Translate this page into:
Useful Parameters in Dynamic Contrast-enhanced Ultrasonography for Identifying Early Response to Chemotherapy in a Rat Liver Tumor Model

*Corresponding author: Ryosuke Taiji, Department of Radiology, Nara Medical University, 840 Shijyocho, Kashihara - 634-8522, Nara, Japan. rtaiji@naramed-u.ac.jp
-
Received: ,
Accepted: ,
How to cite this article: Taiji R, Nishiofuku H, Tanaka T, Minamiguchi K, Fukuoka Y, Saito N, et al. Useful parameters in dynamic contrast-enhanced ultrasonography for identifying early response to chemotherapy in a rat liver tumor model. J Clin Imaging Sci 2021;11:15.
Abstract
Objectives:
The objective of the study is to determine a parameter on the time-intensity curve (TIC) of dynamic contrast-enhanced ultrasonography (DCE-US) that best correlates with tumor growth and to evaluate whether the parameter could correlate with the early response to irinotecan in a rat liver tumor model.
Material and Methods:
Twenty rats with tumors were evaluated (control: Saline, n = 6; treatment: Irinotecan, n = 14) regarding four parameters from TIC: Peak intensity (PI), k value, slope (PI × k), and time to peak (TTP). Relative changes in maximum tumor diameter between day 0 and 10, and parameters in the first 3 days were evaluated. The Mann-Whitney U-test was used to compare differences in tumor size and other parameters. Pearson’s correlation coefficients (r) between tumor size and parameters in the control group were calculated. In the treatment group, relative changes of parameters in the first 3 days were compared between responder and non-responder (<20% and ≥20% increase in size on day 10, respectively).
Results:
PI, k value, PI × k, and TTP significantly correlated with tumor growth (r = 0.513, 0.911, 0.665, and 0.741, respectively). The mean RC in k value among responders (n = 6) was significantly lower than non-responders (n = 8) (mean k value, 4.96 vs. 72.5; P = 0.003).
Conclusion:
Parameters of DCE-US could be a useful parameter for identifying early response to irinotecan.
Keywords
Ultrasonography
Colon cancer
Rats
Biomarkers
Irinotecan
Contrast media
INTRODUCTION
Colorectal cancer (CRC) is the third most common cancer worldwide and the second most common cause of cancer-related death in developed countries.[1] Metastatic CRC is treated by chemotherapy using cytotoxic and/or molecular targeted drugs, among which irinotecan is widely used.[2,3]
The standard method for evaluating treatment response on computed tomography (CT) and magnetic resonance imaging (MRI) is Response Evaluation Criteria in Solid Tumors version 1.1,[4] but as these are based solely on a reduction in tumor size, accurate early evaluation of the efficacy of chemotherapy can be difficult.[5]
Dynamic imaging modalities such as ultrasound (US), CT, and MRI have been used to evaluate the therapeutic effect and to quantify changes in perfusion parameters early after initiation of chemotherapy.[6-8] As a result, various mathematical models have been applied to analyze the chemotherapeutic response.
Compared with other modalities, functional dynamic contrast-enhanced ultrasonography (DCE-US) is a less invasive and more repeatable technique for measuring tumor perfusion; therefore, it has the potential for evaluating tumor response to chemotherapy.[9] The previous studies have reported a significant association of several parameters with tumor response to chemotherapy,[10-14] but with variable results, and no consensus exists regarding the best method for evaluating early response.
Several studies have suggested that structural changes (e.g., necrosis or relative microvessel density [MVD]) induced by chemotherapeutic agents cause alterations in intensity that is apparent on DCE-US before tumor growth can be detected.[15,16] The previous pathological studies have confirmed MVD as an independent predictor of disease-free and overall survival.[17] Indicators of tumor vascular density (relative blood volume and relative blood flow) can be determined from the time-intensity curve (TIC), which portrays the kinetics of microbubble contrast agent flow through the tumor.[18,19] Wei et al. demonstrated excellent correlations between myocardial blood flow and TIC parameters in a mathematical model of DCE-US,[20] using only time- and intensity-related parameters, which correlated with flow velocity and microvessel cross-sectional area, respectively. Therefore, TIC enables the evaluation of the correlation between parameters and structural changes in tumors simpler in the mathematical model. However, to the best of our knowledge, no investigation has applied a mathematical model for evaluating early tumor response on DCE-US. Therefore, we evaluated the correlation in an experimental animal study with clinically used contrasts.
The purpose of this study was to determine the parameter that best correlates with tumor growth on the TIC of DCE-US in a mathematical model and verify whether this parameter could assess early response to chemotherapy in a rat colorectal liver metastasis model.
MATERIAL AND METHODS
Animals
The Institutional Animal Care and Use Committee of our university approved the study (approval number: 12455). All animals were kept under routine laboratory conditions. Twenty male Fisher 344 rats (body weight 240–270 g, age 10–12 weeks; CLEA Japan, Inc., Tokyo, Japan) were randomly assigned into a control group (intravenous saline injection, 1.5 mL, n = 6) or a treatment group (intravenous irinotecan injection [Nippon Kayaku, Tokyo, Japan], 66.7 mg/kg, n = 14).
Cell line and tumor model
A rat colon cancer cell line, RCN-9 (RIKEN Cell Bank, Ibaraki, Japan), was used to make a tumor model. After habituation, an incision was made under isoflurane anesthesia and a suspension of RCN-9 cells (1 × 107 cells/0.2 mL) was injected into the left lobe of the liver. Four weeks after inoculation, US was performed to confirm the presence of a tumor.
