Translate this page into:
Transcatheter Renal Interventions: A Review of Established and Emerging Procedures
Address for correspondence: Dr. Ron C Gaba, Department of Radiology, Division of Interventional Radiology, University of Illinois Hospital and Health Sciences System, 1740 West Taylor Street, MC 931, Chicago, Illinois - 60612, United States. E-mail: rgaba@uic.edu
-
Received: ,
Accepted: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Catheter-based interventions play an important role in the multidisciplinary management of renal pathology. The array of procedures available to interventional radiologists (IRs) includes established techniques such as angioplasty, stenting, embolization, thrombolysis, and thrombectomy for treatment of renovascular disease, as well as embolization of renal neoplasms and emerging therapies such as transcatheter renal artery sympathectomy for treatment of resistant hypertension. Here, we present an overview of these minimally invasive therapies, with an emphasis on interventional technique and clinical outcomes of the procedure.
Keywords
Angioplasty
catheter
embolization
renal
stenting
INTRODUCTION

Transcatheter interventions play an important role in the treatment of renal pathology. With the recent development of catheter-based renal arterial sympathectomy (i.e. renal denervation) for select patients with resistant hypertension, the number of transcatheter therapies available to interventional radiologists (IRs) continues to grow. This article aims to review established and emerging transcatheter renal interventions, with attention to disease epidemiology and pathophysiology, interventional technique, and clinical outcomes of the procedure.
RENAL ARTERY STENOSIS
Epidemiology and pathophysiology
Hypertension affects approximately 30% of the adult population in the United States. Although hypertension is most often “essential” – idiopathic without an identifiable cause – 3–5% of cases are associated with a renovascular etiology.[1] Renal artery stenosis (RAS) is an important and potentially curable cause of hypertension and progressive renal failure.
RAS is defined as narrowing of the renal artery lumen by ≥50%, expressed as a percentage of the diameter of a normal renal vessel.[1] Although RAS is the result of an abnormal process in the arterial wall, it does not typically achieve hemodynamic significance until the luminal cross-sectional area is reduced by 75% or the vessel diameter is narrowed by more than 50%.[1] The most common etiologies of RAS are atherosclerosis and fibromuscular dysplasia (FMD), both of which reduce perfusion pressure in the affected kidney, thereby activating the renin–angiotensin–aldosterone system and increasing the blood pressure.[2] High levels of angiotensin II are also likely contribute to vascular and ventricular hypertrophy, accelerate atherosclerosis, and cause progressive glomerular sclerosis independent of their hemodynamic effect.[3] Treatment of RAS can reverse this process in select patients with renovascular hypertension. Renal revascularization can also provide benefits other than cure of hypertension; these include reduction of blood pressure with lower doses of medications and improvement of renal function in selected patients with ischemic nephropathy.[14]
Interventional technique
The decision to revascularize an affected kidney should be based on the clinical severity of the RAS and the likelihood that intervention will improve the renovascular hypertension. Other factors to consider include patient's age, renal function, unilateral or bilateral disease, solitary kidney, and ability to withstand procedural complications.[1]
Transcatheter treatment options comprise angioplasty with or without stent placement (drug-eluting or otherwise). Renal artery stents are the preferred treatment for ostial stenosis in arteries with a reference diameter ≥6 mm; they are relatively contraindicated if they traverse renal artery branches, and they have no established role in the primary treatment of FMD.[1]
Figure 1 demonstrates a typical renal artery stent case. Aortography typically precedes renal artery selection for angioplasty and stent placement; this allows visualization of the native, untouched appearance of the renal arteries that may help distinguish a true stenosis from catheter-induced spasm. Selective access is then gained into the renal artery using a reverse curve or cobra-shaped catheter, with performance of dedicated arteriography. A curved sheath advanced into the renal artery may afford more stable access. Lesion traversal with a guide wire is followed by either angioplasty and stent placement or primary stent deployment. Stents are typically deployed to the extent of 1–2 mm into the aortic lumen in order to completely eliminate ostial lesions.
