Translate this page into:
Tumors and pseudotumors of the soft tissues: Imaging semiology and strategy

*Corresponding author: Charlinne Paixao, Department Radiology, Musculoskeletal Imaging Unit, CHU Besancon, Besancon, France. charlinne.paixao@orange.fr
-
Received: ,
Accepted: ,
How to cite this article: Paixao C, Lustig J, Causeret S, Chaigneau L, Danner A, Aubry S. Tumors and pseudotumors of the soft tissues: Imaging semiology and strategy. J Clin Imaging Sci 2021;11:13.
Abstract
The aims of this educational review are to learn the semiological basis of soft-tissue lesions and, with the help of diagnostic algorithms, to apply the current recommendations for the management of soft-tissue tumors. Pseudotumors must first be identified and excluded. Among primary tumors, the search for macroscopic fat content on MRI is decisive; since it restricts the diagnostic range to adipocytic tumors. Key imaging features of non-adipocytic tumors are highlighted. When a deep soft-tissue mass is found, therapeutic abstention or simple monitoring is only appropriate when there is diagnostic certainty: This is only the case for typical pseudotumors, typical benign tumors, and fat tumors without atypical criteria. In all other cases, histological evidence is required. If there is any suspicion of soft-tissue sarcoma or any undetermined lesion, the patient should be referred to a sarcoma referral center before biopsy.
Keywords
Soft-tissue sarcoma
Adipocytic tumor
Non-adipocytic tumor
Lipoma
Liposarcoma
INTRODUCTION
The discovery of a soft-tissue mass is a common cause of requests for imaging. Ultrasound is the first-line imaging technique. However, an MRI is mandatory in the absence of a definite ultrasound diagnosis, or in case of a fascia or deep soft-tissue tumor. Malignant soft-tissue lesions, often soft-tissue sarcomas (STS), are rare but not exceptional (incidence ≈ 3/100,000). Histological diagnosis is potentially difficult and often helped by cytogenetic data. The prognosis of malignant soft tissue tumors is all the more unfavorable when the diagnosis is delayed or when initial management is inadequate. The management of suspected soft-tissue tumors should always be discussed in a multidisciplinary meeting.
Pseudotumors must first be identified and eliminated. Among primary tumors, the search for a macroscopic fat component visible on MRI is decisive, since it restricts the diagnostic range to adipocytic tumors.
First, pseudotumors will be discussed. Then, most common characterizable tumors will be detailed by differentiating fatty from non-fatty tumors. Finally, a management strategy will be proposed using diagnostic algorithms [Figures 1-3] and based on current recommendations for the management of soft-tissue tumors.
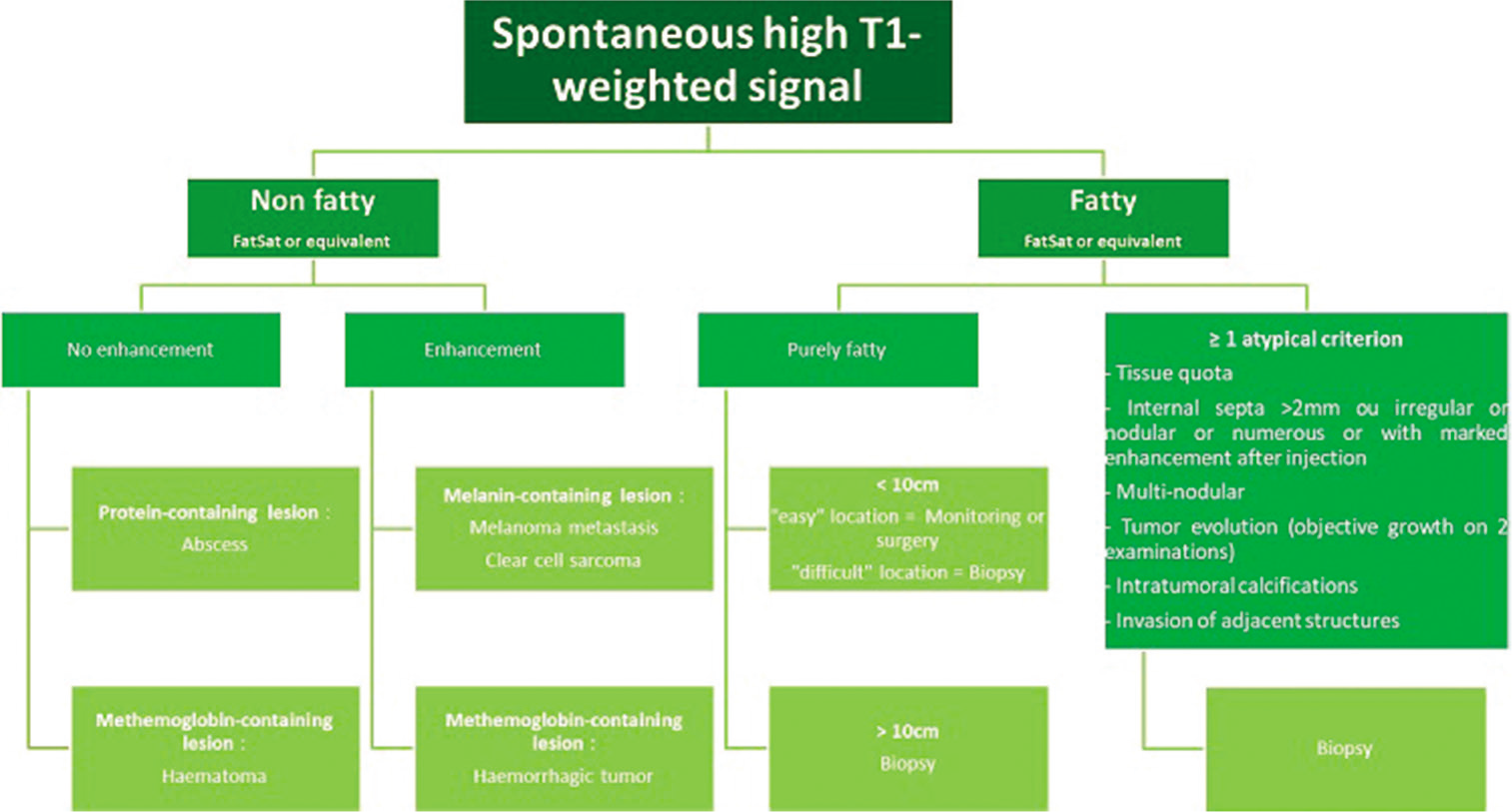
- MRI diagnostic and management algorithm for lesions with spontaneous high signal on T1-weighted images.
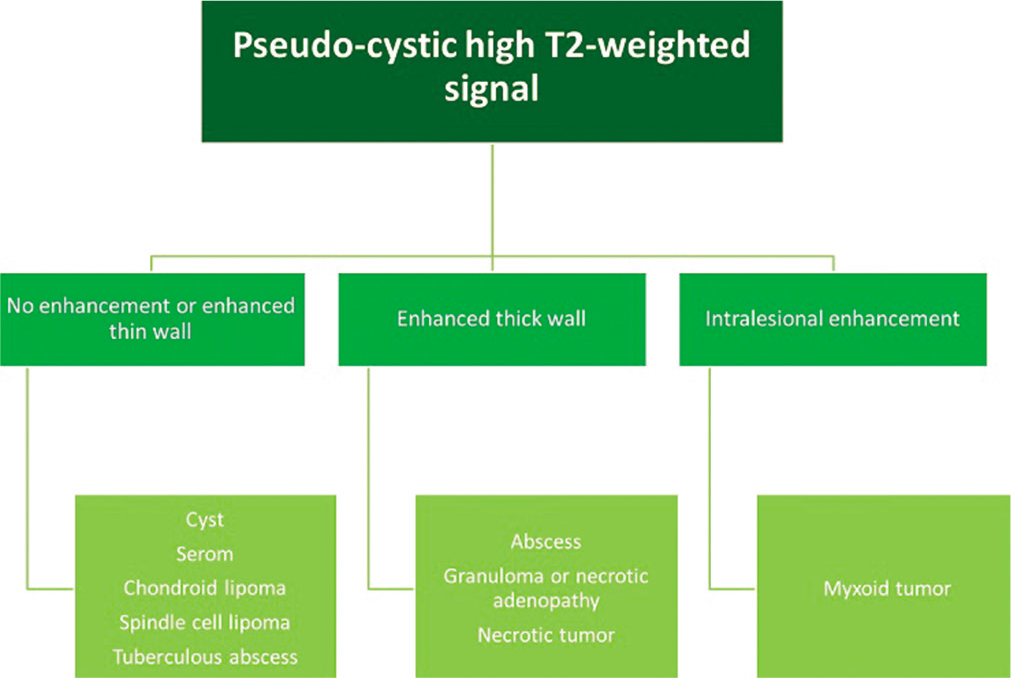
- MRI diagnostic algorithm for lesions with a liquid or pseudo-cystic high signal on T2-weighted images.