Experimental protocol and US techniques
Figure 1 shows the experimental protocol. An intravenous catheter for the injection of contrast medium and saline/ irinotecan was placed through the tail vein, under general anesthesia. DCE-US was used to measure maximum tumor diameter before injection of saline/irinotecan (day 0) and again on days 3 and 10 after injection. Maximum tumor diameter (mm) was measured and DCE-US data were obtained using the following settings: LOGIQ7 diagnostic US system (GE Healthcare, Milwaukee, WI, USA) with a 9L probe, 2D mode as used for B-mode US of the breast, gain 36, dynamic range 51, and rate 31 frames/ second, harmonic mode as used for CEUS of the breast, gain 74, dynamic range 51, rate 18 frames/second, and low mechanical index 0.18 using harmonic imaging. A skilled operator scanned US in a constant procedure and under similar conditions. DCE-US was performed in the plane of maximum tumor diameter. Sonazoid (0.3 mL/kg; Daiichi Sankyo, Tokyo, Japan) was used as the contrast medium, administered intravenously at a flow rate of 2.4 mL/min through a multiprogramming syringe pump (FP-1000, Melquest, Toyama, Japan). DCE-US scanning was performed at 0–85 s after injection from the arterial phase to the parenchymal phase. Imaging parameters (gain, depth, and the injection speed) were the same for all subjects. Besides that, we performed DCE-US to keep the scanning plane as identical as possible under respiratory fluctuation.
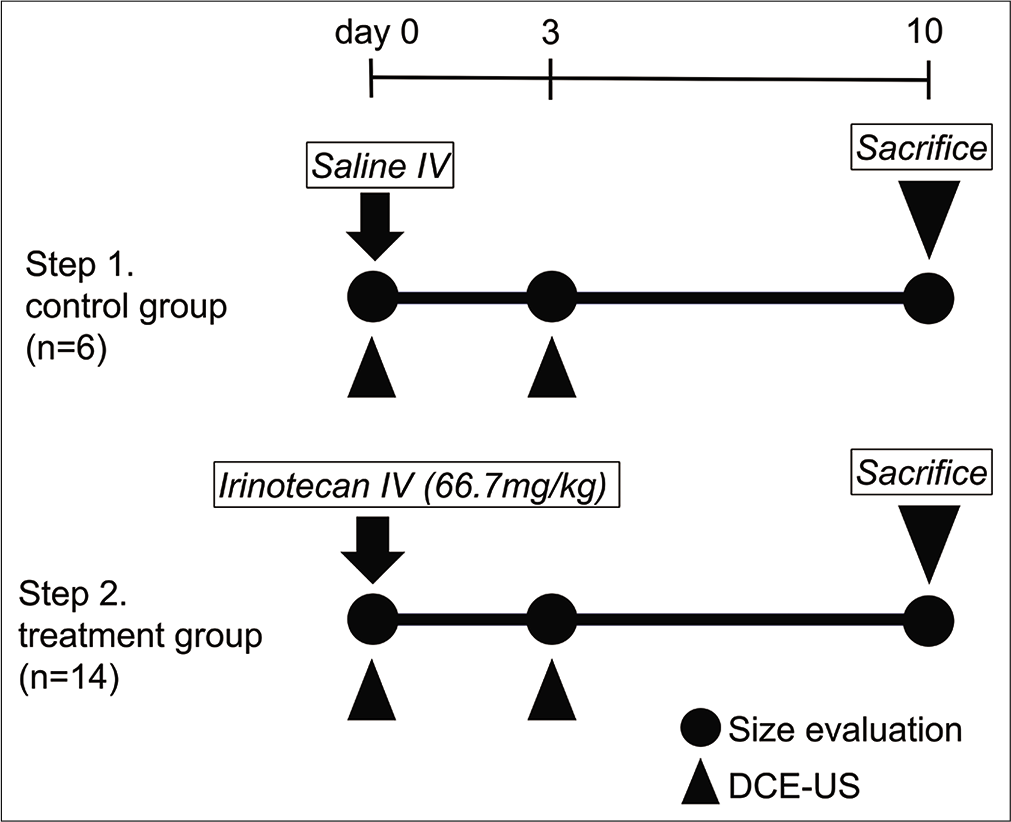
- Experimental protocol. Maximum tumor diameter was measured on days 0, 3, and 10, and DCE-US was performed on days 0 and 3. Saline was injected in the first step (n = 6) and irinotecan in the second step (n=14). DCE-US: Dynamic contrast-enhanced ultrasonography.
Measurement of tumor diameter and parameters on the TIC
Relative change in tumor size was calculated between the tumor sizes recorded on days 0 and 10. The region of interest (ROI) was set in the plane of maximum tumor diameter, and the mean signal intensity of the ROI was measured automatically for every frame. The TIC data were processed by a workstation in LOGIQ7. A fitted curve was obtained for the TICs of the following function, the differentiated function, and the slope at t = 0:
The following four parameters were measured from the fitted curve: Peak intensity (PI, dB), k value, wash-in slope (PI × k, dB/second), and time to peak (TTP, second), as shown in Figure 2. PI was defined by calculating the differential value in signal intensity from the baseline before and after contrast enhancement on DCE-US. The k value was defined as the flow velocity.[20] Relative change of each parameter was calculated on days 0 and 3.
