Translate this page into:
Uncommon Causes of Acute Abdominal Pain – A Pictorial Essay
Address for correspondence: Dr. Mahesh Hariharan, No. 1641, 17th A Main, 1st Stage, 5th Block, HBR Layout, Bengaluru - 560 043, Karnataka, India. E-mail: dr.maheshmax@gmail.com
-
Received: ,
Accepted: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Acute abdomen is one of the most common clinical conditions requiring a radiological investigation. Ultrasound is the primary modality of choice which can diagnose some of the common causes of acute abdomen. However, sometimes the underlying cause for the pain is far more complicated than expected mandating a high degree of suspicion to suggest further investigation with contrast enhanced computed tomography or magnetic resonance imaging. Here, we have compiled a comprehensive series of selected cases to highlight the conditions which can be easily overlooked unless carefully sought for. This article also emphasizes the importance of multimodality approach to arrive at the final diagnosis with an increased overall diagnostic accuracy which in turn improves patient management and prognosis.
Keywords
Acute abdomen
contrast enhanced computed tomography scan
magnetic resonance imaging
plain radiographs
ultrasound

INTRODUCTION
Acute abdominal pain is a very common clinical scenario that requires a radiological examination. Ultrasound (US) has been the most preferred first line investigation, which indeed helps to diagnose some of the common causes. However, sometimes the underlying disease process causing the pain is far more complicated than expected. When US/plain radiographs are nondiagnostic or equivocal high degree of suspicion is required to suspect an uncommon cause and to suggest further investigation with contrast enhanced computed tomography (CECT). Magnetic resonance imaging (MRI) often helps as a problem-solving tool.
In this article, we present a list of such relatively uncommon conditions which require optimal correlation with available clinical history, knowledge to seek an alternate cause, and careful scrutiny of the cross-sectional images which in turn reflects on the diagnostic accuracy.
Epiploic appendagitis
A 50-year-old female patient presented with acute onset of abdominal pain confined to the right lumbar region. High-frequency US done showed a well-defined rounded, noncompressible, hyperechoic lesion surrounded by a hypoechoic margin [Figure 1a]. Multidetector computed tomography (MDCT) showed a fat-density ovoid lesion adjacent to the colon with thin hyperdense rim with surrounding inflammatory fat stranding and adjacent peritoneal thickening. Central hyperdense dot representing a thrombosed vessel was noted [Figure 1b and c].
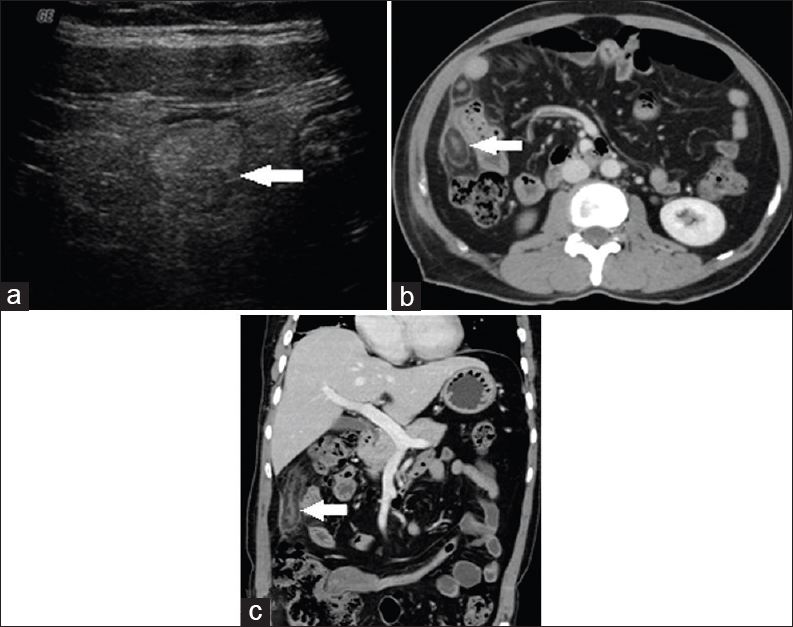
- Case 1: 50-year-old female patient who presented with acute onset of abdominal pain confined to the right lumbar region was diagnosed with epiploic appendagitis. (a) High-frequency ultrasound image shows a well-defined rounded, noncompressible, hyperechoic lesion surrounded by a hypoechoic margin (white arrow). (b) Axial image shows a fat-density ovoid lesion adjacent to colon with thin hyperdense rim with surrounding inflammatory fat stranding and adjacent peritoneal thickening. Central hyper-dense dot representing a thrombosed vessel noted (white arrows). (c) Coronal reformat multidetector computed tomography image shows a fat-density ovoid lesion adjacent to colon with thin hyper-dense rim with surrounding inflammatory fat stranding and adjacent peritoneal thickening. Central hyperdense dot representing a thrombosed vessel noted (white arrow).
Epiploic appendagitis is an uncommon self-limiting ischemic-inflammatory process involving the appendix epiploicae of the colon. Epiploic appendages are small adipose protrusions from the serosal surface of the colon and are predominantly concentrated around the recto-sigmoid region followed by the ileocecal junction, ascending colon, transverse colon, and descending colon in the decreasing order of frequency. This condition usually affects patients in their second to fifth decades with a predilection for women and obese individuals, probably due to larger appendages.[1]
Clinically, patients present with acute abdominal pain. It is essentially indistinguishable clinically from acute appendicitis and diverticulitis (depending on location). The pathogenesis is thought to be due to torsion of a large and pedunculated epiploic appendage or spontaneous thrombosis of the venous outflow, resulting in ischemia and necrosis and associated secondary inflammation.[2]
On US a rounded, noncompressible, hyperechoic lesion, without internal vascularity with hypoechoic peripheral margin is observed.[3] CT appearances are that of a fat attenuation ovoid lesion adjacent to the colon, usually 1–4 cm in diameter[34] with a thin high-density rim[56] with surrounding inflammatory fat stranding and a central hyper-dense dot representing thrombosed vascular pedicle.[4]
On CT, an oval pericolonic fat attenuation nodule with hyperdense ring and surrounding inflammation with a central dot is diagnostic for epiploic appendagitis.