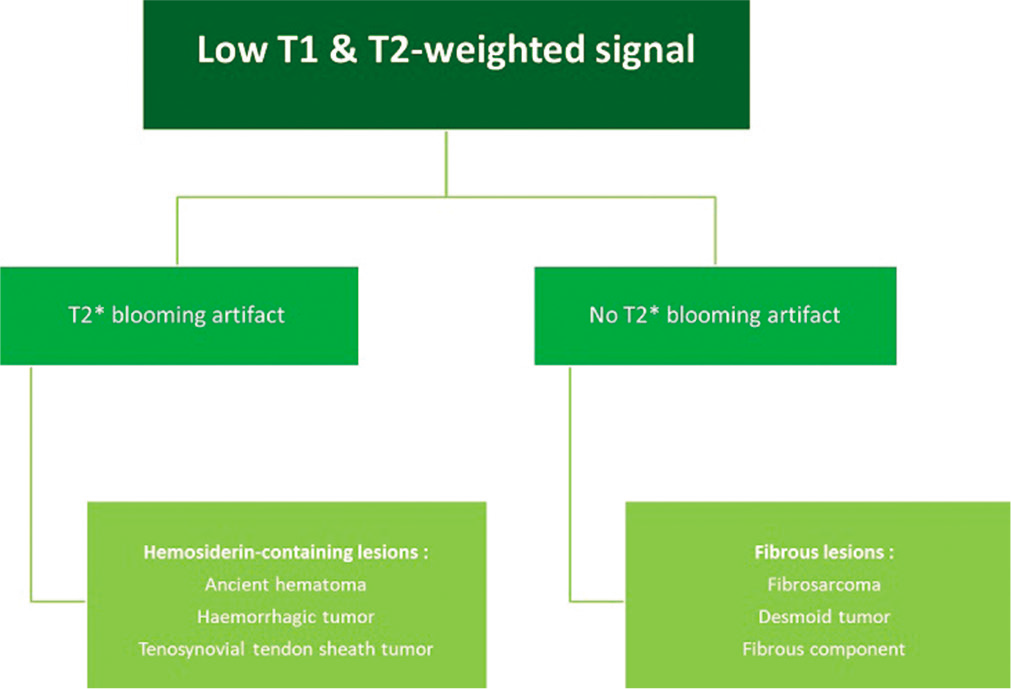
- MRI diagnostic algorithm for low-signal lesions on T1-weighted and T2-weighted images.
PSEUDOTUMORS
Pseudotumors are lesions that mimic a tumor and that are not included in the WHO classification of soft-tissue tumors. Among the pseudotumors, intramuscular hematomas and soft-tissue abscesses both require caution and surveillance because of their sarcoma-like appearance.[1,2]
Hematoma
The ultrasound similarity between a malignant tumor with necrosis and a hematoma should prompt the utmost caution [Figure 4a]. The presence of the following unfavorable factors should be investigated:[3]
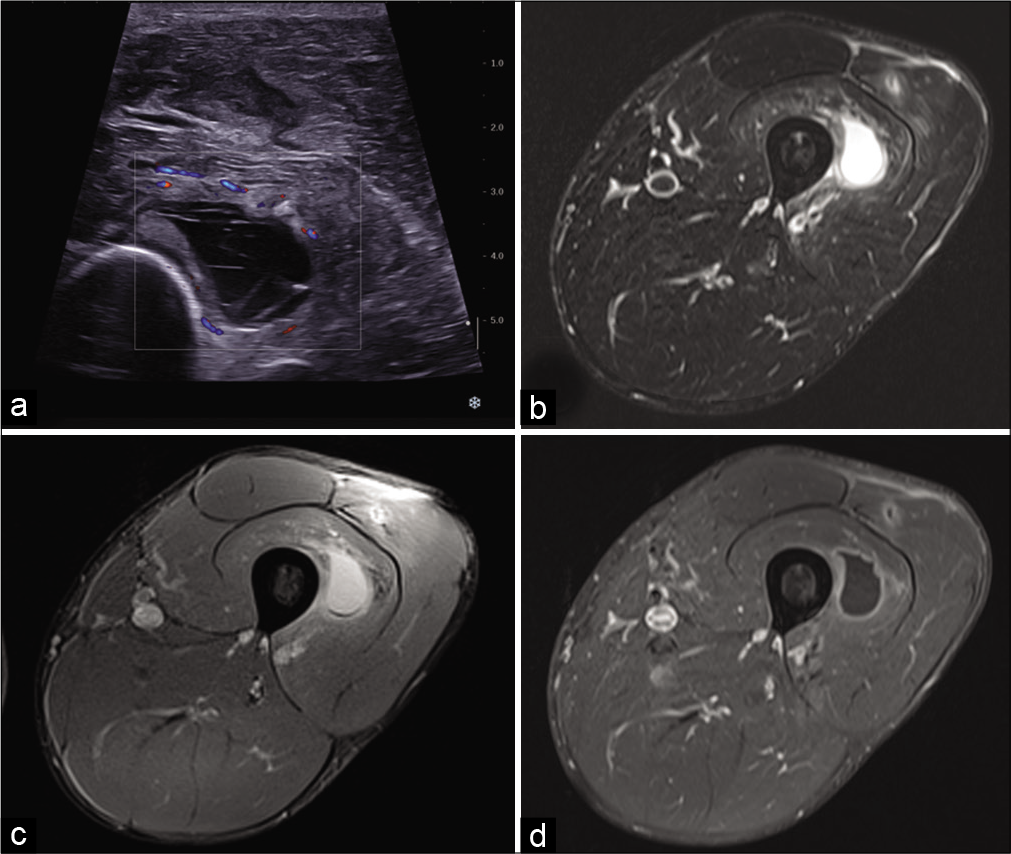
- A 32-year-old man with persistent pain in the left thigh 2 weeks after mild anterior trauma. US: Axial ultrasound image shows an anechoic collection with internal septa located in the vastus intermedius muscle (a). On MRI, the hematoma is well-defined, has a high-signal, and is surrounded by edema on fat-suppressed T2-weighted image (b), presents a low-signal deposit on gradient-echo T2-weighted image (c), and thin peripheral enhancement after injection of gadolinium chelates (d). A small post-traumatic lesion is also visible in the vastus lateralis muscle.
Absence of trauma or mild trauma compared to the size of the hematoma
Occurring in a child or teenager
Intra-lesion vascular flow on color Doppler
Absence of pain and slow progression, although these factors are less reliable because high-grade sarcomas are often painful and progress quickly.
If there is any doubt, an MRI scan [Figure 4b-d] and a biopsy should be performed. If a non-tumorous lesion is suspected, an imaging check-up at 3 weeks can be included in the clinical follow-up. Hematomas may show contrast enhancement, but only finely and peripherally [Figure 4d]. A nodular enhancement or an area of contrast enhancement in part of the lesion should suggest an underlying tumor and the biopsy should focus on that area.
