the angioplasty demonstrates obliteration of the fistula.
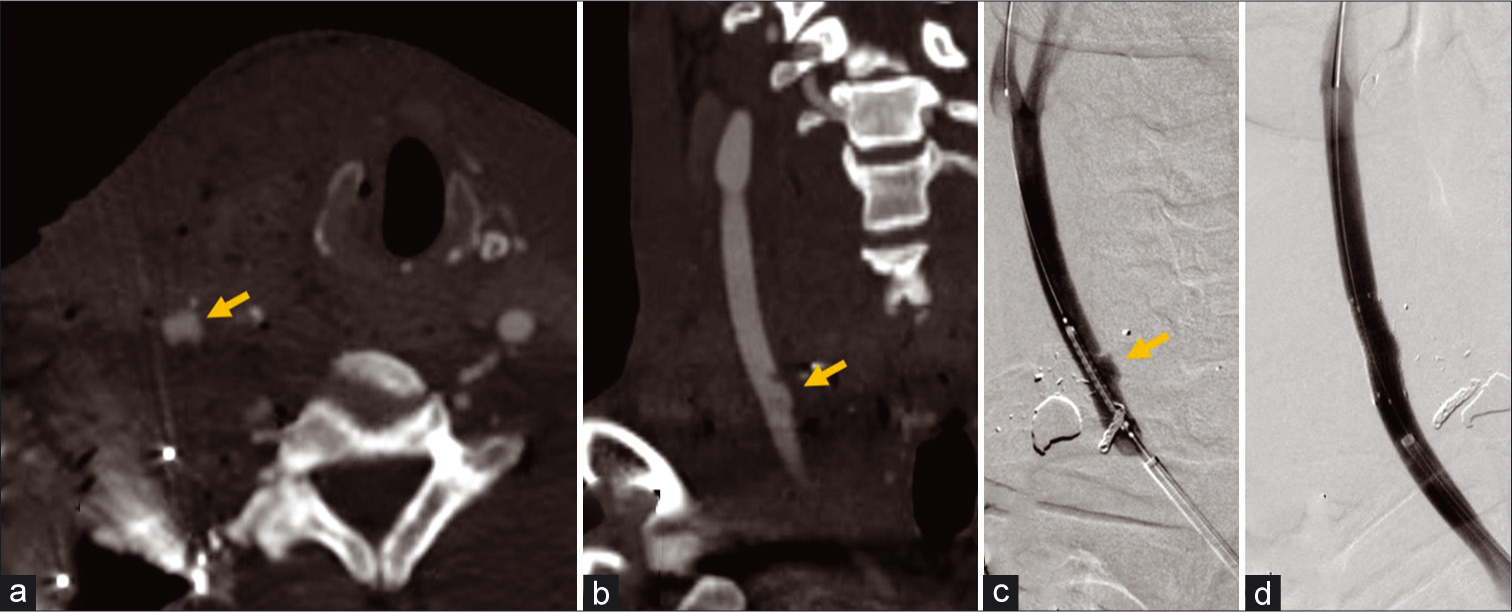
- A 34-year-old male with gunshot to the right side of the neck. (a and b) Axial and coronal CTA MIP images of the neck demonstrate contour irregularity and a vascular outpouching representing pseudoaneurysm (arrows) at the medial aspect of the right common carotid artery. (c) Conventional angiography of the neck provides better resolution of the pseudoaneurysm (arrow). (d) An 8 × 25 mm Viabahn covered stent was placed with successful exclusion and no residual filling of the pseudoaneurysm.
Case 2
A 66-year-old female had arterial placement of a central venous line with complication of an internal jugular vein- common carotid artery AVF and arterial dissection, which was further complicated by a large middle cerebral artery territory embolic stroke [Figure 4]. Endovascular treatment consisted of using a Viabahn covered stent (W. L. Gore, Flagstaff, AZ) to reconstruct the vessel and occlude the fistula. The external carotid artery origin had to be covered, which can be usually done without significant clinical consequences. After a prolonged hospital stay due to the large MCA territory infarct and cardiac comorbidities, the patient was discharged to a rehabilitation facility on dual antiplatelet therapy; after 3 months, her clinical examination remained largely unchanged with a dense left hemiparesis and she was subsequently lost to follow up.