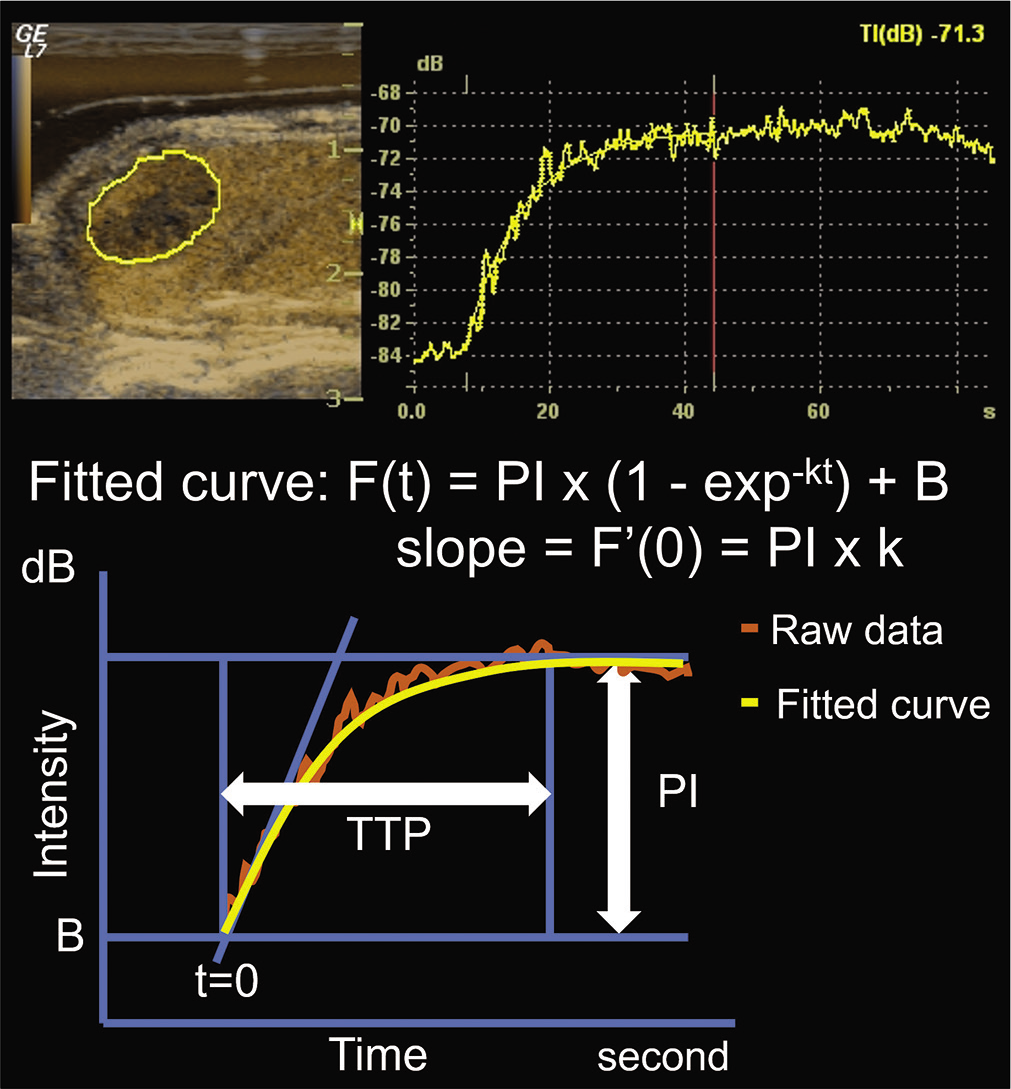
- DCE-US and the fitted curve constructed from the raw data. DCE-US was conducted for 85 s and the following four parameters were extracted from the fitted curve: PI, k value, wash-in slope (PI × k), and TTP. DCE-U: Dynamic contrast-enhanced ultrasonography, PI: Peak intensity, TTP: Time to peak.
In the first step, we evaluated the Pearson’s correlation coefficient between the relative change in tumor size over 10 days and that of each parameter in the control group for 3 days, to identify the most correlative parameter with tumor progression. In the second step, we classified each tumor as either a responder (defined as an increase in tumor size of <20%) or a non-responder (defined as an increase in tumor size of ≥20%) on day 10 compared with the size before treatment. We then compared the responders and the nonresponders in the treatment group in terms of relative change in the most correlated parameter as determined in the first step.
Pathological examination
The animals were euthanized by injection of a lethal dose of pentobarbital immediately after the US examination on day 10, and the livers were extracted. For histological examination, tumor was obtained from the center of the section of maximum tumor diameter. The tumor was cut into 3 mm thick slices and embedded in paraffin, and 2 μm thick slices were then cut, deparaffinized, and stained with hematoxylin and eosin.
The slides were scanned by a microscope (BZ-X710; Keyence, Osaka, Japan) with the following settings: ×40 resolution using a ×4 objective lens and exposure of 8.33 ms. The viable components were delineated by basophilic, purple-stained nuclei. The necrotic components showed predominant eosinophilic, pink-stained cellular material, and/or tissue absence. Tumor necrosis ratio was defined as the ratio of the area of pink stained and/or tissue absence to the whole tumor, as calculated by the BZ-II Analyzer software Hybrid Cell Count tool (Keyence, Osaka, Japan) as previously in the literature.[21]
Statistical analysis
SPSS version 25 software (SPSS Inc., Chicago, IL, USA) was used for all statistical analyses. The Mann-Whitney U-test was used to compare differences in tumor size and other parameters between the responder and non-responder groups. Pearson’s correlation coefficient with P value was calculated. P < 0.05 was considered statistically significant.