Abscess
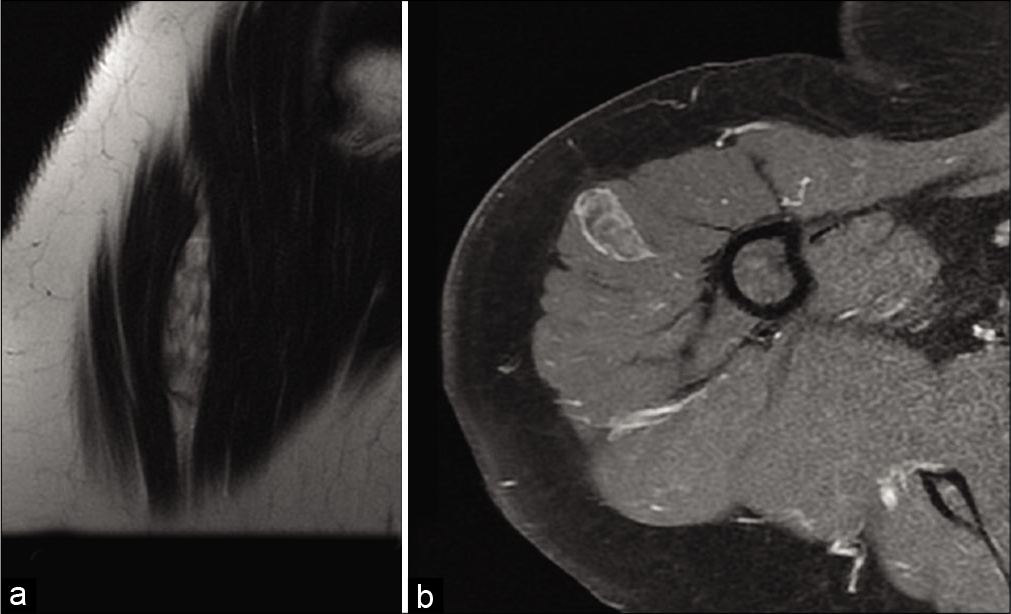
On ultrasound,[4] an abscess appears as a heterogeneous collection, with hyperechoic peripheral infiltration related to edema and peripheral hyperemia on color Doppler. If the abscess has a “falsely solid” appearance, mobilization shows fluctuation or displacement of its content. In the chronic stage, the liquefied center is anechogenic with posterior reinforcement [Figure 5a], the wall is echogenic and thick. Calcifications and air bubbles can be detected. Similarly, the ultrasound appearance of a high-grade sarcoma with necrosis may simulate an abscess. The absence of general clinical and biological signs of infection should prompt caution. If there is any doubt, MRI and sampling of the lesion (possibly percutaneous microbiopsies under radiological control) should be performed.
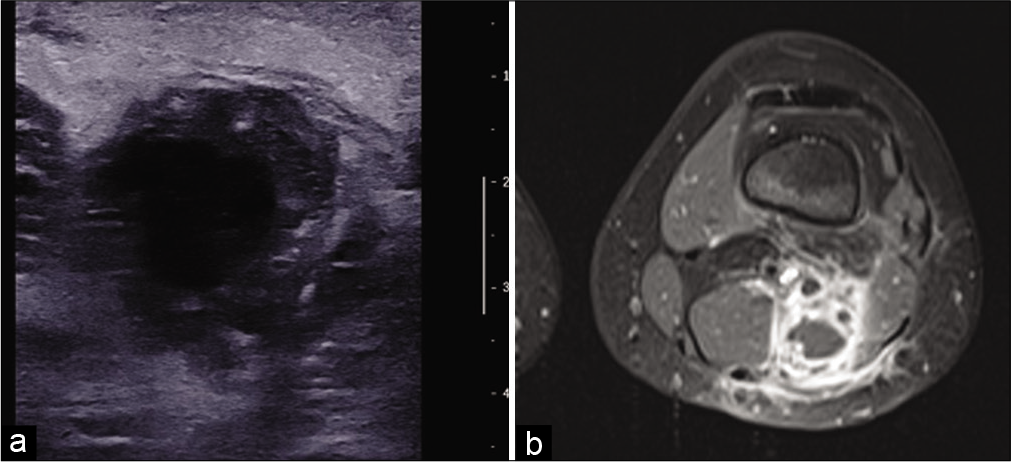
- An 11-year-old girl with a popliteal fossa abscess consulting for pain and swelling with inflammatory syndrome. US: Axial ultrasound image of an abscess with hypoechoic and thick-walled, hyperechoic and heterogeneous content (a). MRI: On fat-saturated T1-weighted MRI image after injection of gadolinium chelates (b), the abscess, located between the semi-membranous and biceps muscles, has a low signal center, intense peripheral enhancement, and ill-defined peripheral enhancement due to perilesional edema.
On MRI, the center of an abscess typically has a low signal on T1-weighted (T1w) images (although a high signal is possible), high signal on T2w, and a high signal on diffusion-weighted with apparent diffusion coefficient (ADC) restriction.[5] It has an outer shell that enhances after injection of contrast material[6] and marked peri-lesional edema [Figure 5b]. Occasionally, a visible border in discrete high signal on T1w images is indicative of granulation tissue.
Others pseudotumors
Others pseudotumors mainly include: Muscles and accessory muscle bundles, muscle hernia, Morel-Lavallee syndrome [Figure 6], circumscribed myositis ossificans [Figure 7], elastofibroma [Figure 8], synovial or mucoid cyst, cytosteatonecrosis, and parietal endometriosis following cesarean section and foreign body granuloma.
- A 22-year-old man with Morel-Lavallee syndrome who presented with left hip pain 4 weeks after a motorcycle accident. MRI: Collection with a low signal on T1-weighted (a) and high signal on T2-weighted (b) coronal images, between the hypodermic fat and the peripheral deep fascia of the left gluteus maximus muscle, typical of a Morel-Lavallee syndrome (arrows).