Case 3
A 34-year-old man presented after a gunshot wound to the right base of the neck (Zone 1) with a right common carotid pseudoaneurysm (Grade 3 injury) [Figure 5]. The pseudoaneurysm was treated with a heparin-bonded Viabahn covered stent with immediate vessel wall reconstruction and exclusion of the pseudoaneurysm. A follow-up CTA after 6 months demonstrated patency of the stent and continued exclusion of the pseudoaneurysm; clopidogrel was stopped after 6 months, and the patient remains on aspirin monotherapy.
VASCULAR INJURIES OF THE HEAD
Vascular injuries in the head typically occur from blunt trauma with calvarial or skull base fractures injuring adjacent vessels or from penetrating trauma. According to the American College of Radiology Appropriateness Criteria for imaging for head trauma, CTA or MRA should be considered to screen for suspected intracranial arterial injury in trauma patients with neurologic symptoms that are not explained by a diagnosed injury, blunt trauma patients with epistaxis that is suspected to be from an arterial injury, and those with other risk factors for intracranial arterial injury, including Glasgow Coma Scale ≤8, skull base fracture extending through a carotid canal, diffuse axonal injury, and LeFort 2 or 3 facial fracture patterns.[14] Penetrating gunshot injuries to the head are especially lethal with up to 71% of patients dying at the scene and up to 90% dying before reaching the hospital; there is a reported survival of only 51% in those who reach a hospital.[15,16] Initial screening CTA should be performed in penetrating head trauma from high-velocity gunshot wounds or blast-type injuries given the higher probability of vascular injuries such as pseudoaneurysms.[17]
Vascular injuries in the head may manifest in several forms. This could include a spectrum of injuries from vessel wall dissection similar to as described in the neck, including luminal narrowing from intramural hematoma, pseudoaneurysms, occlusion, and extravasation. Pseudoaneurysms will manifest as vascular outpouchings from a parent vessel on angiography; in the setting of penetrating trauma with bone or metal debris present, careful comparison should be made between noncontrast CT and post-contrast CTA images to distinguish between hyperdense debris and a true contrast-enhancing pseudoaneurysm. Although traumatic intracranial aneurysms/pseudoaneurysms are rare, accounting for <1% of all cerebral aneurysms, they have an especially high incidence in penetrating trauma and are reported to occur in 20–50% of these injuries.[15,18] Risk of rupture of untreated traumatic intracranial pseudoaneurysms is highest within the first 1–3 weeks with mortality up to 50% resulting from rupture.[18] A traumatic AVF could occur if an arterial laceration occurs immediately adjacent to a vein with a resulting luminal communication. CTA may demonstrate early contrast filling of dilated adjacent veins at the site of fistula. Skull base fractures violating the carotid canal create a risk for direct carotid-cavernous fistulas, which are communications between the internal carotid artery and cavernous sinus; symptoms of this often develop on a delayed basis and could include sequela of cavernous sinus venous congestion such as chemosis, vision loss, and proptosis.[19]
An intracranial vascular injury could also be presumed if there is an expanding intracranial hematoma. For example, epidural hematomas are from an arterial source in 85% of cases and are often caused by temporal calvarial fractures lacerating adjacent middle meningeal arteries.[19] A slow extravasation may not be detectable on imaging, but if there is concern for an underlying active hemorrhage, CTA should be performed to exclude a traumatic vascular injury.
We present a series of cases of traumatic arterial injury in the head from both blunt and penetrating trauma and with depiction of the intervention used for each of these patients.