RESULTS
The mean maximum tumor diameter on day 0 was 12.3 mm (95% confidence interval [CI]: 10.4–14.2 mm) in the control group and 14.2 mm (95% CI: 12.6–15.9 mm) in the treatment group. There was no significant difference in mean maximum tumor diameter before treatment between the responder (14.6 mm, 95% CI: 12.6–16.9 mm) and non-responder (13.9 mm, 95% CI: 11.2–16.7 mm) groups (P = 0.702).
DCE-US parameters indicating tumor progression
In the first step, all six tumors in the control group increased in size between days 0 and 10. Table 1 lists tumor sizes, DCE-US parameters, and the correlation coefficients for the control group. The mean maximum tumor diameter was 12.3, 12.8, and 19.2 mm on days 0, 3, and 10, respectively. The relative change in tumor size was +4.25% on day 3 and +57.1% on day 10. There was no significant difference in size between days 0 and 3 (P = 0.66). Tumor size on day 10 was significantly larger than that on day 0 (P = 0.0001). The mean relative change in k value and in TTP between days 0 and 3 was 96.3% (95% CI: 44.8–147.9) and −37.4% (95%CI: −50.9–−23.8), respectively. The relative change in PI and in PI × k between days 0 and 3 was 4.19% (95%CI: −10.6–19.0) and 140% (95% CI: −0.08–280), respectively. The correlation coefficients were 0.911 for k value, −0.741 for TTP, 0.513 for PI, and 0.665 for PI × k [Table 2]. The k value had a strong positive correlation and TTP had a strong negative correlation with tumor progression.
| Mean value | Relative change(%) | ||||
|---|---|---|---|---|---|
| Day 0 | Day 3 | Day 10 | Day 0–3 | Day 0–10 | |
| Tumor size(mm) | 12.3±0.79 | 12.8±0.73 | 19.2±0.78 | 4.25±0.71 | 57.2±3.50 |
| TTP(second) | 34.6±1.30 | 21.8±2.34 | −37.4±5.77 | ||
| k value | 0.13±0.021 | 0.24±0.038 | 96.3±22.0 | ||
| PI(dB) | 19.7±1.05 | 20.3±0.81 | 4.19±6.32 | ||
| Slope(dB/second) | 2.52±0.50 | 4.92±0.89 | 140±59.9 | ||
Data are means±standard deviations. DCE-US: Dynamic contrast-enhanced ultrasonography, TTP: Time to peak, PI: Peak intensity
| Correlation coefficient(r) | P | |
|---|---|---|
| TTP | −0.741 | 0.033 |
| k value | 0.911 | 0.010 |
| PI | 0.513 | 0.875 |
| Slope | 0.665 | 0.279 |
DCE-US: Dynamic contrast-enhanced ultrasonography, TTP: Time to peak, PI: Peak intensity
Assessment of tumor response
In the second step, there were six responders and eight nonresponders in the treatment group on day 10 [Figure 3]. Table 3 lists the mean tumor sizes and the mean relative changes for these two groups. Table 4 and Figure 4 show the mean parameters and the mean relative changes in the two groups. Mean growth ratios on days 3 and 10 were +2.81% and +5.62% in the responder group and +5.47% and +36.8% in the non-responder group, respectively. There was a significant difference between them in relative change of size on day 10 (P = 0.0004). On the other hand, there was no significant difference between the two groups in terms of relative change of size on day 3 (P = 0.602).