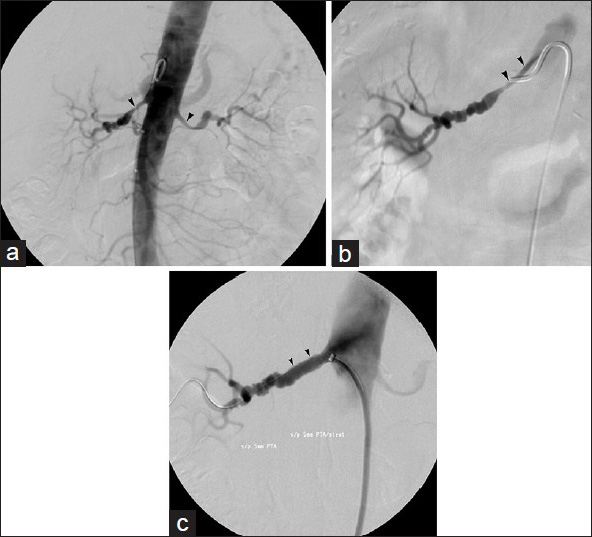
- 78-year-old woman with medically refractory hypertension and renal artery stenosis. (a) Abdominal aortogram exhibits stenosis of proximal renal arteries bilaterally (arrowheads). (b) Right renal arteriogram better delineates stenosis (arrowheads). (c) Final right renal arteriogram performed after primary stent deployment shows marked improvement in the renal artery caliber (arrowheads).
Procedure clinical outcomes
Technically successful intervention is defined by less than 30% residual stenosis at the narrowest point of the vascular lumen and restoration of the pressure gradient to below the selected threshold for intervention.[1] Technical success rates vary based on the chosen intervention. A meta-analysis by Rees reported 99% technical success following stent placement, compared with 55% for ostial and 70% for non-ostial stenoses treated by angioplasty.[5] Clinical success following renal revascularization depends, in part, on the etiology, location, and extent of the underlying stenosis. Only a small percentage of patients with atherosclerotic RAS are reported as cured following treatment. In a recent multicenter randomized controlled trial, Cooper et al., found no difference in the occurrence of adverse renal or cardiovascular events among patients randomized to medical therapy alone or in conjunction with renal artery stenting.[6] Outcomes are more favorable in patients with FMD, however. A meta-analysis by Martin et al., found a mean cure rate of 44% following transcatheter treatment of RAS secondary to FMD (majority with the"medial fibroplastic" type).[1] Davidson et al., reported that younger age, milder hypertension, and shorter duration of hypertension were statistically significant independent variables predicting clinically successful results from angioplasty in patients with FMD.[7]
Complication rates following transcatheter treatment of RAS range from 5 to 15%. Minor access site complications such as hematoma and pseudoaneurysm formation are the most common (3–5%), while uncommon complications include renal artery dissection (5%), renal failure (5%), cholesterol embolization syndrome (1%), need for salvage surgical intervention (<1%), and death (0.5%).[2]
RENAL ARTERY (PSEUDO) ANEURYSM
Epidemiology and pathophysiology
A true renal artery aneurysm (RAA) is defined as an expansion of all layers of the arterial wall, whereas a renal artery pseudoaneurysm (RAP) is an expansion of the renal artery with focal disruption of the arterial wall.[8] RAAs are uncommon, with an incidence of ≤1%. In women with FMD, however, the incidence may approach 10%.[9] Other etiologies of RAAs and/or RAPs include degenerative aneurysms, vasculitis, trauma, and percutaneous renal interventions.[10] RAAs are typically asymptomatic. Symptoms arise from rupture, embolization of the peripheral arterial bed, arterial thrombosis, or renal failure. RAAs are associated with hypertension in up to 73% of cases and hematuria in up to 30% of cases.[1011] The risk of rupture varies with the size and location of the aneurysm. Most are saccular and non-calcified and tend to occur at the bifurcation of the main renal artery.[10] The increasingly common use of cross-sectional imaging such as computed tomography (CT) has led to the detection and treatment of asymptomatic RAAs, while the incidence of iatrogenic RAPs has climbed in recent years due to the widespread use of percutaneous renal interventions.[8]