Case 1
A 32-year-old male presented after a self-inflected gunshot injury to the head and was found to have a traumatic pseudoaneurysm of the left anterior cerebral artery A3 segment, pericallosal artery [Figure 6]. This was treated endovascularly with flow diversion using a Pipeline embolization device (PED; Medtronic; Minneapolis, Minnesota, USA). This reconstructive type of treatment enables treatment of the pseudoaneurysm and preservation of the parent vessel.[20] Deconstructive techniques include open surgical treatment with trapping of the injured segment or endovascular embolization techniques such as with liquid embolics or coils and would result in sacrifice of the parent vessel with high risk for downstream infarct. This patient underwent a cranioplasty after 3 months and made an excellent recovery and is able to live independently. A follow-up CTA showed patency of the flow diverting stent, clopidogrel was stopped after 6 months, and the patient remains on aspirin monotherapy.
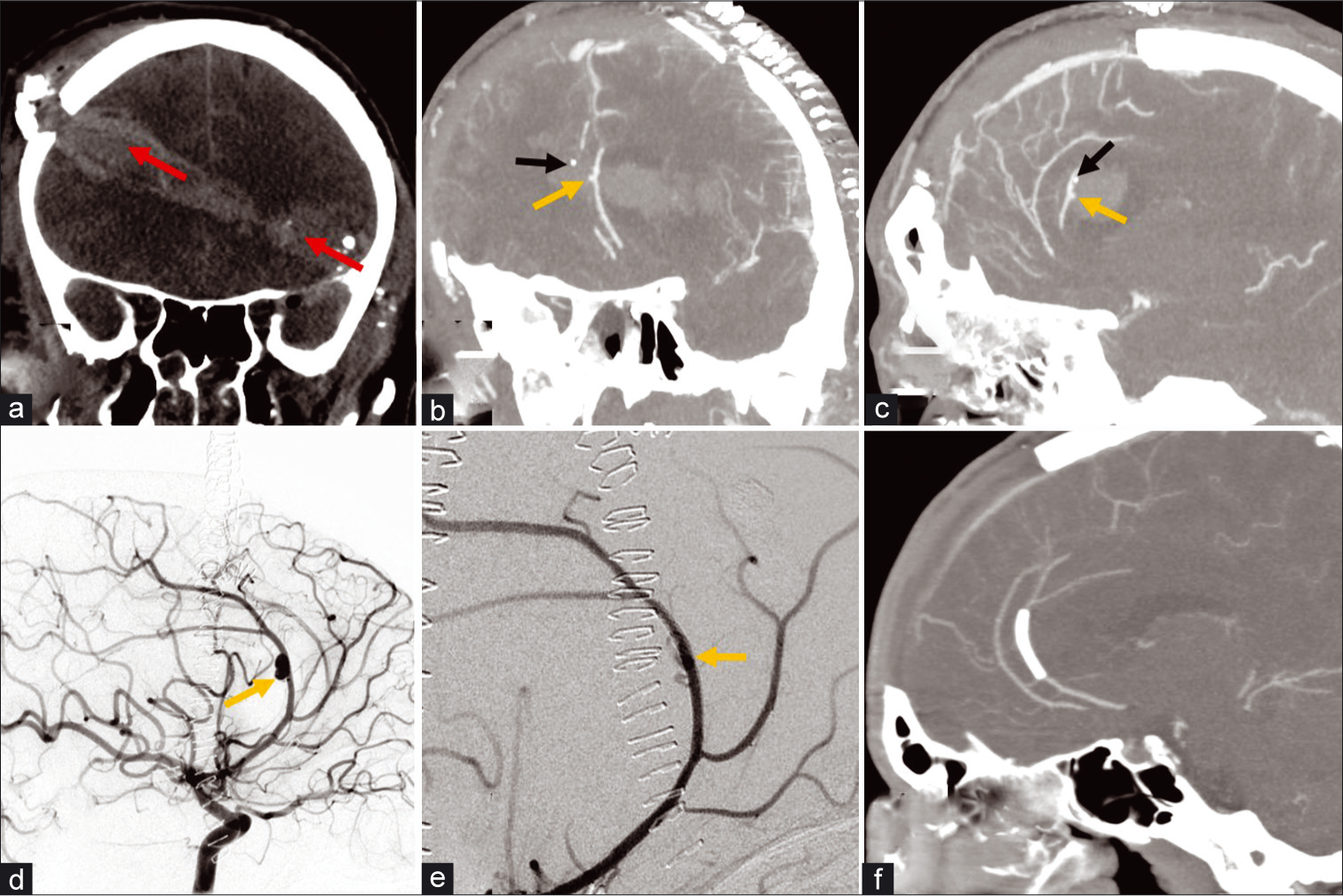
- A 32-year-old male with self-inflicted gunshot wound to the head. (a) Non-contrast CT coronal MIP demonstrates a bullet trajectory from the left temporal aspect through the right frontal region with a hematoma along trajectory (red arrows) and blowout fracture wound on the right. (b and c) CTA coronal and sagittal MIP images of the head obtained after a decompressive craniectomy demonstrate a contrast outpouching (yellow arrow) from the left pericallosal artery or left ACA A3 segment consistent with an pseudoaneurysm; a punctate hyperdense focus nearby (black arrow) with attenuation greater