Omental infarction
A 40-year-old middle-aged man presented with acute onset of abdominal pain in the right upper quadrant. US was equivocal, hence, a CECT was performed. Coronal reformatted images showed a heterogeneous area of omental fat stranding with dense halo and swirling of omental vessels between the anterior abdominal wall and the colon [Figure 2a and b].
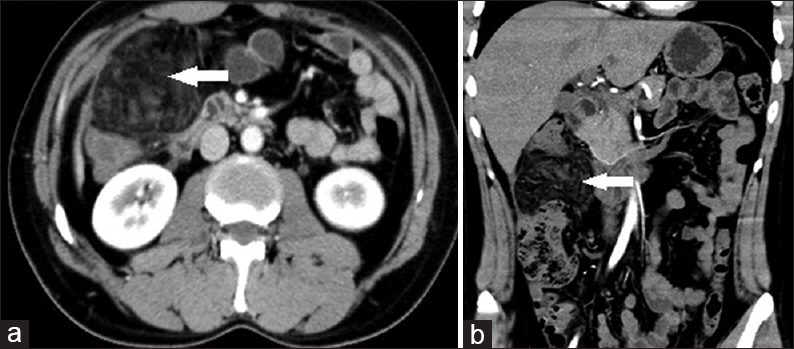
- Case 2: 40-year-old middle-aged man presented with acute onset of abdominal pain in the right upper quadrant was diagnosed with omental infarction. (a) Axial image shows a heterogeneous area of omental fat stranding with dense halo and swirling of omental vessels between anterior abdominal wall and colon (white arrows). (b) Coronal reformat multidetector computed tomography image shows a heterogeneous area of omental fat stranding with dense halo and swirling of omental vessels between anterior abdominal wall and colon (white arrows).
Omental infarction is an uncommon cause of pain in the abdomen that is usually localized to the right upper or central quadrants.[5] It results from interrupted arterial supply to the omentum, often due to torsion of right epiploic vessels (in more than 90% of cases) and the resultant vascular compromise. It is usually encountered in healthy individuals such as athletes due to low omental blood flow. The omentum may infarct without torsion, and this is referred as primary idiopathic segmental infarction.[6] Other risk factors being postabdominal surgery, blunt abdominal trauma and in rare cases implicated superior mesenteric artery (SMA) thrombosis, in which case it is localized to the site of initial insult. The diagnosis is made after excluding appendicitis or diverticulitis. It is a self-limiting condition and has a nonspecific clinical presentation and is usually managed conservatively.
On US, a focal hyperechoeic area which is nonmobile and noncompressible confined to the omental fat with tenderness on graded compression is seen. On CT, a heterogeneous focal area of fat stranding with hyper-dense peripheral halo and swirling of omental vessels are noted.[7] It is usually larger than 5 cm, which helps distinguishing it from epiploic appendagitis.[5]
On CT a heterogeneous fatty mass with hyperdense streaks located between anterior abdominal wall and colon is diagnostic of this condition.
Mesenteric panniculitis
A 45-year-old patient presented with vague upper abdominal pain that had persisted for 3 months. The patient had no other significant past medical or surgical history.
US showed no significant abnormality. CECT was suggested which showed an increased attenuation of the jejunal mesentry producing “misty mesentry” sign. Also noted were mesenteric vessels and nodes with peripheral fat halo [Figure 3a and b].
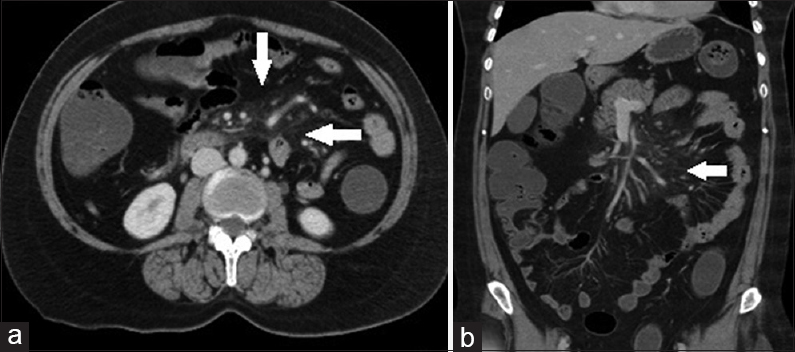
- Case 3: 45-year-old patient presented with vague upper abdominal pain that had persisted for 3 months was diagnosed with mesenteric panniculitis. (a) Axial contrast enhanced computed tomography image shows an increased attenuation of the jejunal mesentery producing “Misty mesentery” sign. Also seen are mesenteric vessels and nodes with peripheral fat halo (white arrows). (b) Coronal reformat multidetector computed tomography image shows an increased attenuation of the jejunal mesentery producing misty mesentery sign. Also, note mesenteric vessels and nodes with peripheral fat halo (white arrows).
Sclerosing mesenteritis/mesenteric panniculitis is an uncommon, complex mesenteric inflammatory disorder of unknown etiology. It has a greater predilection to elderly males in their sixth or seventh decade.[8] Most patients are asymptomatic, but may present with nonspecific abdominal pain, altered bowel habits, weight loss, and occasionally intermittent partial bowel obstruction. Abdominal tenderness and palpable mass may be seen on per abdominal examination.[9] It is often an isolated condition but may be associated with other conditions such as lymphoma, gastric carcinoma, recent abdominal surgery, systemic inflammatory condition, and other autoimmune conditions.[10]
Mostly, the central small bowel mesentry or root of mesentry is affected. Sigmoid mesocolon and omentum can also be involved sometimes.