Interventional technique
Multiple transcatheter techniques have been developed to successfully treat RAAs and RAPs. The goal of treatment is to exclude the aneurysm sac from the circulation while preserving sufficient blood flow to the affected kidney. Accepted indications for intervention include size greater than 2.0 cm, renovascular hypertension, dissection, localized symptoms (e.g. flank pain and/or hematuria), distal embolization, and female patients in childbearing age.[12] Published treatment options include occlusion of inflow and outflow arteries, direct occlusion of the aneurysm sac itself (for lesions with a narrow neck), and exclusion of the aneurysm with a stent graft. Coils are the most commonly employed embolic agent; glue, thrombin, and particles are less commonly used.[812131415] In contrast to visceral vessel embolization – which requires inflow and outflow vessel occlusion during coiling to prevent collateral reconstitution of (pseudo) aneurysms – inflow vessel occlusion is sufficient for renal arteries, which are terminal vessels [Figure 2].
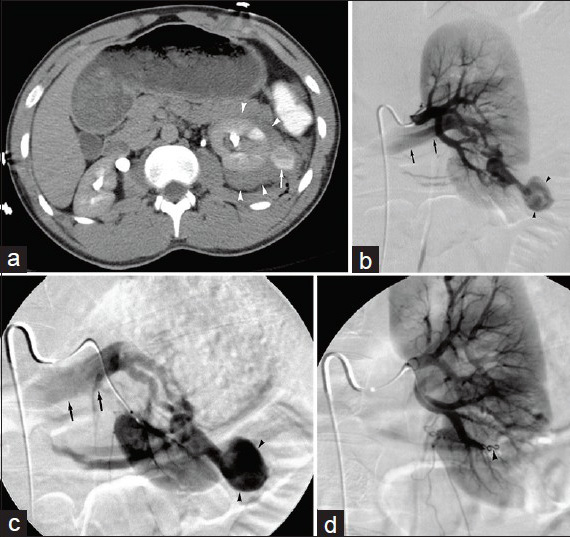
- 40-year-old man suffered penetrating flank trauma resulting in renal artery pseudoaneurysm. (a) Contrast-enhanced axial CT image reveals left peri-renal hematoma (arrowheads) and focal round high attenuation pseudoaneurysm (arrow). (b) Main left renal arteriogram and (c) selective left renal arteriogram demonstrate traumatic renal artery pseudoaneurysm (arrowheads). Note the presence of arteriovenous fistula, with early filling of renal vein (arrows). (d) Completion arteriogram following coil embolization (arrowhead) of feeding artery shows no filling of pseudoaneurysm.
Procedure clinical outcomes
Technical success rates for RAA or pseudoaneurysm embolization range from 94 to 100%.[812131415] In patients with clinical symptoms prior to treatment, clinical success approaches 100%. Overall, the clinical success rates range from 87.5 to 100%.[812131415] Noted complications include intra-procedural aneurysm rupture, distal thromboembolic events, non-target embolization, coil migration, and end-organ infarction.[812131415]
RENAL ARTERIOVENOUS FISTULAS AND MALFORMATIONS
Epidemiology and pathophysiology
Renal arteriovenous fistulas (AVFs) and arteriovenous malformations (AVMs) represent abnormal communications between renal arterial and venous branches. The abnormal communications are classified as acquired, congenital, or idiopathic. Acquired communications or AVFs represent approximately 75% of cases and result from iatrogenic causes (e.g. percutaneous biopsy), trauma, or neoplasm.[16] Congenital renal malformations or AVMs constitute approximately 20% of the total. The remaining 5% represent idiopathic malformations, which show characteristics of AVFs, but without an identifiable cause.