than contrast represents bone debris confirmed with presence on the non-contrast images. (d) Conventional angiography lateral projection of the left ICA injection demonstrates increased size of the ACA aneurysm (arrow). (e) The pseudoaneurysm was treated using flow diversion with a pipeline embolization device and subsequent injection demonstrated slowed filling and contrast stagnation within the pseudoaneurysm (arrow) and preservation of parent vessel. (f) Follow-up CTA sagittal MIP image of the head demonstrates successful occlusion of pseudoaneurysm and preserved flow in the ACA.
Case 2
A 55-year-old male suffered a blunt head injury from a fall with multicompartmental intracranial hemorrhage, including an expanding intraparenchymal hematoma discovered to be caused by a pseudoaneurysm in the distal left ACA frontopolar branch, which was treated with parent vessel embolization [Figure 7]. The parent vessel was sacrificed as other endovascular techniques would not have been feasible due to the distal location of the pseudoaneurysm and very small caliber of the vessel as well as usually limited clinical consequences of stroke in this location. Unfortunately, despite craniectomy, pseudoaneurysm embolization, and intensive care, the patient’s neurological examination continued to decline, and eventually, he succumbed to his injuries.
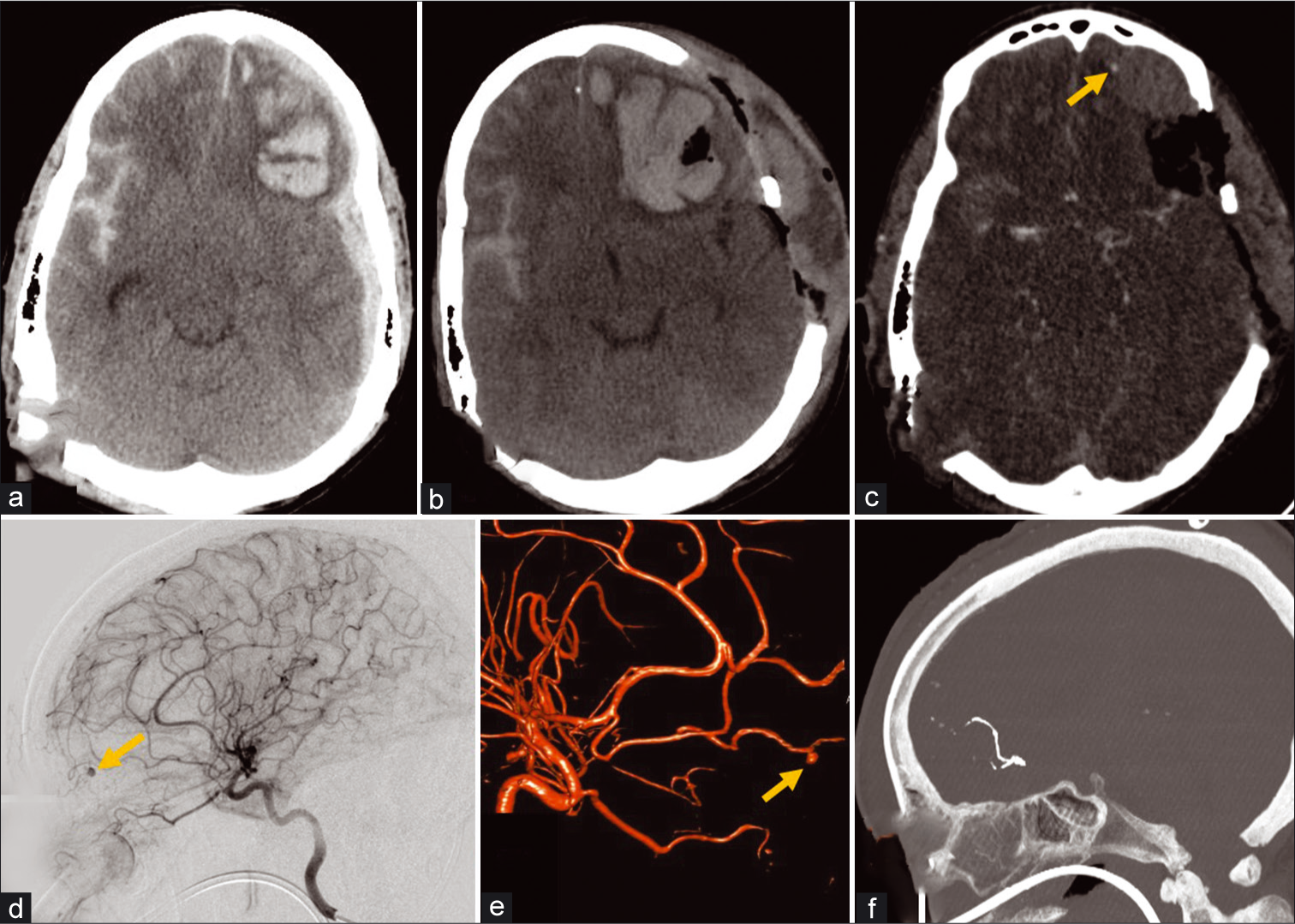