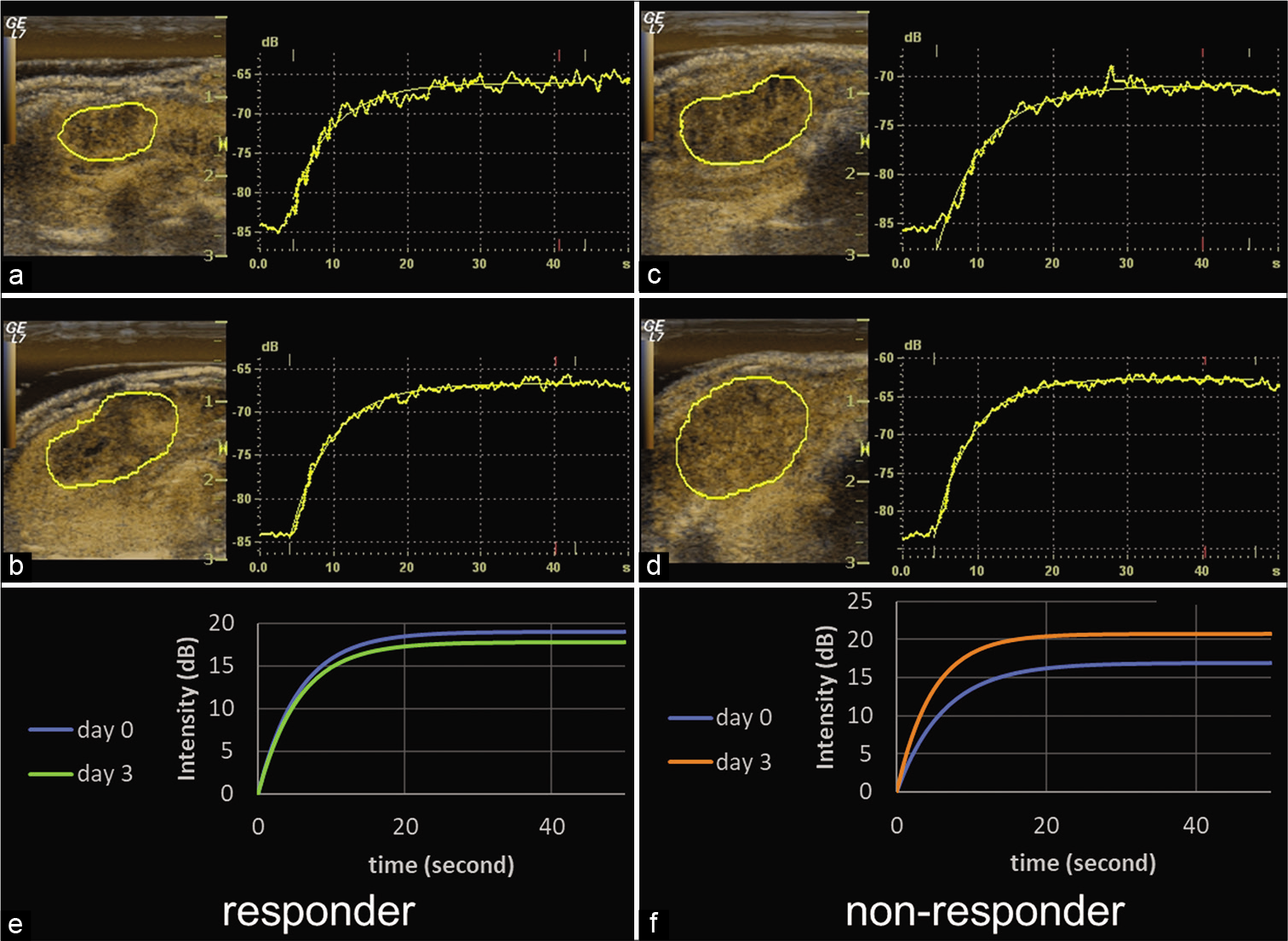
- Representative responder and non-responder fitted curves. Time-intensity curves on days 0 and 3 and fitted curves after modelization at baseline for a responder (a, b, and e, respectively) and a non-responder (c, d, and f, respectively). In the responder, TTP shortened 1.2 s and PI decreased 1.2 dB. In the non-responder, TTP shortened 5.2 s and PI increased 3.9 dB. The relative change of TTP in the non-responder was shorter than in the responder. TTP: Time to peak, PI: Peak intensity.
| Mean tumor size(mm) | Relative change(%) | ||||
|---|---|---|---|---|---|
| Day 0 | Day 3 | Day 10 | Day 0–3 | Day 0–10 | |
| Total(n=14) | 14.2±0.80 | 14.8±0.83 | 17.4±1.09 | 4.33±0.71 | 23.4±5.25 |
| Responder(n=6) | 14.6±0.99 | 15.0±0.93 | 15.4±1.10 | 2.81±1.32 | 5.62±3.70 |
| Non-responder(n=8) | 13.9±1.25 | 14.7±1.33 | 18.9±1.56 | 5.47±0.52 | 36.8±4.80 |
Data are means±standard deviations
| Responder(n=6) | Non-responder(n=8) | |||
|---|---|---|---|---|
| Day 0 | Day 3 | Day 0 | Day 3 | |
| TTP(second) | 28.3±2.81 | 28.9±8.60 | 32.67±1.80 | 23.3±2.75 |
| k value | 0.157±0.017 | 0.160±0.015 | 0.133±0.013 | 0.226±0.022 |
| PI(dB) | 12.7±2.89 | 16.3±2.56 | 11.7±1.43 | 16.4±1.69 |
| Slope(dB/second) | 2.20±0.64 | 2.79±0.75 | 1.63±0.33 | 3.69±0.52 |
Data are means±standard deviations. TTC: Time-intensity curve, TTP: Time to peak, PI: Peak intensity
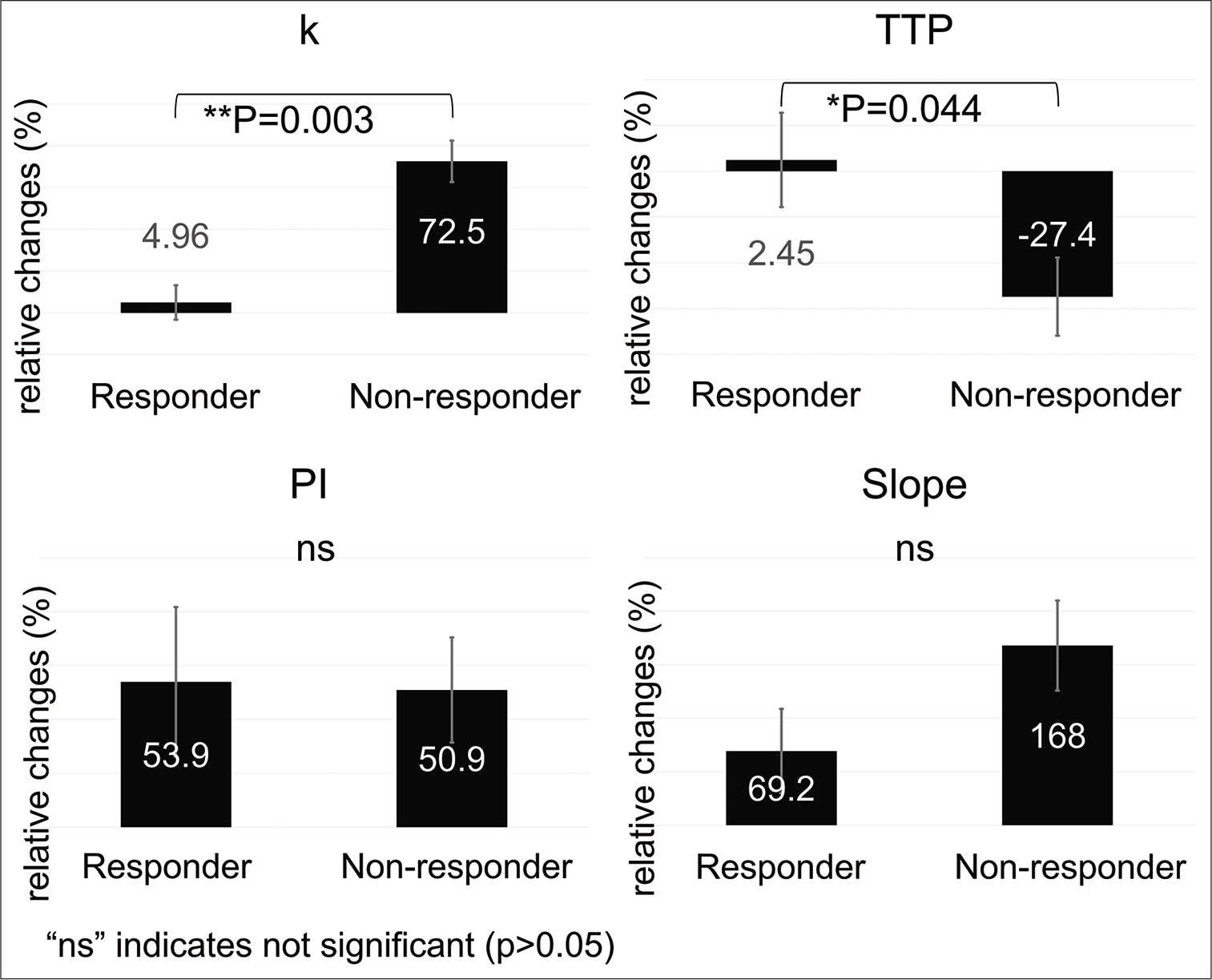
- Relative change in the four DCE-US parameters between days 0 and 3 (second step). The k value was significantly lower in the responder group (4.96%) than in the non-responder group (72.5%, P=0.003). Significant difference was also found for TTP between the responders (2.45%) and non-responders (−27.4%) groups (P = 0.044). DCE-US: Dynamic contrast-enhanced ultrasonography, TTP: Time to peak, PI: Peak intensity. The error bars represent the standard deviation of each data. *: P<0.05, **: P<0.01.
In contrast, the mean relative change in k value in the responder group on day 3 was significantly lower than that in the non-responder group (4.96 vs. 72.5, P = 0.003, Figure 4). In addition, the mean relative change in TTP on day 3 was significantly longer in the responder group than in the non-responder group (2.45 vs. −27.4, P = 0.044). There was no significant difference in PI or PI × k (53.9 vs. 50.8, P = 0.92; 69.2 vs. 168, P = 0.12, respectively). In summary, there were significant differences in k value and TTP between the responder and non-responder groups on day 3 even though there was no significant difference in size.