On imaging, US demonstrate distortion of the mesenteric root with decreased echogenicity with evident mass effect. Halo of sparing around vessels may be also seen as a region of hyperechoic fat.[11] On CT, well-defined or ill-defined mass-like lesion confined to the mesentry with surrounding ground glass attenuation also known as the “misty mesentry” sign with a thin pseudocapsule is observed.[8] The traversing mesenteric vessels and soft tissue nodules have a spared fat halo also referred to as the “fat ring” sign. Punctate or coarse calcifications, as well as small subcentimeter lymph nodes, may be present within the region.
The treatment is often supportive, as the disease is typically self-limiting. In severe conditions corticosteroids and tamoxifen may be effective.
On CT fibrofatty mesenteric mass encasing mesenteric vessels with preserved fat halo around vessels with subcentimeter lymph node is diagnostic of mesenteric panniculitis.
Acute superior mesenteric vein thrombosis with extension into portal vein
A 23-year-old lady staff nurse came to the emergency department with acute onset of retching, nausea, intractable vomiting and severe epigastric pain. No other significant past history was elicited. Preliminary US examination revealed echogenic intraluminal thrombus within the superior mesenteric vein (SMV) [Figure 4a and b]. Contrast CT was performed which showed intraluminal filling defects suggestive of acute thrombosis within the SMV with extension into the portal vein and its branches. Mild bowel wall thickening and hypoenhancement were noted [Figure 4c and d].
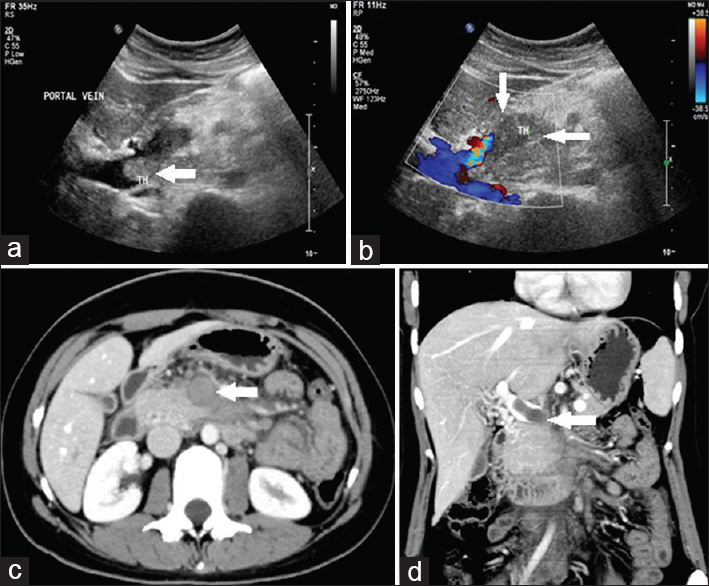
- Case 4: 23-year-old lady staff nurse came to the emergency department with acute onset of retching, nausea, intractable vomiting and the severe epigastric pain was diagnosed with acute superior mesenteric vein thrombosis with extension into the portal vein. (a) Grayscale image shows echogenic intraluminal thrombus within the superior mesenteric vein with extension into the portal vein (white arrow). (b) Color Duplex image shows echogenic intraluminal thrombus within the superior mesenteric vein with extension into the portal vein (white arrows). (c) Axial contrast enhanced computed tomography image shows intraluminal filling defects suggestive of acute thrombosis within the superior mesenteric vein with extension into the portal vein and its branches (white arrow). Mild bowel wall thickening, dilatation, and hypo-enhancement are also noted. (d) Coronal reformat multidetector computed tomography image shows intraluminal filling defects suggestive of acute thrombosis within the superior mesenteric vein with extension into the portal vein and its branches (white arrow). Mild bowel wall thickening, dilatation, and hypo-enhancement are also noted.
Acute SMV thrombosis is a relatively uncommon cause of intestinal ischemia, accounting for only 5–15% of all cases.[12] Spontaneous splanchnic vein thrombosis without any predisposing factors is termed as primary mesenteric venous thrombosis which accounts for 20–40% of cases.[13] Patients with known medical conditions or predisposing factors such as pancreatitis, hypercoagulability states, cirrhosis, or surgery are termed as cases of secondary mesenteric venous thrombosis which constitutes the majority of cases.
Patients usually present with acute colicky abdominal pain which is out of proportion to the physical findings, abdominal distension, nausea, vomiting, and constipation, with or without bloody diarrhea. Diffuse, intermittent abdominal pain may be present from several days to as long as weeks.[14] Despite complete thrombosis of the SMV, small bowel necrosis does not occur frequently probably due to persistent arterial supply and some venous drainage through collaterals.[12] Progression of ischemia eventually leads to bowel wall necrosis, perforation, sepsis, and shock. The nonspecific signs and symptoms can delay diagnosis, contributing to the poor clinical outcome. High morbidity and mortality rates create an important role for imaging in aiding early detection.
Plain radiographs demonstrate a nonspecific ileus pattern with dilated, fluid-filled loops of bowel. Thumbprinting, separation of bowel loops due to mesenteric thickening, pneumatosis intestinalis, and mesenteric or portal venous gas may occasionally be seen which indicate late-stage disease.[15] US with color Doppler is noninvasive and inexpensive and usually demonstrate intraluminal thrombus, thickened bowel wall, free intraperitoneal fluid. The US is reported to have approximately 70% sensitivity.[13]
CT is the most accurate modality with an excellent sensitivity of up to 90%.[15] The most common manifestation of bowel ischemia is bowel wall thickening. The thickened bowel wall may appear hyperdense which may be due to intramural venous engorgement and hemorrhage. Portomesenteric venous thrombosis is seen as well-defined intraluminal filling defects with central low attenuation surrounded by rim-enhancing venous walls.[16] Other ancillary findings are bowel dilatation, absent or poor enhancement of the bowel wall and less commonly intestinal pneumatosis. The appearance of mesenteric or portal venous gas suggests dissection of intramural gas into the venous system. Free intraperitoneal air from perforation of an infarcted bowel segment may also be seen.