- A 52-year-old male with blunt head injury after a ground level fall. (a) Initial non-contrast CT demonstrates left frontal intra- axial hematoma, left hemispheric subdural hematoma, and right subarachnoid hemorrhage. (b) Follow-up non-contrast CT examination after decompressive craniectomy demonstrates enlargement of the left frontal intra-axial hematoma. (c) CTA of the head demonstrates a punctate focus of contrast enhancement at the anterior periphery of the left frontal hematoma likely representing either active contrast extravasation or pseudoaneurysm (arrow). (d) Conventional angiography lateral projection of the left ICA injection and reconstructed 3D rotational angiography image (e) demonstrate the CTA finding to represent a left ACA frontopolar branch pseudoaneurysm (arrows). (f) Post-procedure CTA sagittal MIP image of the head shows hyperdense Onyx material used to embolize the parent frontopolar branch vessel with no residual filling of the pseudoaneurysm.
Case 3
A 39-year-old male presented after blunt head injury from a motor vehicle collision with right temporal calvarial fracture and epidural hematoma, which did not require surgical evacuation given a benign neurological examination and low hematoma volume with only mild focal mass effect [Figure 8]. Further evaluation with CTA revealed a traumatic AVF supplied by the right middle meningeal artery. The lesion was treated with Onyx (Micro Therapeutics, Irvine, CA) embolization of the fistula and middle meningeal artery given high risk of rupture and enlargement of the epidural hematoma if left untreated. Immediately after the embolization, the patient’s headaches improved significantly and he was discharged home 2 days after the embolization procedure. A non-contrast head CT after 2 months showed complete resolution of the epidural hematoma, and the patient was cleared to return to work.