The animals showed gradually reducing body weight at each evaluation day. Reduction in body weight did not show significant differences between the control and treatment groups (mean 7.7% and 6.3%, P = 0.40, respectively).
Pathological examination
The mean tumor necrosis ratio was 5.3% in the control group and 17.7% in the treatment group and was significantly higher in the treatment group compared with the control group (P = 0.004). In contrast, the mean tumor necrosis ratio was 23.2% and 12.4% in the responder and non-responder groups, respectively, and was significantly higher in the responder group than in the non-responder group (P = 0.013).
DISCUSSION
Our study demonstrated that k value and TTP correlated strongly with tumor progression and could assess early tumor response to chemotherapy in a rat colorectal tumor model. The results suggest that time-related parameters (k value and TTP) are more suitable than the intensity-related parameter (PI) for assessing early tumor response on day 3 after treatment.
The previous studies have shown that early changes in TTP may predict tumor growth[12,13,22,23] and that TTP was elongated in a treatment group compared with a control group.[13,14] It has been suggested that TTP elongation is caused by a decrease of MVD within the tumor.[24] Wang et al. reported that chemotherapy using cytotoxic agents decreases tumor MVD.[25] The element flow velocity in a capillary is proportional to the fourth power of the radius and inversely proportional to length.[26] Changes in MVD can influence capillary flow velocity.[27]
Wei et al. reported k value as the flow velocity and TTP as inversely proportion to k value.[20] TTP (second) and PI (dB) are independent of each other in the fitted curve. The flow is also proportional to the slope (PI × k), derived by the following exponential function:
Likewise, the present results demonstrated that k value and TTP showed opposite correlations with tumor progression.