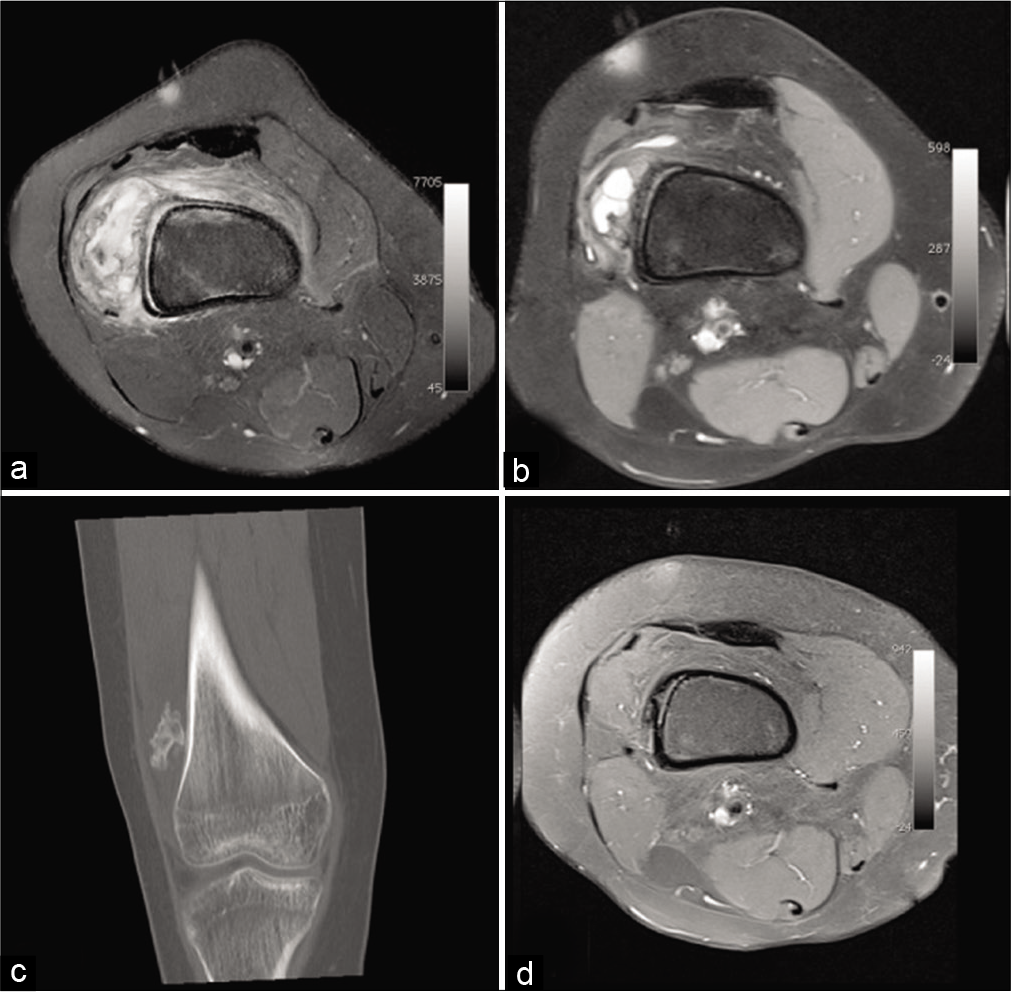
- A 16-year-old girl presenting with increasing knee pain for 15 days and swelling. Natural evolution of a circumscribed myositis ossificans of the right thigh. MRI: Worrying pseudotumoral appearance on initial fat saturated proton density weighted axial MRI image (a). 7 months later on fat saturated T1-weighted axial image after injection (b), the size of the lesion and peri lesional infiltration decreased. The coronal CT reformat performed on the same day (c) shows ossification. Three years later, on fat saturated T1-weighted MRI image after injection (d), it has incorporated itself into the bone cortex.
- A 53-year-old woman with bilateral elastofibromas below the scapular tip, undergoing MRI for bilateral scapulalgia. Typical laminated appearance on T1-weighted axial MRI image (arrows).
ADIPOCYTIC TUMORS
MRI is the reference method for the evaluation of deep fatty tumors. It is particularly discriminating when it enables the detection of macroscopic fat with high signal on T1w and T2w images, and low signal on sequences with fat signal suppression (Fat saturation, Water-only Dixon, Short Tau Inversion Recovery [STIR]) [Figure 1].
Lipoma
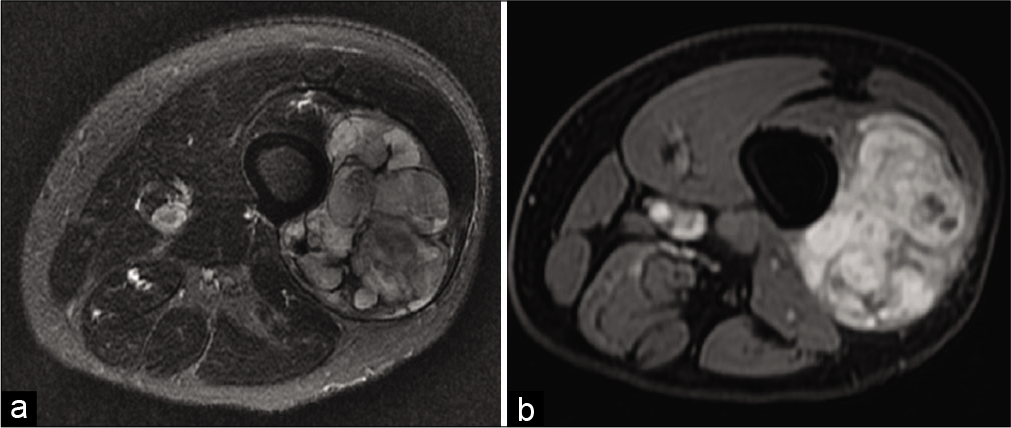
This is the most common soft-tissue tumor. Conventional lipoma is well defined, finely encapsulated, in a lobular arrangement. It is more often unilobular than liposarcoma.[7] It presents a purely fatty signal in two thirds of cases [Figure 9a]. In one-third of cases, there is a nonfat component: calcifications, adipocyte necrosis, inflammatory infiltrate, vessels and phleboliths (angiolipoma), smooth muscle fibers (myolipoma), or myxoid areas.

- A 47-year-old woman with a conventional lipoma consulting for swelling of the right buttock. MRI: Lipoma of the right gluteus maximus muscle (arrow) showing a purely fatty signal on T1-weighted MRI image (a), low signal and no enhancement on fat saturated T1-weighted axial images after injection (b).
Its septa are thin (<2 mm), and it does not show enhancement after contrast injection [Figure 9b]. Muscle fibers may be included in the lipoma, which is almost pathognomonic for benignity.
Liposarcoma
Liposarcoma is the second most common STS after undifferentiated pleomorphic cell sarcoma (14–18% of all soft-tissue malignancies). There are five major histologic types: Well-differentiated liposarcoma, dedifferentiated liposarcoma, myxoid liposarcoma, pleomorphic liposarcoma, and spindle cell liposarcoma. Liposarcoma is extremely rare in neonates and children. About 85–90% of patients present a painless mass. Liposarcoma develops mainly at the expense of deep soft tissue, especially in the intermuscular spaces. Its two preferred sites are the thigh and the retroperitoneum. It is rare in the extremities (1–2%).
Calcifications are visible on radiography in 10% of cases.[8] Ossifications are rare.
On CT and MRI, liposarcoma is a large tumor with polylobulated contours. It is quite limited, because it tends to grow by expansion rather than infiltration.[7] Liposarcoma is usually multinodular in shape with small satellite nodules adjacent to the main tumor.[9]
The appearance of liposarcoma depends on the degree of differentiation. The more differentiated the tumor, the fattier the signal: A well-differentiated liposarcoma can simulate a lipoma [Figure 10]. A signal intensity ratio on STIR images between the lesion and adjacent fat <1.4 and a maximum diameter <11 cm would be an additional argument in favor of a lipoma over atypical lipomatous tumors/well-differentiated liposarcomas.[10] The slightest sign of atypia should lead to referral to a sarcoma center for image-guided percutaneous microbiopsy [Figure 1].[11] A nodular non-fatty component of more than 2 cm in an atypical adipose tumor is in favor of a dedifferentiated liposarcoma.[12] Pleomorphic, dedifferentiated, and myxoid liposarcomas may not contain a macroscopic fat component visible on imaging.[13] Myxoid liposarcoma is characterized by low density pseudocystic areas on CT scan, with a T1w and T2w pseudocystic signal on MRI that is intensely enhanced after contrast injection.[14] Fatty areas account for less than 10% of the mass. If MRI is performed without contrast injection, myxoid liposarcoma may be confused with cystic lesions and operated inappropriately.

- A 55-year-old man with liposarcoma consulting for swelling of the right thigh. MRI: T1-weighted (a) and fat saturated T2-weighted coronal MRI images (b). Fatty tumor of the right thigh whose signal is heterogeneous (arrows) and higher than that of subcutaneous fat (arrowhead). Biopsy confirmed the diagnosis of well-differentiated liposarcoma.
Hibernoma
Hibernoma is a very rare benign tumor that occurs in adults aged 20–40 years. It is composed of brown fat present in newborns that is similar to that of hibernating animals. Hibernoma is most frequently reported in the thigh, in the superficial soft tissues more than in deep tissue.[15]
Hibernoma is frequently discovered incidentally on PET-CT due to its high hypermetabolism. On ultrasound, hibernoma is hyperechoic, but heterogeneous with hyperemia and arterial-like flows on Doppler.[16]
On CT, its density is intermediate, between that of fat and muscle, and heterogeneous due to the presence of fibrous septa.