The triad of low attenuation filling defect in the SMV, thickening of the small bowel wall and the presence of peritoneal fluid are indicators of imminent bowel infarction Intraluminal thrombus within the SMV with bowel wall thickening is diagnostic.
Gallstone ileus
A 55-year-old man presented to the emergency department with complaints of acute onset of colicky abdominal pain, vomiting, and abdominal distension. Patient had a long standing history of upper quadrant pain for more than 3 years for which he was on intermittent medication.
CECT scan was done which revealed pneumobilia with air within the gallbladder lumen as well [Figure 5a]. A fistulous tract was seen extending from the gall bladder to the duodenum – cholecystoenteric fistula. Significantly dilated small bowel loops were noted. A well-defined nonenhancing hyperdense (HU 100–140) lesion measuring 45 mm × 16 mm with surrounding hypodensity [Figure 5b coronal reformat] was noted within the distal jejunal lumen with proximal dilatation of bowel loops. Focal transitional dilatation at the site of obstruction was noted with surrounding bowel wall thickening.
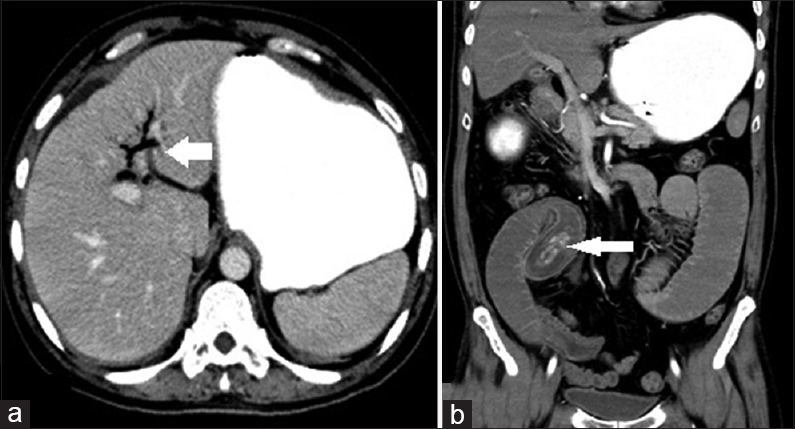
- Case 5: 55-year-old man presented to the emergency department with complaints of acute onset of colicky abdominal pain, vomiting, and abdominal distension was diagnosed with Gallstone ileus. (a) Axial contrast enhanced computed tomography image shows pneumobilia-air within the intrahepatic biliary radicles (white arrow). (b) Coronal reformatted computed tomography image shows a large calculus within the distal jejunal lumen causing proximal obstruction (white arrow).
Gallstone ileus is an uncommon cause of a mechanical small bowel obstruction. It occurs when a gallstone passes into the small bowel lumen and impacts to cause mechanical obstruction. However, in the elderly, it accounts for up to 25% of nonstrangulated bowel obstruction. Women are more frequently affected explained by the higher incidence of cholelithiasis.[17]
Patients usually present with a background of the long history of recurrent right upper quadrant pain in keeping with repeated inflammatory events.[18] The acute presentation is that of a small bowel obstruction, with colicky abdominal pain, abdominal distension, and vomiting.
Repeated attacks of cholecystitis result in adhesion of the gallbladder to the small bowel (usually duodenum) with eventual fistula formation (cholecystoenteric fistula) and passage of gallstones into the lumen of the bowel. The most common site of entry by erosion is thought to be to the duodenum. The site of obstruction is usually the terminal ileum because it is the narrowest portion of the small bowel.[17]
The radiographic features on a plain film consist of pneumobilia, small bowel obstruction, and gallstones usually in the right iliac fossa (described as Riggler's triad). Appearances of Riggler's triad are better seen on CT scan. The presence of bulging of the bowel just before the transition point is often seen. The size, the number of stones and their morphology (cylindrical or faceted) can be depicted in detail, and this plays a role in the prognosis.[1920] In addition, the site of fistulization is often visible. The presence of free fluid, free gas, portal venous gas, or mural gas is signs of more advanced disease and poorer prognosis. CT allows a correct diagnosis of gallstone ileus with greater accuracy.[21] The information obtained on CT is used to make a rapid diagnosis and helps in deciding whether surgical or conservative treatment may be most effective.[22] Surgery is definitive, with removal of the stone (enterolithotomy) and fistula repair, accompanied by a cholecystectomy.
On CT mechanical small bowel obstruction due to impacted gallstones within the gut is diagnostic of gallstone ileus.
Acute superior mesenteric artery thrombosis
A 76-year-old elderly gentleman, a chronic smoker with history of cardiovascular compromise came to the emergency department with complaints of severe abdominal pain, distension, and vomiting. Emergency US revealed gaseous distension of bowel loops and no ascites. CECT was ordered on an emergency basis which revealed intra-luminal thrombus causing complete occlusion of celiac axis and superior mesentric artery [Figure 6a]. Dilated small bowel loops with nonenhancing segments of bowel wall were noted. Intramural gas indicating pneumatosis intestinalis [Figure 6b] was also noted as a consequence of severe bowel ischemia.