Due to baseline fluctuation, analysis error occasionally occurs in determining the peak of a fitted logistic curve (as used in our study); consequently, the TTP obtained from a fitted curve can be a subjective parameter. In addition, in the DCE-US technique, the flow rate of the contrast agent cannot be controlled if the bolus injection is performed manually. To improve objectivity, we used an automatic injector, which greatly improved the accuracy of the raw data and the fitted curve. Therefore, we consider that k value is the most accurate of the four parameters studied.
Some studies have reported reduced PI in treatment groups.[12,13] However, others have shown that PI was not a significantly effective parameter for predicting tumor growth.[10,28] In the present study, there was no significant difference in PI between the responder and non-responder groups. Since slope is proportional to PI, there was also no significant difference in slope.
The lack of significant correlation between PI and tumor growth in the present study could be caused by a change in the depth of ROIs due to tumor growth and by saturation of signal intensity due to the high concentration of Sonazoid. In our study, the factors affecting PI were the movement of the rat liver lobes in the abdominal cavity and inconsistent ROI depth across the evaluation times.
The pathophysiologic mechanism for changes in tumor perfusion following chemotherapy is probably associated with the requirements associated with microvessel changes.[25] In our study, the necrotic rate was significantly higher in the responder group than in the non-responder group. It is known that the response to chemotherapy causes cytotoxic tumor cell death resulting in reduced concentrations of tissue endothelial growth factor and therefore apoptosis of immature endothelial cells, with secondary vascular shutdown.[29] Most cytotoxic agents impair the endothelial cells as well as the tumor cells.[30] This mechanism acts to decrease tumor perfusion, thus leading to changes in the DCE-US parameters.
Assessment of MVD to predict tumor response has been reported in several studies, using perfusion CT,[22] dual-energy CT,[31] and DCE-MRI.[32,33] A previous study has shown a correlation of DCE-US parameters with those of DCE-MRI and with immunohistochemistry.[32] Therefore, DCE-US is a worthwhile modality for evaluating changes in tumor perfusion. Further comparative studies of DCE-US and these other modalities are needed to confirm the relative advantages of each technique.
There are some limitations to our study. First, as a potential limitation of the DCE-US technique in animal experimentation, there were fluctuations in intensity due to respiratory variation. Since those factors can introduce variability and fail to obtain the generalizability of results, operators are required to be adequately trained so that subsequent scans could be performed consistently in DCEUS qualification.[34] When applying our method in a clinical examination, the evaluation of CEUS is generally affected by the patient’s body shape and background liver and tumor location. Second, in our study, only two-dimensional US images were obtained in the maximum plane; therefore, the tumor may not have been evaluated as a whole. Third, only single-tumor cases were evaluated in this study, but it could be difficult to assess multiple or fused lesions with US in clinical examination. Finally, pathological MVD in each group was not evaluated on day 3 because it is impossible to confirm both the changes in MVD and tumor size in the same individual. However, it has been demonstrated that cytotoxic chemotherapy induces changes in MVD at an early point.[25] Further prospective and clinical investigations are mandatory to confirm the validity of k value in DCEUS for the early assessment of tumor response to systemic chemotherapy.
CONCLUSION
DCE-US could be a helpful method for evaluating the efficacy of the use of cytotoxic agents for the treatment of liver tumors. Among perfusion parameters, k value obtained from DCE-US appears to be particularly effective as a parameter for detecting early tumor response to chemotherapy in a rat colorectal liver metastasis model.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Financial support and sponsorship
This study was supported by Grant-in-Aid for Young Scientists (B) (Project/Area Number: 17K16477) from Japan Society for the Promotion of Science.
Conflicts of interest
There are no conflicts of interest.
References
- Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424.
- [CrossRef] [PubMed] [Google Scholar]
- NCCN guidelines insights: Colon cancer, Version 2.2018. J Natl Compr Cancer Netw. 2018;16:359-69.
- [CrossRef] [PubMed] [Google Scholar]
- ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016;27:1386-422.
- [CrossRef] [PubMed] [Google Scholar]
- New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228-47.
- [CrossRef] [PubMed] [Google Scholar]
- Pathological evidence of necrosis in recurrent renal mass following treatment with sunitinib. Int J Urol. 2007;14:1095-7. discussion 1097
- [CrossRef] [PubMed] [Google Scholar]
- Dynamic contrast-enhanced ultrasound for quantification of tissue perfusion. J Ultrasound Med. 2015;34:179-96.
- [CrossRef] [PubMed] [Google Scholar]
- Dynamic contrast-enhanced perfusion area detector CT for non-small cell lung cancer patients: Influence of mathematical models on early prediction capabilities for treatment response and recurrence after chemoradiotherapy. Eur J Radiol. 2016;85:176-86.
- [CrossRef] [PubMed] [Google Scholar]
- Equivalent cross-relaxation rate imaging and diffusion weighted imaging for early prediction of response to bevacizumab-containing treatment in colorectal liver metastases-preliminary study. Clin Imaging. 2017;41:1-6.
- [CrossRef] [PubMed] [Google Scholar]
- Contrast-enhanced ultrasound of the liver: Optimizing technique and clinical applications. AJR Am J Roentgenol. 2018;210:320-32.