On MRI, it presents a fat signal different from that of subcutaneous fat: An intermediate signal between muscle and fat on T1w images (“dirty white appearance”), slightly heterogeneous, close to that of fat on T2w and intense in STIR.[16] Fat saturation only partially suppresses its signal.[17] The lesion is partitioned by typically central fibrous septa. The presence of large intra-tumoral vessels is an inconsistent but useful sign, since it is absent in liposarcomas[18] [Figure 11]. There is a rare myxoid variant with a pseudo-liquid signal on T2w images.

- A 36-year-old woman with a hibernoma who presented with a subcutaneous mass of the left thigh. MRI appearance of a hibernoma of the posterior compartment of the left thigh on T1-weighted axial image (a), T2-weighted sagittal image (b), and fat saturated T1-weighted axial image after injection (c). The brown fat of the hibernoma (arrows) has a lower signal on T1-weighted and T2-weighted images than that of the subcutaneous fat. There are also large intra lesional vessels enhanced after injection (arrowhead).
As the lesion is hypervascular, percutaneous biopsy presents a risk of hemorrhage.
Intramuscular hemangioma
Intramuscular hemangioma is a benign vascular tumor composed of capillary proliferation and vessels. This term should not be used for purely cavernous forms, which are vascular malformations. Intramuscular hemangioma often has a macroscopic fat component.[19] Other signs are:
On standard radiography, the presence of phleboliths
On ultrasonography, a heterogeneous multi-cystic lesion with serpiginous images with very low or even undetectable flow on color Doppler
On MRI, a polylobulated, well-delimited, heterogeneous lesion with predominantly high signal on T1w and T2w images, and the presence of intra lesional vessels that are highly enhanced after injection.
NON-ADIPOCYTIC TUMORS
When the lesion does not contain a macroscopic fatty component, imaging can still help in the diagnosis, by showing a melanocytic [Figure 1], myxoid [Figure 2], or fibrous component [Figure 3].
Melaninic tumor: Clear cell sarcomas
This is a melanocytic sarcoma generally affecting women between the ages of 25 and 40. The preferred topography is the limbs (especially the lower limbs, at the foot), opposite a tendon, ligament, or fascia. It has the MRI signal of melanin in about 50% of cases [Figure 1]. It is often non-encapsulated, with blurred borders, homogeneous, without necrosis or peri-lesional edema, and enhances after injection.[20]
Myxoid tumors
Myxoid tumors show pseudo-liquid high signal on T2w images and enhancement after gadolinium injection [Figure 2]. In the presence of a deep soft-tissue mass presenting a myxoid signal, the following entities should be considered: Intramuscular myxoma [Figure 12], myxoid chondrosarcoma, myxofibrosarcoma [Figure 13], myxoid liposarcoma (see above), or intramuscular neurogenic tumor (schwannoma or neurofibroma).
- A 66-year-old woman with intramuscular myxoma who presented with palpable swelling of the right deltoid muscle. MRI: High T2-weighted signal and septa (a). It presents weak internal enhancement, with enhancement of its pseudo-capsule on fat saturated T1-weighted axial images after injection (b).
- A 42-year-old man with myxofibrosarcoma consulting for a painful mass in the right thigh. MRI: On fat saturated T2-weighted axial image (a), this fibrosarcoma is very heterogeneous due to fibrous, myxoid, and tissue content, and areas of hemorrhagic necrosis. Therefore, on fat saturated T1-weighted axial image after injection (b), enhancement is also heterogeneous.
Neurogenic tumors should be considered in the presence of a mass centered on a nerve that shows the split fat sign (thin layer of periwound fat) and the target sign (central fibrocellular zone and peripheral myxoid zone).[21]
Fibrous tumors
Nodular fasciitis
Nodular fasciitis is the most common benign fibrous soft-tissue tumor. Its spontaneous growth is limited. It occurs mostly in young adults and with no predominance in either sex. Clinically, it is a single mass of less than 3 cm, firm and painful, growing rapidly in 2–3 months, and it may disappear spontaneously. The nodule is always unique and mainly affects the upper limb.[22] Its location with regard to the fascia makes it possible to distinguish between subcutaneous, intramuscular (mimicking a sarcoma), or intermuscular forms.
On ultrasonography, nodular fasciitis is a hypoechoic mass that is often heterogeneous. On MRI, it is a homogeneous T1w isosignal mass with a heterogeneous, and moderately high signal on T2w images, which enhances diffusely and homogeneously or peripherally after gadolinium injection.[23] The subcutaneous form is the most frequent, forming rounded nodules developed from the deep peripheral fascia and extending subcutaneously. There are often characteristic extensions that produce a “fascial tail sign,” an evocative but non-specific sign. Intermuscularly, the tumor develops in the fascia, often with star-shaped contours, mimicking an inflammatory, or aggressive lesion.[22,24] It may have a central cystic zone, which is a sign of the “inverted target.”[23,24]
Desmoid tumor
The desmoid tumor is part of the group of deep aggressive fibromatosis. Its location is deep, subaponeurotic, unlike superficial plantar and palmar fibromatoses. It affects all ages but especially young women. It presents slow and painless growth, which explains its large size (>10 cm). It is rarely multifocal and is limited to a single limb most of the time. It does not metastasize, but is locally aggressive.
It can be distinguished as follows:
The abdominal parietal form, the most frequent, mainly affecting young women (context: Pregnancy or surgical scar)
The extra-abdominal form occurring mainly at the root of the limbs, especially the shoulder[25]
And the intra-abdominal form.
The ultrasound appearance, which is not very specific, shows a poorly delimited hypoechoic mass with posterior acoustic shadowing and possible hypervascularization.