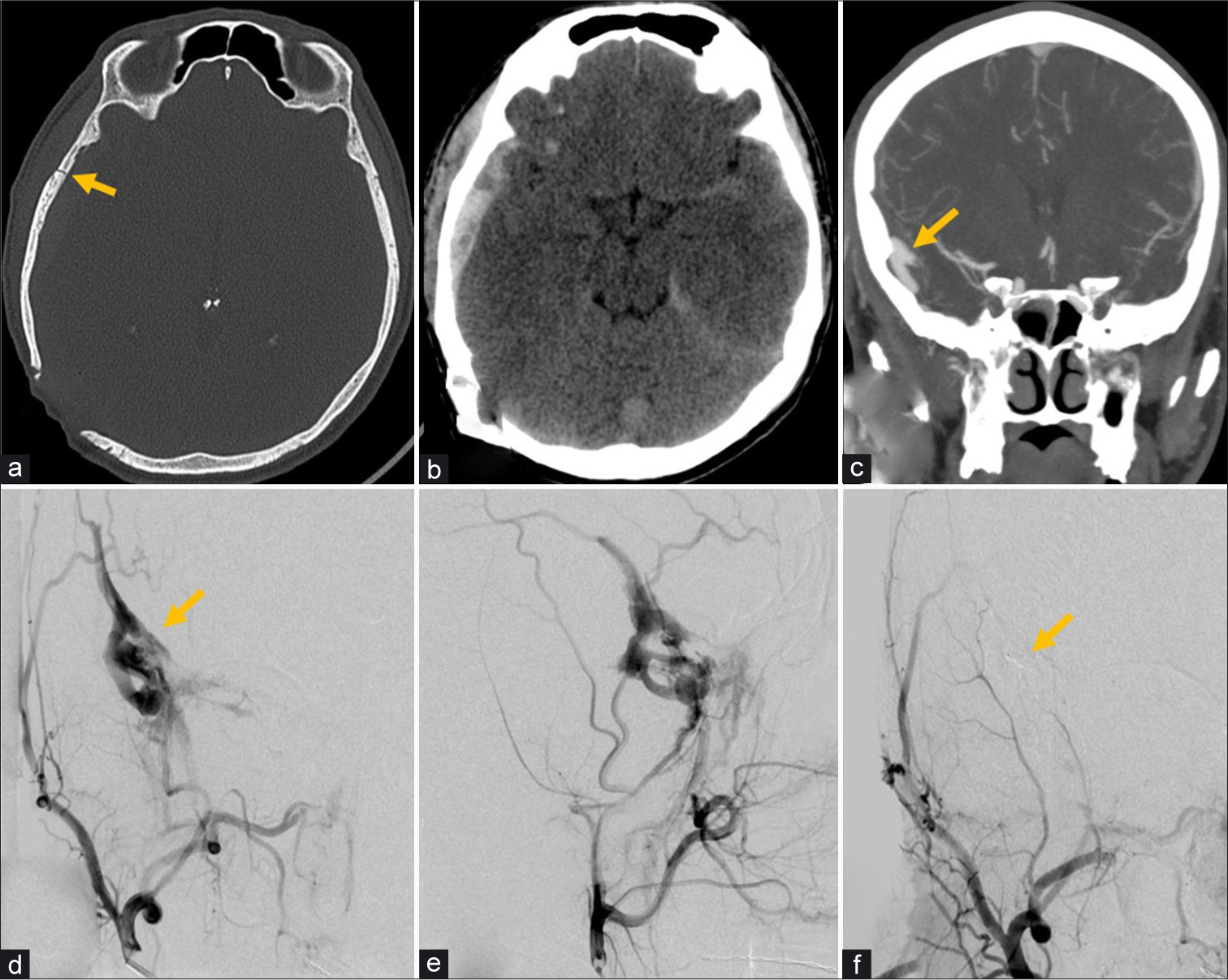
- A 39-year-old male with head injury from motor vehicle collision. (a) Non-contrast CT bone window image of the head demonstrates a non-displaced fracture (arrow) of the right temporal calvarium. (b) CT brain window image of the head demonstrates a right temporal extra-axial hematoma deep to the fracture, left cisternal subarachnoid hemorrhage, and left tentorial subdural hemorrhage. (c) CTA coronal MIP image of the head demonstrates tubular vessel-like enhancement in the right temporal extra-axial space concerning for arteriovenous fistula (arrow). (d and e) Conventional angiography selective right internal maxillary artery injection AP (d) and lateral projections (e) confirm middle meningeal arteriovenous fistula (arrow) with associated prominent early venous drainage. (f) AP view right external carotid artery injection demonstrates Onyx embolization (arrow) of the middle meningeal artery with occlusion of the fistula.