- [CrossRef] [PubMed] [Google Scholar]
- Advanced hepatocellular carcinoma: Early evaluation of response to bevacizumab therapy at dynamic contrast-enhanced US with quantification-preliminary results. Radiology. 2011;258:291-300.
- [CrossRef] [PubMed] [Google Scholar]
- Validation of dynamic contrast-enhanced ultrasound in predicting outcomes of antiangiogenic therapy for solid tumors: The French multicenter support for innovative and expensive techniques study. Invest Radiol. 2014;49:794-800.
- [CrossRef] [PubMed] [Google Scholar]
- Early prediction of response to sorafenib in patients with advanced hepatocellular carcinoma: The role of dynamic contrast enhanced ultrasound. J Hepatol. 2013;59:1014-21.
- [CrossRef] [PubMed] [Google Scholar]
- Early quantitative evaluation of a tumor vasculature disruptive agent AVE8062 using dynamic contrast-enhanced ultrasonography. Invest Radiol. 2008;43:100-11.
- [CrossRef] [PubMed] [Google Scholar]
- Early response to anti-tumoral treatment in hepatocellular carcinoma-can quantitative contrast-enhanced ultrasound predict outcome? Ultraschall Med. 2013;34:38-46.
- [CrossRef] [PubMed] [Google Scholar]
- Utility of contrast-enhanced ultrasonography with perflubutane for determining histologic grade in hepatocellular carcinoma. Ultrasound Med Biol. 2015;41:3070-8.
- [CrossRef] [PubMed] [Google Scholar]
- To predict progression-free survival and overall survival in metastatic renal cancer treated with sorafenib: Pilot study using dynamic contrast-enhanced Doppler ultrasound. Eur J Cancer. 2006;42:2472-9.
- [CrossRef] [PubMed] [Google Scholar]
- Microvessel quantitation and prognosis in invasive breast carcinoma. Hum Pathol. 1992;23:755-61.
- [CrossRef] [Google Scholar]
- Dynamic contrast-enhanced ultrasound parametric maps to evaluate intratumoral vascularization. Invest Radiol. 2015;50:212-7.
- [CrossRef] [PubMed] [Google Scholar]
- Quantification of myocardial blood flow with ultrasound-induced destruction of microbubbles administered as a constant venous infusion. Circulation. 1998;97:473-83.
- [CrossRef] [PubMed] [Google Scholar]
- Use of a glass membrane pumping emulsification device improves systemic and tumor pharmacokinetics in rabbit vX2 liver tumor in transarterial chemoembolization. J Vasc Interv Radiol. 2020;31:347-51.
- [CrossRef] [PubMed] [Google Scholar]
- Response evaluation of chemotherapy in metastatic colorectal cancer by contrast enhanced ultrasound. World J Gastroenterol. 2012;18:541-5.
- [CrossRef] [PubMed] [Google Scholar]
- Advanced hepatocellular carcinoma: Early evaluation of response to targeted therapy and prognostic value of perfusion CT and dynamic contrast enhanced-ultrasound, Preliminary results. Eur J Radiol. 2013;82:e205-11.
- [CrossRef] [PubMed] [Google Scholar]
- BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64:7099-109.
- [CrossRef] [PubMed] [Google Scholar]
- Assessment of early tumor response to cytotoxic chemotherapy with dynamic contrast-enhanced ultrasound in human breast cancer xenografts. PLoS One. 2013;8:e58274.
- [CrossRef] [PubMed] [Google Scholar]
- Mathematical modelling of flow through vascular networks: Implications for tumour-induced angiogenesis and chemotherapy strategies. Bull Math Biol. 2002;64:673-702.
- [CrossRef] [PubMed] [Google Scholar]
- Mathematical modeling of tumor-induced angiogenesis. Annu Rev Biomed Eng. 2006;8:233-57.
- [CrossRef] [PubMed] [Google Scholar]
- Arterial-phase contrast-enhanced ultrasonography for evaluating anti-angiogenesis treatment: A pilot study. World J Gastroenterol. 2011;17:1045-50.
- [CrossRef] [PubMed] [Google Scholar]
- Blood vessel maturation: Vascular development comes of age. J Clin Invest. 1999;103:157-8.
- [CrossRef] [PubMed] [Google Scholar]
- A comparative analysis of low-dose metronomic cyclophosphamide reveals absent or low-grade toxicity on tissues highly sensitive to the toxic effects of maximum tolerated dose regimens. Cancer Res. 2004;64:3994-4000.
- [CrossRef] [PubMed] [Google Scholar]
- Spectral computed tomography in advanced gastric cancer: Can iodine concentration non-invasively assess angiogenesis? World J Gastroenterol. 2017;23:1666-75.
- [CrossRef] [PubMed] [Google Scholar]
- Correlation of perfusion MRI and 18F-FDG PET imaging biomarkers for monitoring regorafenib therapy in experimental colon carcinomas with immunohistochemical validation. PLoS One. 2015;10:e0115543.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of dynamic contrast-enhanced magnetic resonance imaging and contrast-enhanced ultrasound for evaluation of the effects of sorafenib in a rat model of hepatocellular carcinoma. Magn Reson Imaging. 2019;57:156-64.
- [CrossRef] [PubMed] [Google Scholar]
- Quantitative contrast-enhanced ultrasound imaging: A review of sources of variability. Interface Focus. 2011;1:520-39.
- [CrossRef] [PubMed] [Google Scholar]