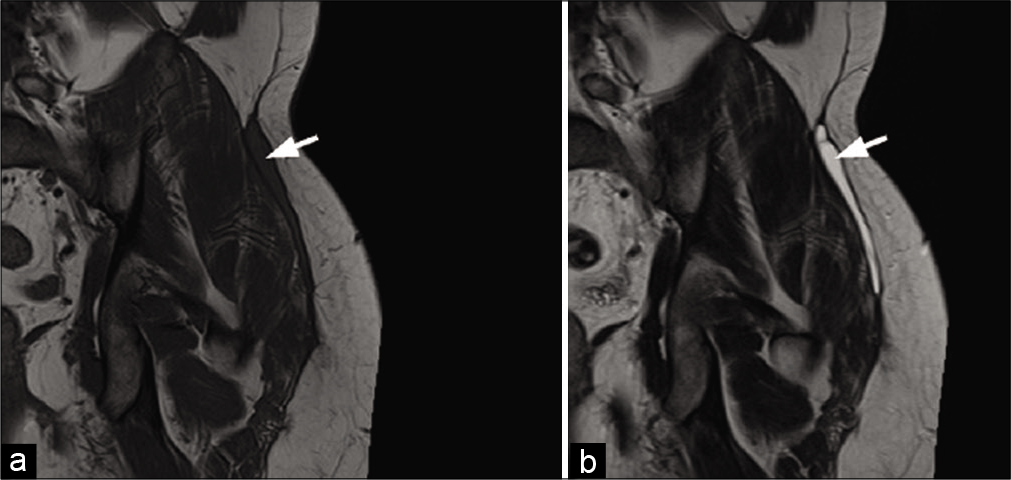
On MRI, the extra-abdominal form is typically intermuscular and can be surrounded by fat (“split fat sign”), like a neurogenic tumor. However, it also frequently infiltrates the muscles. Its signal is variable and often heterogeneous. Depending on the degree of maturation, it is hyperintense (young lesion with significant cellularity) or hypointense on T2w images [chronic fibrous form rich in collagen, Figure 3].[26,27] The presence of hypointense bands within the mass on all sequences is quite frequent and evocative (corresponding to dense collagen patches). The enhancement is moderate to high. Erosion or periosteal apposition of the facing bone is possible, but the medullary canal is not invaded. A linear extension of these lesions is often observed along the fascia, giving the appearance of a “deer antler” or “fascial tail sign” [Figure 14].[28]
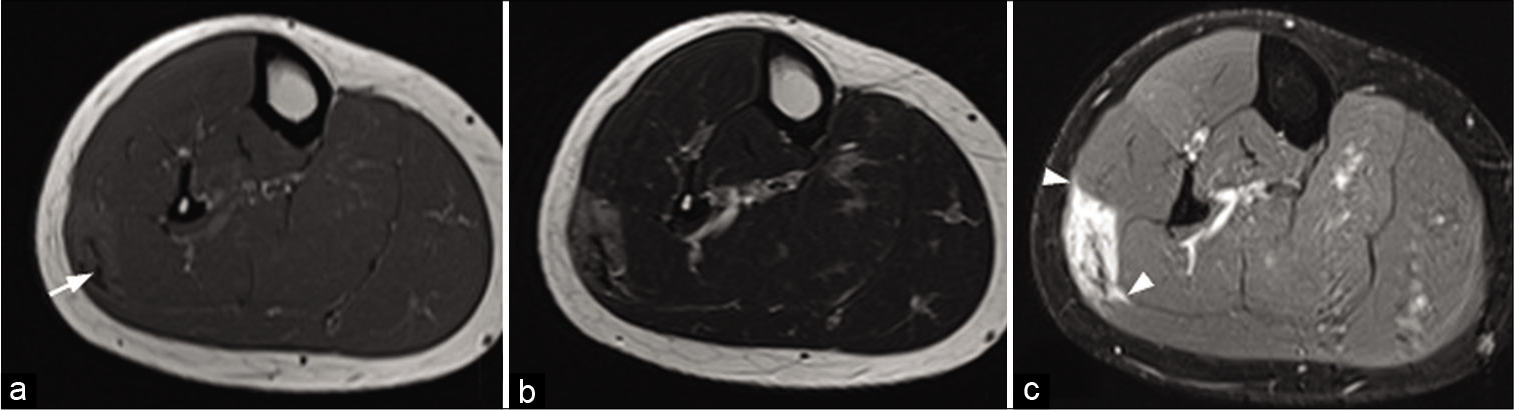
- A 30-year-old woman with a desmoid tumor who presented palpable swelling of the right leg. MRI: T1-weighted axial image (a), T2-weighted axial image (b), and fat saturated T1-weighted image after injection (c) of a desmoid tumor of the superficial posterior compartment of the right leg. It presents an intermediate signal with a central fibrous area (arrow) with low signal regardless of weighting. Although non-specific, its extension along the fascia (arrowheads), clearly visible after injection (c), is suggestive of the diagnosis.
Because recurrences are common even after complete surgical removal, treatment options include monitoring, discontinuation of oral contraceptives, and possibly systemic therapies or cryoablation under imaging control.[29]
Fibrosarcoma
Fibrosarcoma is a rare sarcoma that affects middle-aged subjects, and usually develops in the deep soft parts of the extremities, trunk or cervicofacial area. Its signal depends on its content (myxoid [Figure 2], fibrous [Figure 3], and necrotic).
Inflammatory myofibroblastic tumors
These tumors were previously referred to as “inflammatory pseudotumors.” Also known as plasma cell granulomas, they are rare neoplasms. They occur in children or young adults and can occur in any part of the body. They consist of a single or multinodular mass of variable size that may include tissular, myxoid, hemorrhagic, necrotic, or calcified areas. The treatment is surgical excision. Imaging is non-specific.
DISEASE MANAGEMENT
When a deep soft-tissue mass is found, therapeutic abstention or simple monitoring is only appropriate when there is diagnostic certainty: This is only the case for typical pseudotumors, typical benign tumors, and fat tumors without atypical criteria measuring less than 10 cm [Figure 1]. In all other cases, imaging can suggest diagnostic hypotheses, but histological evidence by percutaneous microbiopsy under imaging control will be essential.
The essential elements of the imaging report
The report must include the following elements:
Position of the lesion in relation to the deep peripheral fascia (i.e., superficial or deep)
The exact compartmental location
Relationships (vascular, nervous, bone, and joint)
Size in all three dimensions
Form and boundaries
And the composition or matrix.
The conclusion must mention one or more diagnostic hypotheses, and specify if necessary the elements of subsequent management, for example, complementary further imaging examinations, monitoring, and/or discussion in a multidisciplinary meeting.
Ultrasound
As a first-line examination, ultrasound confirms the deep site of the lesion and provides semiological information.
Typical benign lesions that do not require further exploration are:[30]
Simple cysts
Well-defined synovial bursae or cysts, with purely fluid content, without a solid component, anechogenic with posterior enhancement and without internal vascularization
Superficial, homogeneous, well-defined, encapsulated, compressible, and stable for at least 6 months
Vascular malformations, stable for at least 6 months
Granulomas consistent with clinical history
Muscular hernias
And Morton’s neuromas.
Post-traumatic muscle injury requires caution and control at 3–6 weeks is essential.
Ultrasound “red flags” are as follows:
Deep localization. A peripheral lesion in intimate contact with, or crossing the deep peripheral fascia is also suspect
Anarchic vascularization
Heterogeneity
Areas of necrosis
Hypoechoicity.
If there is any doubt about the diagnosis, the ultrasound must be supplemented by MRI.[21]
MRI
MRI indications are broad. MRI should be carried out immediately if there is a clinical suspicion of malignancy: Large, fixed or deep lesion, persistent post-traumatic swelling, increase in lesion size, or bone or joint damage. It is indicated as soon as a “red flag” is observed on ultrasound, when the lesion is not typically benign on ultrasound (see above), if the size of the mass exceeds 5 cm, and for any recurrent tumor at an operated site.[30]
MRI is the reference examination for the characterization of the tumor matrix according to its signal on T1w [Figure 1], T2w [Figure 2], T2*w [Figure 3] images, and its enhancement after injection of contrast medium. The search for macroscopic fat component visible on MRI is decisive, since it restricts the diagnostic range to adipocytic tumors and a few benign tumors containing fat.
MRI is essential for the assessment of loco regional extension. The field of view must encompass the entire lesion and peripheral edema with a margin. A skin marker is useful. The analysis is essentially based on high-resolution axial sections. However, at least two orthogonal acquisition planes or 3D-acquisition are required to accurately measure the lesion.
All non-fatty tumors, purely fat tumors larger than 10 cm, and fat tumors with any atypical criteria require pathological evidence (except for genetic exceptions).[11] In the case of a deep, purely fatty tumor of less than 10 cm, with a location that is “easy” for the surgeon to access, surveillance or surgery must be discussed in a multidisciplinary meeting. In addition to the criteria for atypical fat tumors [Figure 1], some MRI signs are suggestive of a malignant lesion:[31,32]
Heterogeneity on T2w images (necrosis)
Arterial enhancement after gadolinium injection[32]
Retroperitoneal location
Erosion of the adjacent bone
Spread to a vascular pedicle in contact.