Renal AVFs and AVMs represent distinct pathological entities, which have bearing on technical approach and interventional management. AVFs typically show a focal, abnormal direct communication between a renal artery branch and renal venous channel. In contrast, AVMs classically demonstrate inflow renal arteries supplying a vascular nidus or tangle of vessels, which drain via outflow renal veins.
Acquired renal AVFs – including those found in renal allografts – usually heal spontaneously. However, symptomatic acquired renal AVFs may present with hypertension, hematuria, and renal failure.[17] When sufficiently large, renal AVFs can result in a high cardiac output state and may present with heart failure.[16] Renal AVMs do not typically show spontaneous regression.
Interventional technique
Historically, large, symptomatic renal AVFs were treated with total or partial nephrectomy because of the risk of non-target (pulmonary) embolization associated with endovascular treatment.[16] With current transcatheter techniques, however, endovascular treatment is now preferred in most patients. Reported techniques include embolization with coils (in one case with the use of a constrained stent), stent grafts, detachable balloons, liquid occlusive agents, and silk suture.[1718192021222324] Vascular plug devices may also be used, and afford benefits of large vessel occlusion, precision in deployment, positional stability, and ability to reconstrain. For vessel occlusion using metallic coils, a microcatheter is advanced as close to the arteriovenous communication as possible, where coils are deployed.
The therapeutic approach to renal AVMs may parallel that for renal AVFs – particularly in cases showing a single inflow vessel (cavernous AVM) – with closure of arterial inflow vessels using coils or vascular plug devices [Figure 3]. However, the presence of multiple arterial feeders (cirsoid AVM) may reduce technical success rates and increase the incidence of clinical recurrence, especially compared to renal AVF embolization, due to persistence of arterial inflow. An interventional approach aimed at treating the vascular nidus is thus typically warranted for complex AVM obliteration; this may be achieved using liquid agents such as ethanol or glue, and multiple sequential treatment sessions may be required.
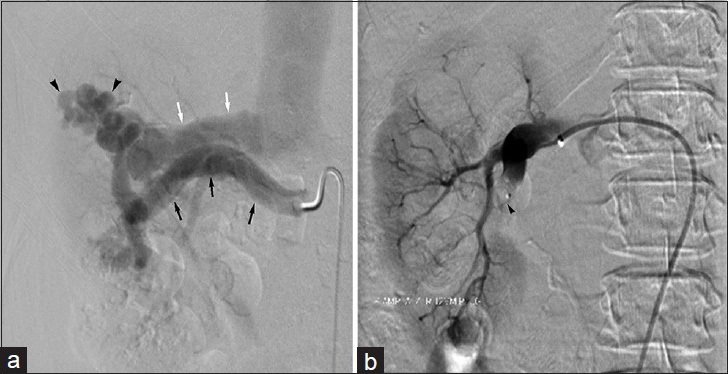
- 60-year-old woman with intermittent right flank pain found to have congenital renal arteriovenous malformation. (a) Right renal arteriogram demonstrates arteriovenous malformation (arrowheads). Note hypertrophy of main renal artery (black arrow) and early renal venous drainage (white arrow). (b) Final right renal arteriogram following vascular plug (arrowhead) embolization displays no residual filling of the renal arteriovenous malformation.