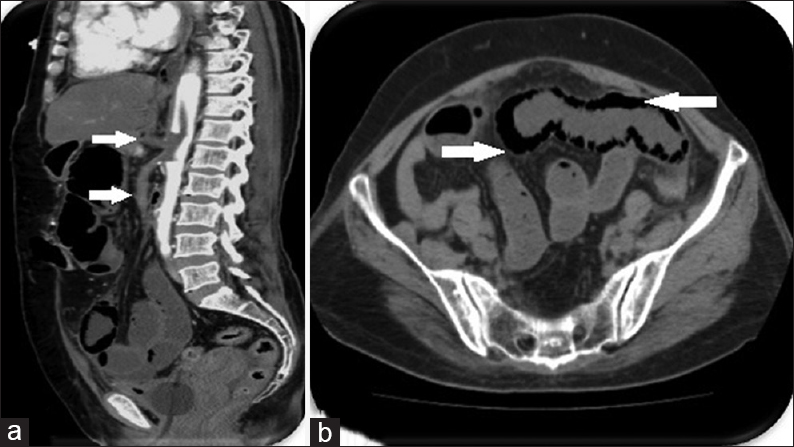
- Case 6: 76-year-old elderly gentleman, a chronic smoker with history of cardiovascular compromise came to the emergency department with complaints of severe abdominal pain, distension, and vomiting was diagnosed with acute mesenteric artery thrombosis. (a) Sagittal reformat multidetector computed tomography image shows complete occlusion of celiac axis and superior mesenteric artery by thrombus (white arrow). (b) Axial contrast enhanced computed tomography image shows nonenhancing bowel wall with intramural gas – complication of intestinal ischemia leading to pneumatosis intestinalis (white arrow).
Acute SMA occlusion can result in acute mesenteric ischemia which can be a life-threatening event as a major portion of the small bowel and right colon is supplied by SMA. It is relatively uncommon and affects elderly patients with other co-morbidities. Clinical presentation is variable and nonspecific, and the diagnosis is often delayed.
The onset of symptoms is typically acute and consists of severe abdominal pain that is disproportionate to the findings on physical examination.[23]
The causes implicated being[2324] acute embolic arterial occlusion in ~50%, acute thrombosis superimposed on preexisting atherosclerosis in ~30%, aortic dissection with the involvement of the SMA origin and slow flow. Predisposing risk factors are heart failure, atrial fibrillation, coronary heart disease, arterial hypertension, and peripheral arterial occlusion.[25]
In acute mesenteric ischemia, US is often negative due to distended bowel loops. However, color Doppler evaluation of the mesenteric circulation with an experienced US operator can identify or, at least, suspect the vascular pathology.[26] When acute occlusive mesenteric ischemia is suspected, biphasic CECT with multiplanar reconstruction is the diagnostic tool of choice.[27] CT angiography is more specific and will often demonstrate the site of embolic occlusion as a central filling defect within the SMA or may show an abrupt point where the artery is not opacified, with poor or absent collaterals. CT may demonstrate bowel dilatation and wall thickening, as well as ascites and mesenteric edema in the early stages, whereas pneumatosis, pneumoperitoneum, and intravascular gas are seen in later stages. In cases of embolic occlusion, other abdominal organs such as the kidneys may show evidence of infarction.
Intraluminal thrombus within the SMA with associated bowel dilatation, wall thickening, and hypoenhancement are diagnostic.
Right ovarian dermoid cyst with torsion
A 28-year-old recently married lady presented with acute onset of excruciating pain in the right lower quadrant. She had no associated vomiting, fever, distension, or bleeding per vagina. US examination showed a well-defined echogenic solid lesion in the right adnexa extending to the midline with no obvious internal vascularity [Figure 7a and b]. Further MRI T1-weighted axial, T2-weighted sagittal and SPectral Attenuated Inversion Recovery (T2 fat suppressed) images showed a well-defined lobulated mass with fat intensity and calcific foci arising from the right ovary suggestive of a dermoid cyst. Visualized right ovary showed multiple small follicles in the periphery. Torsion of the pedicle with displacement of the mass anteriorly was appreciated [Figure 7c–e] with a moderate amount of free fluid in the pouch of Douglas.
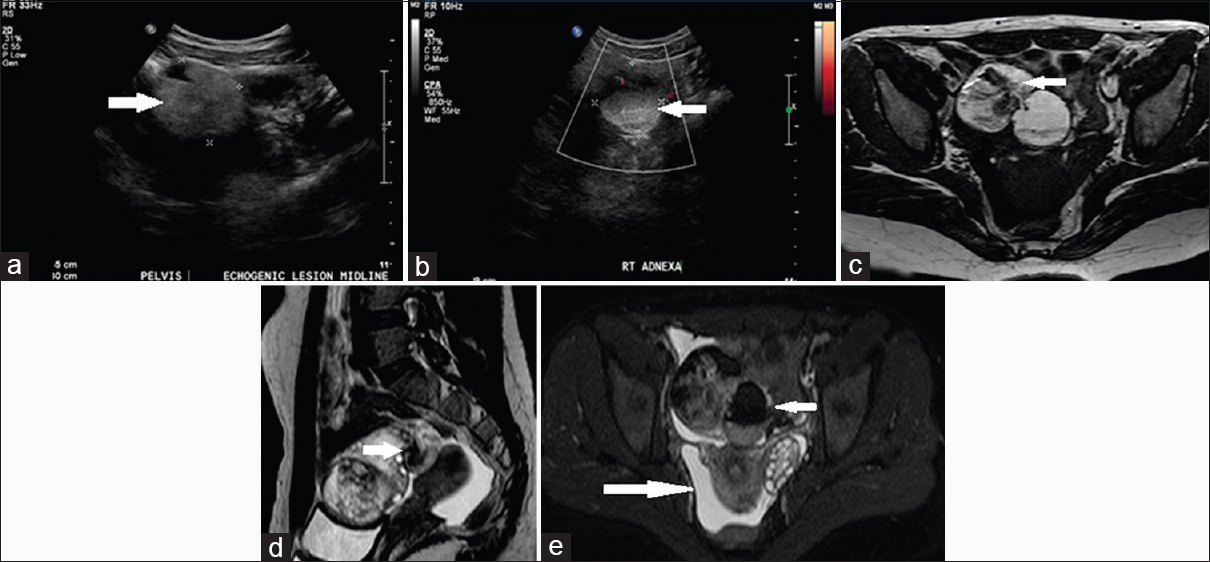
- Case 7: 28-year-old recently married lady presented with acute onset of excruciating pain in the right lower quadrant. She had no associated vomiting, fever, distension, or bleeding per vagina was diagnosed with right ovarian dermoid cyst with torsion. (a) Gray-scale ultrasound image shows a well-defined echogenic solid lesion in the right adnexa (white arrow). (b) Color Doppler ultrasound image shows no obvious internal vascularity (white arrow). (c) T1-weighted axial magnetic resonance image shows a well-defined lobulated mass with fat intensity and calcific foci arising from the right ovary (white arrow). (d) T2-weighted Sagittal magnetic resonance imaging shows a well-defined lobulated adnexal mass with fat intensity and calcific foci with multiple small follicles in periphery. Torsion of the pedicle with a displacement of the mass anteriorly is observed (white arrow) with moderate fluid in pouch of Douglas. (e) Axial SPectral Attenuated Inversion Recovery (T2 suppressed) image shows signal suppression within the right ovarian lesion (short white arrow) and fluid in pouch of Douglas (long arrow).