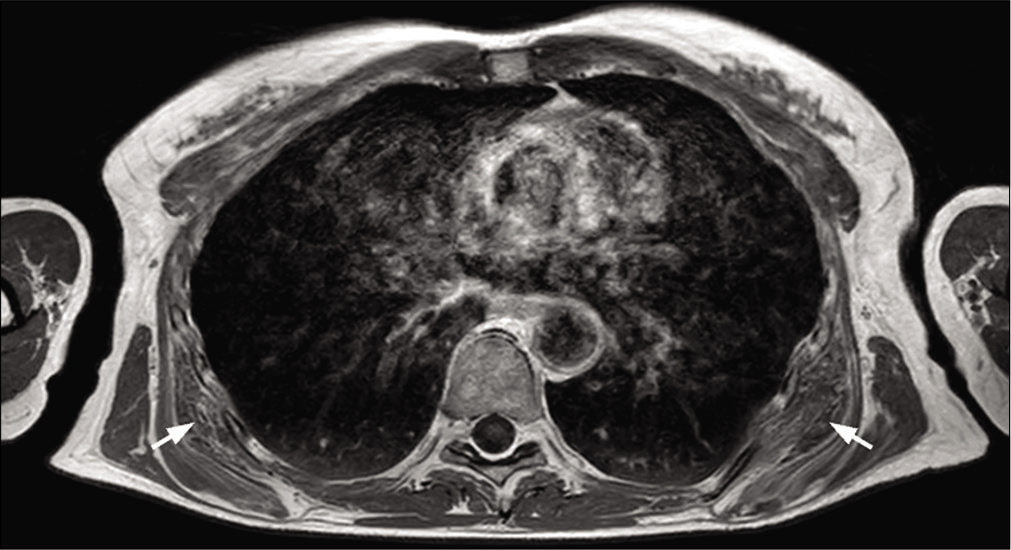
The regularity or otherwise of the lesion limits is not a discriminating criterion.[33] Many studies have focused on diffusion imaging. The difference between an abscess [Figure 15] and a necrotic tumor [Figure 16] could be due to diffusion-weighted imaging. Both would have a high diffusion-weighted signal, but the abscess would have a low ADC, unlike the necrotic sarcoma, which would have a high ADC.
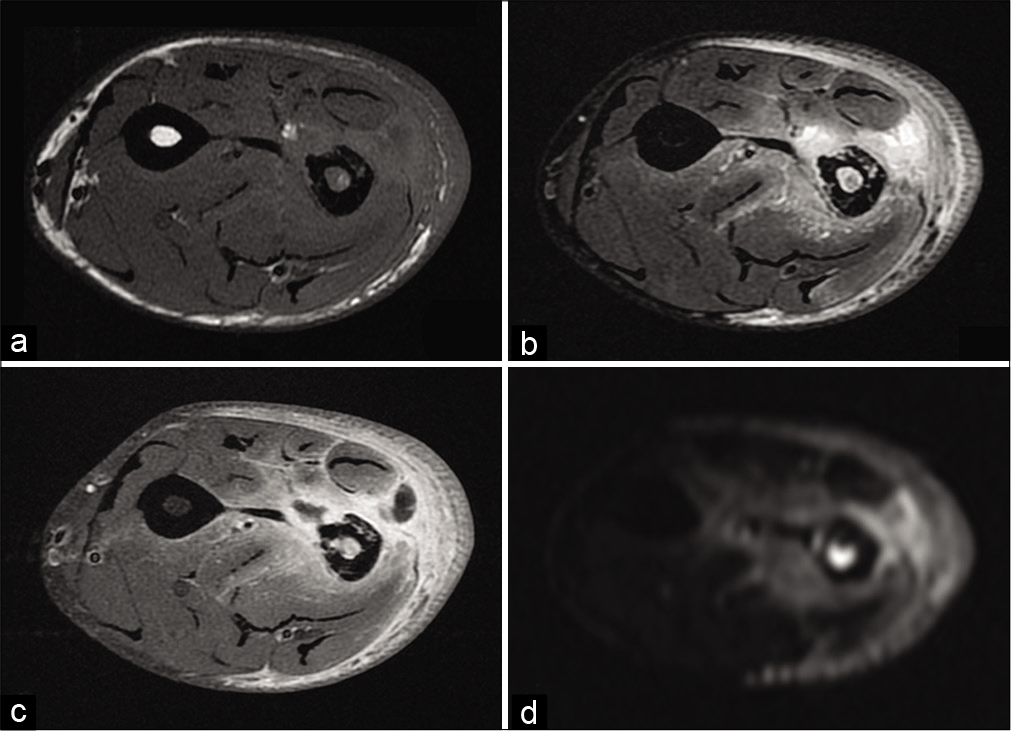
- A 38-year-old man with osteitis and abscess who presented for left forearm pain without fever. T1-weighted MR image (a), fat-suppressed T2-weighted image (b), fat-suppressed T1-weighted image after injection of gadolinium chelates (c) and diffusion weighted image (d) of radial osteitis: Cortical erosion of low T1-weighted signal and high diffusion-weighted signal of the medullary. Deep muscular abscess (short radial extensor of the carpus, short extensor, and long abductor of the thumb) have low T1-weighted signal (a), high T2-weighted signal (b), peripheral enhancement (c), and high diffusion-weighted signal (d). Bacteriological analysis found Staphylococcus aureus.
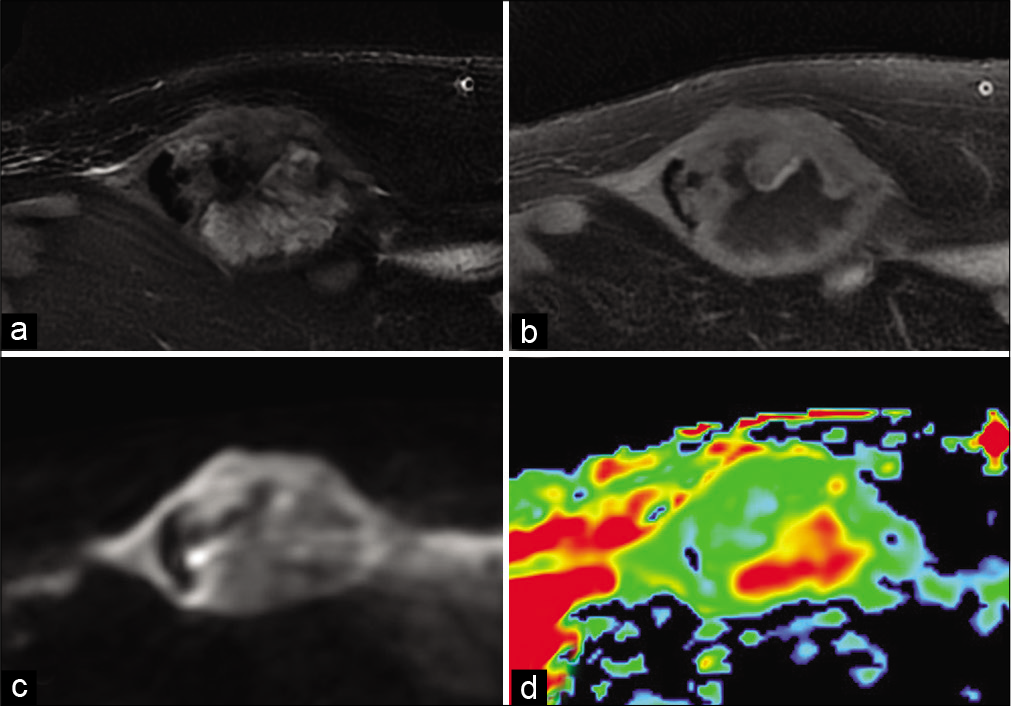
- A 72-year-old man with dedifferentiated spindle cell sarcoma who presented for growing abdominal wall mass. Fat-suppressed T2-weighted MR image (a), fat-suppressed T1-weighted image after gadolinium chelate injection (b), diffusion-weighted image (c) and Apparent Diffusion Coefficient (d). Moderately high diffusion-weighted image with high ADC of the necrotic portion and low ADC of the tissue portion.
Beyond its usefulness in abscess, diffusion imaging would help distinguish benign from malignant tumors. The ADC of malignant tumors is lower than that of benign tumors. However, there is a large overlap, which precludes sufficient diagnostic reliability.[34]
Multidisciplinary management and sampling recommendations
If there is any suspicion of STS and any undetermined lesion, the patient should be referred to a sarcoma referral center before biopsy.[35] Percutaneous biopsy should be performed after MRI and the multidisciplinary meeting.[36] The biopsy path, defined in agreement with the surgeon, must take into account the compartmental anatomy of the limb, so as not to transform an intracompartmental lesion into an extracompartmental lesion. It should be in the axis of the limbs or ribs. Numerous samples must be taken with a coaxial needle (≥16 Gauge) from the various lesion components, avoiding necrotic areas, and major vascular-nerve axes. A tattoo is sometimes performed to mark the biopsy site. In addition to pathological analysis, molecular biology analysis should also be systematically performed[37] and centralized review in a sarcoma referral center.[38] During the operation, the surgeon will have to resect the path and the mass in a single block to achieve complete macroscopic and microscopic margin-negative “R0” resection.