CONCLUSION
A range of traumatic vascular injuries may occur in the head and neck as a result of penetrating or blunt trauma, and familiarity with this pathology and its appearance on cross-sectional imaging is critical to help direct appropriate management in a timely manner. Endovascular neurointerventional deconstructive and reconstructive techniques offer minimally invasive treatment options for these injuries.
Declaration of patient consent
Patient’s consent not required as patient’s identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Head and neck neurovascular trauma: Clinical and angiographic correlation. Interv Neuroradiol. 2015;21:108-13.
- [CrossRef] [PubMed] [Google Scholar]
- Penetrating and blunt trauma to the neck: Clinical presentation, assessment and emergency management. B-ENT. 2016;26:69-85.
- [Google Scholar]
- Clinical and radiographic outcomes following traumatic grade 3 and 4 carotid artery injuries: A 10 year-retrospective analysis from a level 1 trauma center. The Parkland carotid and vertebral artery injury survey. J Neurosurg. 2015;122:610-15.
- [CrossRef] [PubMed] [Google Scholar]
- Natural history and management of blunt traumatic pseudoaneurysms of the internal carotid artery: The Harborview algorithm based off a 10-year experience. Ann Surg. 2016;263:821-6.
- [CrossRef] [PubMed] [Google Scholar]
- Cerebrovascular dissections: A review. Part II: Blunt cerebrovascular injury. Neurosurgery. 2011;68:517-30.
- [CrossRef] [PubMed] [Google Scholar]
- Western trauma association critical decisions in trauma: Screening for and treatment of blunt cerebrovascular injuries. J Trauma. 2009;67:1150-53.
- [CrossRef] [PubMed] [Google Scholar]
- Blunt carotid arterial injuries: Implications of a new grading scale. J Trauma. 1999;47:845-53.
- [CrossRef] [PubMed] [Google Scholar]
- Western trauma association critical decisions in trauma: Penetrating neck trauma. J Trauma Acute Care Surg. 2013;75:936-40.
- [CrossRef] [PubMed] [Google Scholar]
- Endovascular repair of traumatic cervical internal carotid artery injuries: A safe and effective treatment option. AJNR Am J Neuroradiol. 2013;34:1219-26.
- [CrossRef] [PubMed] [Google Scholar]
- Pipeline embolization device for treatment of high cervical and skull base carotid artery dissections: Clinical case series. J Neurointerv Surg. 2016;8:722-8.
- [CrossRef] [PubMed] [Google Scholar]
- The endovascular management of penetrating carotid artery injuries: Long-term follow-up. Eur J Vasc Endovasc Surg. 2009;38:267-72.
- [CrossRef] [PubMed] [Google Scholar]
- Randomized clinical trial of open-cell vs closed-cell stents for carotid stenting and effects of stent design on cerebral embolization. J Vasc Surg. 2011;54:1310-6.
- [CrossRef] [PubMed] [Google Scholar]
- Emergency endovascular management of penetrating gunshot injuries to the arteries in the face and neck: A case series and review of the literature. J Neurointerv Surg. 2014;6:42-6.
- [CrossRef] [PubMed] [Google Scholar]
- ACR appropriateness criteria head trauma. J Am Coll Radiol. 2016;13:668-79.
- [CrossRef] [PubMed] [Google Scholar]
- Current concepts in penetrating and blast injury to the central nervous system. World J Surg. 2015;39:1352-62.
- [CrossRef] [PubMed] [Google Scholar]
- Penetrating traumatic brain injury: A review of the current evaluation and management concepts. J Neurol Neurophysiol. 2015;6:1-7.
- [CrossRef] [Google Scholar]
- Traumatic pseudoaneurysms of the head and neck: Early endovascular intervention. J Vasc Surg. 2007;46:1227-33.
- [CrossRef] [PubMed] [Google Scholar]
- Use of pipelineTM embolization device for the treatment of traumatic intracranial pseudoaneurysms: Case series and review of cases from literature. Clin Neurol Neurosurg. 2018;169:154-60.
- [CrossRef] [PubMed] [Google Scholar]