In torsion of the ovary, ipsilateral fallopian tube twists with the vascular pedicle, resulting in vascular compromise. Adnexal torsion is well-known but infrequently encountered clinical entity, and patients often present with abdominal pain that may mimic acute abdomen. Adnexal torsion should be considered when an ovarian mass is discovered in the appropriate clinical setting. Of the various ovarian neoplasms, benign cystic teratoma is considered to be the most common cause of adnexal torsion, occurring in 3.5–16.1% of cases.
US is usually the first examination performed in an emergency setting. Sonographic features include enlarged hypo or hyperechoic ovary with peripherally displaced follicles.
On color Doppler little or no intra-ovarian venous flow (common), absent arterial flow (less common), and absent or reversed diastolic flow may be seen. Other features include pelvic free fluid (in more than 80% of cases) and “Whirlpool” sign of twisted vascular pedicle. The underlying ovarian lesion can often be found. The uterus may be slightly deviated toward the torted ovary; Normal vascularity does not exclude intermittent torsion as normal Doppler flow can be found due to dual supply from both the ovarian and uterine arteries.[28]
Although ultrasonography and color Doppler US have been reported to be useful in detecting adnexal torsion, CT and MRI may also be useful in making the preoperative diagnosis of adnexal torsion, especially in subacute cases.[29]
Bulky ovary with peripherally placed follicles with absent color flow on Doppler images should raise the suspicion of ovarian torsion. The “Whirpool” sign (twisted vascular pedicle) is diagnostic.
Small bowel volvulus with coexisting lymphagioma being the possible cause
A 5-year-old boy presented with a history of progressive on and off pain in the abdomen for 2 days, associated mild abdominal distension and two episodes of vomiting. He had a similar episode 1 year ago, but this subsided on its own. No other relevant investigations done.
On the US, a fluid filled multiloculated pelvic mass with thin septations with associated dilated small bowel loops and increased peristalsis was seen in Figure 8a–c. On color Doppler “Whirlpool” sign was noted which strongly raised a suspicion of midgut volvulus [Figure 9a and b]. On MDCT, multiloculated cystic lesion with thin septations with characteristic “Whirlpool” sign was seen in Figure 10a–c.
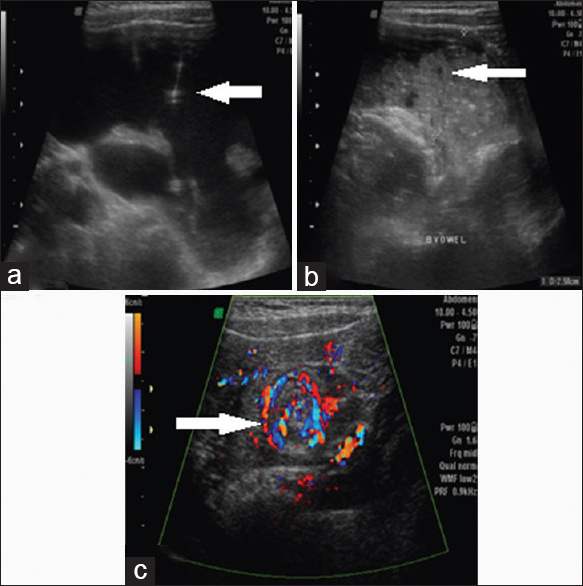
- Case 8: 5-year-old boy presented with a history of progressive on and off pain in the abdomen for 2 days, associated mild abdominal distension and two episodes of vomiting and was diagnosed with small bowel volvulus with coexisting lymphangioma being the possible cause. (a) Grayscale axial ultrasound image shows a fluid-filled multiloculated pelvic mass with thin septations (white arrow). (b) Gray-scale axial ultrasound image shows dilated small bowel loops (white arrow). (c) Color Duplex ultrasound image shows typical whirlpool sign noted strongly raising a suspicion of midgut volvulus (white arrow).