CONCLUSION
The discovery of a deep soft-tissue mass is a problem commonly encountered by radiologists. In line with the adage whereby “the ignorant asserts, the learned doubts, the wise thinks,” should be referred to a referral center for multidisciplinary care, in which oncologists, radiologists, pathologists, surgeons, and radiotherapists specialized in the management of STS will collaborate.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Pseudo-Tumeurs des Tissus Mous, in Imagerie Musculosquelettique: Pathologies Générales Netherlands: Elsevier, Masson; 2013.
- [Google Scholar]
- Soft tissue sarcomas or intramuscular haematomas? Eur J Radiol. 2009;72:44-9.
- [CrossRef] [PubMed] [Google Scholar]
- Musculoskeletal infections: Ultrasound appearances. Clin Radiol. 2005;60:149-59.
- [CrossRef] [PubMed] [Google Scholar]
- Diffusion-weighted magnetic resonance imaging for the evaluation of musculoskeletal tumors. Magn Reson Imaging Clin N Am. 2011;19:159-80.
- [CrossRef] [PubMed] [Google Scholar]
- Imaging of musculoskeletal soft tissue infections. Skeletal Radiol. 2010;39:957-71.
- [CrossRef] [PubMed] [Google Scholar]
- MRI findings in intramuscular lipomas. Skeletal Radiol. 1999;28:145-52.
- [CrossRef] [PubMed] [Google Scholar]
- From the archives of the AFIP: Imaging of musculoskeletal liposarcoma with radiologic-pathologic correlation. Radiographics. 2005;25:1371-95.
- [CrossRef] [PubMed] [Google Scholar]
- Liposarcoma of the extremities: MR and CT findings in the histologic subtypes. Radiology. 1993;186:455-9.
- [CrossRef] [PubMed] [Google Scholar]
- Quantitative signal intensity ratios to distinguish between subfascial lipoma and atypical lipomatous tumor/well-differentiated liposarcoma using short-tau inversion recovery (STIR) MRI. Diagn Interv Imaging. 2020;101:383-90.
- [CrossRef] [PubMed] [Google Scholar]
- Strategy for the diagnosis of an adipocytic soft tissue tumor of the adult. J D'imagerie Diagnostique Interv. 2018;1:265-83.
- [CrossRef] [Google Scholar]
- World Health Organization classification of bone and soft tissue tumors: Modifications and implications for radiologists. Semin Musculoskelet Radiol. 2007;11:201-14.
- [CrossRef] [PubMed] [Google Scholar]
- Imaging of fatty tumors: Distinction of lipoma and well-differentiated liposarcoma. Radiology. 2002;224:99-104.
- [CrossRef] [PubMed] [Google Scholar]
- Myxoid liposarcoma: Appearance at MR imaging with histologic correlation. Radiographics. 2000;20:1007-19.
- [CrossRef] [PubMed] [Google Scholar]
- The morphologic spectrum of hibernoma: A clinicopathologic study of 170 cases. Am J Surg Pathol. 2001;25:809-14.
- [CrossRef] [PubMed] [Google Scholar]
- Hibernoma: Imaging characteristics of a rare benign soft tissue tumor. Skeletal Radiol. 2001;30:590-5.
- [CrossRef] [PubMed] [Google Scholar]
- Imaging of hibernomas: A retrospective study on twelve cases. Clin Sarcoma Res. 2011;1:1-12.
- [CrossRef] [PubMed] [Google Scholar]
- Hibernoma: Report emphasizing large intratumoral vessels and high T1 signal. Skeletal Radiol. 2006;35:547-50.
- [CrossRef] [PubMed] [Google Scholar]
- Vascular anomalies: What a radiologist needs to know. Pediatr Radiol. 2010;40:895-905.
- [CrossRef] [PubMed] [Google Scholar]
- MR imaging of clear cell sarcoma (malignant melanoma of the soft parts): A multicenter correlative MRI-pathology study of 21 cases and literature review. Skeletal Radiol. 2000;29:187-95.
- [CrossRef] [PubMed] [Google Scholar]
- Imaging of soft-tissue musculoskeletal masses: Fundamental concepts. Radiographics. 2016;36:1931-48.
- [CrossRef] [PubMed] [Google Scholar]
- Nodular fasciitis of the breast and knee in the same patient. AJR Am J Roentgenol. 2002;178:1426-8.
- [CrossRef] [PubMed] [Google Scholar]
- MRI characteristics of nodular fasciitis of the musculoskeletal system. Skeletal Radiol. 2013;42:975-82.
- [CrossRef] [PubMed] [Google Scholar]
- Nodular fasciitis: Correlation of MRI findings and histopathology. Skeletal Radiol. 2002;31:155-61.
- [CrossRef] [PubMed] [Google Scholar]
- Extraabdominal desmoid tumor: A study of 83 Cases. Clin Orthop Relat Res. 2000;375:207-13.
- [CrossRef] [Google Scholar]
- Imaging of intra-and extraabdominal desmoid tumors. Radiographics. 1991;11:959-68.
- [CrossRef] [PubMed] [Google Scholar]
- New concepts in understanding evolution of desmoid tumors: MR imaging of 30 lesions. Eur Radiol. 1997;7:1013-9.
- [CrossRef] [PubMed] [Google Scholar]
- Imaging of musculoskeletal fibromatosis. Radiographics. 2001;21:585-600.
- [CrossRef] [PubMed] [Google Scholar]
- Percutaneous cryoablation of extraabdominal desmoid tumors: A 10-year experience. AJR Am J Roentgenol. 2016;207:190-5.
- [CrossRef] [PubMed] [Google Scholar]
- Soft tissue tumors in adults: ESSR-approved guidelines for diagnostic imaging. Semin Musculoskelet Radiol. 2015;19:475-82.
- [CrossRef] [PubMed] [Google Scholar]
- Statistical analysis of MRI parameters predicting malignancy in 141 soft tissue masses. Röfo. 1992;156:587-91.
- [CrossRef] [PubMed] [Google Scholar]
- MR imaging of soft-tissue masses: Role of gadopentetate dimeglumine. J Magn Reson Imaging. 1994;4:485-90.
- [CrossRef] [PubMed] [Google Scholar]
- Principles of staging of soft-tissue sarcomas. Clin Orthop Relat Res. 1993;289:19-31.
- [CrossRef] [Google Scholar]
- Diffusion-weighted MR imaging in musculoskeletal diseases: Current concepts. Diagn Interv Imaging. 2015;96:327-40.
- [CrossRef] [PubMed] [Google Scholar]
- Soft tissue and visceral sarcomas: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25(Suppl 3):iii102-12.
- [CrossRef] [PubMed] [Google Scholar]
- Territorial inequalities in management and conformity to clinical guidelines for sarcoma patients: An exhaustive population-based cohort analysis in the RhôneAlpes region. Int J Clin Oncol. 2014;19:744-52.
- [CrossRef] [PubMed] [Google Scholar]
- Incidence of sarcoma histotypes and molecular subtypes in a prospective epidemiological study with central pathology review and molecular testing. PLoS One. 2011;6:e20294.
- [CrossRef] [PubMed] [Google Scholar]
- Sarcoma: Concordance between initial diagnosis and centralized expert review in a population-based study within three European regions. Ann Oncol. 2012;23:2442-9.
- [CrossRef] [PubMed] [Google Scholar]