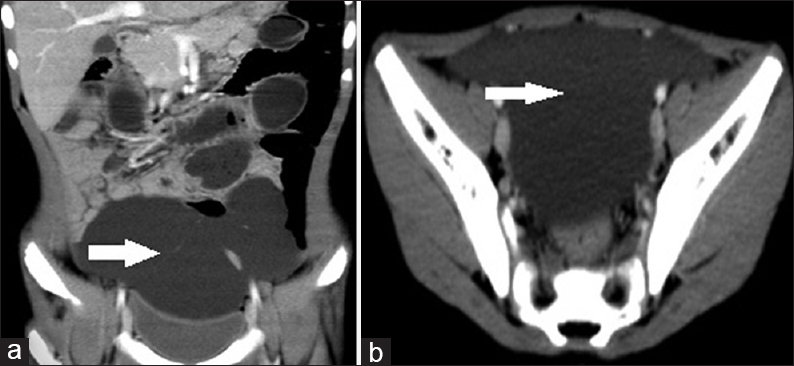
- Case 9: 5-year-old boy presented with a history of progressive on and off pain in the abdomen for 2 days, associated mild abdominal distension and two episodes of vomiting and was diagnosed with small bowel volvulus with coexisting lymphangioma being the possible cause. (a) Coronal reformat multidetector computed tomography image shows showed fluid attenuation multiloculated lesion with thin septations (white arrow). (b) Axial contrast enhanced computed tomography image shows fluid attenuation multiloculated lesion with thin septations (white arrow).
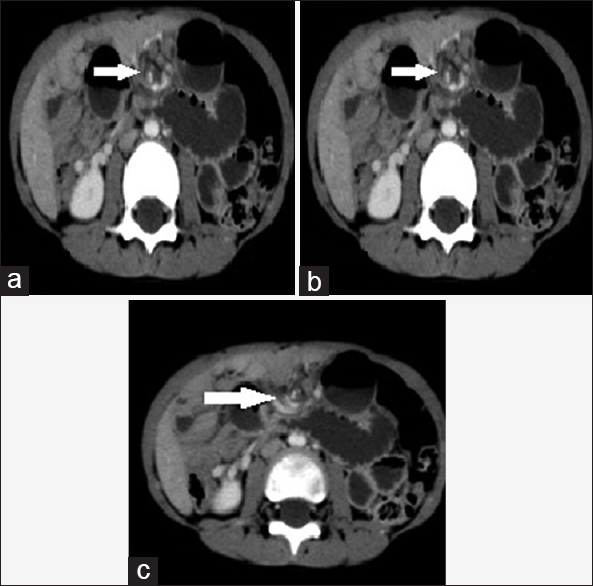
- Case 10: 5-year-old boy presented with a history of progressive on and off pain in the abdomen for 2 days, associated mild abdominal distension and two episodes of vomiting and was diagnosed with small bowel volvulus with coexisting lymphangioma being the possible cause. (a) Axial multidetector computed tomography image shows characteristic “Whirlpool” sign (white arrow). (b) Axial reformat multidetector computed tomography image shows characteristic “Whirlpool” sign (white arrow). (c) Axial reformat multidetector computed tomography image shows characteristic “Whirlpool” sign (white arrows).
Small bowel volvulus is a rare cause of intestinal obstruction. There are two categories of small bowel volvulus. Primary type is defined as torsion of a segment of the small bowel mesentery without evidence of any predisposing anatomical abnormalities. Secondary type is precipitated by underlying anatomical abnormalities, including postoperative adhesion, malrotation, congenital bands, intussusception, colostomy, fistula, tumors, and Meckel's diverticulum.[30]
In our case, small bowel volvulus and a closed loop obstruction was induced by the rotation of the mesenteric lymphangioma.
Lymphangioma is a congenital malformation of the lymphatic vessels and usually seen in pediatric age group. Approximately, 95% of lymphangiomas are found in the neck and axilla, and the other 5% occur in the mediastinum and abdominal cavity, including the mesentery and retroperitoneum.[30]
The typical CT feature of mesenteric lymphangioma is a multiloculated fluid-filled mass. MDCT with thin slice multiplanar reformation is helpful to diagnose and also to look for the complications such as volvulus, as was noted in our case.
There are two theories explaining the relevance between small bowel volvulus and lymphangioma. The flaccid and mobile characteristics of a mesenteric lymphangioma cause it to rotate, and this induces small bowel volvulus. Longstanding or intermittent volvulus causes lymphatic obstruction, and this forms lymphatic cysts as a result. The latter case generally forms a unilocular cystic mass without internal septa.[3132]
In our case, a multilocular mesenteric cyst was the primary pathology that secondarily caused volvulus of the small bowel.
Lymphangioma is a rare congenital entity which could predispose to mesenteric and small bowel volvulus.
Aortic dissection
A 50-year-old patient came to the emergency department with sudden onset of severe chest pain with a tearing sensation in the interscapular region radiating down to the epigastric region. Electrocardiogram ruled out myocardial infarction, and a clinical suspicion of aortic dissection was made.
CT aortogram was performed which revealed acute Stanford type B dissection [Figure 11a–c].

- Case 11: 50-year-old patient came to the emergency department with sudden onset of severe chest pain with a tearing sensation in the interscapular region radiating down to the epigastric region diagnosed as due to aortic dissection. (a) Coronal reformat multidetector computed tomography image shows dissection involving the descending thoracic aorta distal to the left subclavian artery (white arrow). (b) multi-detector computed tomography axial image shows dissection involving the descending thoracic aorta distal to the left subclavian artery (white arrow). (c) multidetector computed tomography with computed tomography aortogram shows dissection involving the descending thoracic aorta distal to the left subclavian artery (white arrow).
Acute aortic dissection is a life-threatening emergency and requires prompt diagnosis. Aortic dissection is defined as the separation of the layers within the aortic wall. It begins with a tear in the intimal layer which then propagates and creates a false lumen filled with blood. Mortality is still high despite advances in diagnostic and therapeutic modalities.
The diagnosis of acute aortic dissection requires a high index of suspicion and involves the following: History and physical examination, Imaging studies, Electrocardiography, complete blood count, serum chemistry studies, and cardiac marker assays.
The Stanford classification system for dissections is based on the need for surgical intervention.
Stanford type A dissection involves the ascending thoracic aorta, and the dissection flap may extend into the descending aorta. Type A dissections account for 60-70% of cases and typically require urgent surgical intervention to prevent extension into the aortic root, pericardium, or coronary arteries. If untreated, type A dissections are associated with a mortality rate of over 50% within 48 h.[33]
Stanford type B dissection involves the descending thoracic aorta distal to the left subclavian artery and accounts for 30-40% of cases. Management takes the form of medical treatment of hypertension unless there are complications due to extension of the dissection such as end-organ ischemia or persistent pain that would necessitate surgical intervention.[33]
Initial bedside portable chest radiography may reveal widening of the mediastinum with associated left-sided pleural effusion in the case of rupture.
If the patient is hemodynamically stable, then a contrast CT aortogram may be performed which will the show double barrel aorta with the presence of the intimal flap. The false lumen may distend with blood and cause compression of the true lumen with occlusion of the branch vessels arising from the true aortic lumen. It also helps to determine if hypothermic circulatory arrest is necessary for surgery.
MRI is the most sensitive method for diagnosing aortic dissection in a hemodynamically stable patient. The imaging findings are similar to those on CT.
Acute aortic dissection is a life-threatening emergency and requires prompt diagnosis. The Stanford dissection classification helps to determine the need for surgical intervention.
CONCLUSION
This interesting compilation is to emphasize the importance of having an insight of relatively uncommon conditions that clinically mimic other common causes of acute abdominal pain. It also provides an in-depth understanding of such conditions, additionally reinforcing the importance of good clinical history, higher degree of suspicion in equivocal cases and multimodality approach, thereby increasing the overall diagnostic accuracy which influences the patient prognosis and management by and large.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
Dr. Kapil Shirodkar, MBBS, DMRD, Secondary DNB Resident, Department of Radiology and Imaging, Apollo Hospitals, Bangalore, Karnataka, India.
Available FREE in open access from: http://www.clinicalimagingscience.org/text.asp?2016/6/1/4/177548
REFERENCES
- Epiploic appendagitis: An entity frequently unknown to clinicians - Diagnostic imaging, pitfalls, and look-alikes. AJR Am J Roentgenol. 2009;193:1243-51.
- [Google Scholar]
- Radiologic-pathologic conference of Keller Army Community Hospital at West Point, the United States Military Academy: Torsion of the epiploic appendage. AJR Am J Roentgenol. 2003;180:748.
- [Google Scholar]
- Primary epiploic appendagitis: Clinical, US, and CT findings in 14 cases. Radiology. 1994;191:523-6.
- [Google Scholar]
- Imaging manifestations of abdominal fat necrosis and its mimics. Radiographics. 2011;31:2021-34.
- [Google Scholar]
- Torsion of abdominal appendages presenting with acute abdominal pain. Ann Saudi Med. 2000;20:211-3.
- [Google Scholar]
- Disproportionate fat stranding: A helpful CT sign in patients with acute abdominal pain. Radiographics. 2004;24:703-15.
- [Google Scholar]
- CT evaluation of mesenteric panniculitis: Prevalence and associated diseases. AJR Am J Roentgenol. 2000;174:427-31.
- [Google Scholar]
- Current Surgical Diagnosis and Treatment. New York(NY-): McGraw-Hill Medical; 2006.
- [Google Scholar]
- General case of the day. Mesenteric panniculitis with extensive inflammatory involvement of the peritoneum and intraperitoneal structures. Radiographics. 1999;19:1083-5.
- [Google Scholar]
- Clinical imaging of the small intestine. New York, NY: Springer-Verlag; 2001. ISBN:0387953884
- [Google Scholar]
- Mesenteric venous thrombosis: Still a lethal disease in the 1990s. J Vasc Surg. 1994;20:688-97.
- [Google Scholar]
- Treatment of acute superior mesenteric vein thrombosis with percutaneous techniques. AJR Am J Roentgenol. 2003;181:1305-7.
- [Google Scholar]
- Mesenteric venous thrombosis: Diagnosis and noninvasive imaging. Radiographics. 2002;22:527-41.
- [Google Scholar]
- CT appearance of intestinal ischemia and intramural hemorrhage. Radiol Clin North Am. 1994;32:845-60.
- [Google Scholar]
- Gallstone ileus. Review of the literature and presentation of thirty-four new cases. Am J Surg. 1975;129:552-8.
- [Google Scholar]
- Gallstone ileus analysis of radiological findings in 27 patients. Eur J Radiol. 2004;50:23-9.
- [Google Scholar]
- A case of gallstone ileus with a spontaneous evacuation. Am J Gastroenterol. 2002;97:1259-60.
- [Google Scholar]
- Surgical Emergencies. Oxford, UK: Wiley-Blackwell; 1999.
- CTA and MRA in mesenteric ischemia: Part 1, Role in diagnosis and differential diagnosis. AJR Am J Roentgenol. 2007;188:452-61.
- [Google Scholar]
- Cough-induced intercostal lung herniation successfully diagnosed with imaging techniques. Recenti Prog Med. 2012;103:523-5.
- [Google Scholar]
- CT diagnosis of acute mesenteric ischemia from various causes. AJR Am J Roentgenol. 2009;192:408-16.
- [Google Scholar]
- Diagnosis of ovarian torsion with color Doppler sonography: Depiction of twisted vascular pedicle. J Ultrasound Med. 1998;17:83-9.
- [Google Scholar]
- Mesenteric lymphatic malformation associated with small-bowel volvulus - Two cases and a review of the literature. Pediatr Radiol. 2002;32:362-5.
- [Google Scholar]
- Chronic midgut volvulus with mesenteric lymphangioma: A case report. Pediatr Radiol. 1998;28:611.
- [Google Scholar]
- Multidetector CT of aortic dissection: A pictorial review. Radiographics. 2010;30:445-60.
- [Google Scholar]