Procedure clinical outcomes
Technical success ranges from 95 to 100%, although these figures are based on case reports and small case series.[1718192021222324] As noted previously, AVMs may be associated with reduced technical success rates compared to AVFs, given the potential for persistent patency due to multiplicity of arterial feeders. Complications following renal AVF or AVM embolization include non-target embolization, coil migration, access site complications, and hematuria.[1718192021222324]
RENAL VEIN THROMBOSIS
Epidemiology and pathophysiology
Renal vein thrombosis (RVT) is a well-known complication of nephrotic syndrome, most commonly membranous glomerulonephritis. Other causes of RVT include extension of thrombus from the inferior vena cava and iliofemoral veins, as well as hypercoagulable states such as antiphospholipid antibody syndrome, factor V Leiden mutation, recent surgery, and malignancy. Acute RVT may also been seen following renal transplantation, either in the immediate postoperative period as a result of technical factors or as a late complication associated with the recurrence of nephrotic syndrome or hypercoaguable states.[25]
Acute RVT is an uncommon condition with a clinical presentation that may vary based on the extent of disease (e.g. unilateral or bilateral RVT, associated renal failure). Clinical manifestations include flank/abdominal pain (73%) and gross hematuria (36%), as well as non-specific symptoms such as nausea/vomiting, anorexia, and fever.[26] The signs and symptoms of RVT may also represent the first clinical manifestations of renal cell carcinoma (RCC) in some patients, while bilateral RVT may present with acute renal failure.
Interventional technique
The standard treatment for RVT remains anticoagulation in eligible patients; however, therapeutic recommendations regarding the use of anticoagulation vary greatly.[26] Transcatheter treatments have also been described with clinical success in patients with symptomatic RVT and associated renal failure.[25] The goal of endovascular treatment in patients with RVT is prompt relief of renal venous obstruction and preservation of renal function.[27] Although anticoagulation may be associated with a survival advantage in patients with RVT,[26] catheter-directed thrombolysis with or without thrombectomy can be a valuable adjunct in select patients with RVT and renal failure. Patients with contraindications to thrombolytic agent infusion are excluded from such procedures; selected contraindications to catheter-directed thrombolysis include previous history of stroke, active bleeding or known bleeding disorder, and recent major surgery or trauma.[28] Reported techniques for endovascular therapy in native and transplanted kidneys complicated by RVT include catheter-directed thrombolysis via arterial, venous, and arteriovenous routes with or without percutaneous thrombectomy devices.[25272930313233] For catheter-directed thrombolysis, a multiple side hole infusion catheter is typically advanced into the affected renal vein. Recombinant tissue plasminogen activator is then infused at a rate approximating 0.5–1.0 mg/h. Systemic intravenous heparin may also be administered to prevent thrombus propagation; subtherapeutic doses are acceptable when used in combination with thrombolytic therapy.[34] Patients are monitored closely during the entirety of thrombolytic therapy, and hematologic parameters as well as fibrinogen levels are checked. The progress of catheter-directed thrombolysis is routinely assessed by daily venography. Thrombolysis is continued until complete or near-complete clot dissolution is achieved with concomitant symptom improvement. Mechanical methods, such as balloon maceration of clot, may also be concomitantly used.
Procedure clinical outcomes
Published technical success rates – though based on individual case reports and small retrospective case series – approach 100% in appropriately selected patients.[25272930313233] Clinical success is defined as the resolution of pre-procedural symptoms and improvement in renal function. Kim et al., reported improvement in mean glomerular filtration rate from 30.8 ± 23.0 to 64.2 ± 52.4 ml/min/1.73 m2 following catheter-directed therapy in seven cases.[25] Weger et al., described a patient with preserved renal function 18 months after successful catheter-directed treatment for bilateral RVT.[27]
Review of the literature revealed no reports of minor or major complications associated with this procedure. However, known complications following catheter-directed thrombolysis in other settings include minor or major bleeding, thromboembolism, and vascular access site complications.[35]
EMBOLIZATION OF RENAL TUMORS
Catheter-directed embolotherapy can play an important role in the management of both benign and malignant renal tumors.
ANGIOMYOLIPOMA
Epidemiology and pathophysiology
Renal angiomyolipomas (AMLs) are benign tumors that account for up to 3% of renal masses. They are composed of varying proportions of fat, smooth muscle, and thick-walled vessels. Most AMLs are sporadic, and approximately one-tenth are associated with the tuberous sclerosis (TS) complex. Sporadic AMLs tend to occur as unilateral, solitary tumors in middle-aged women. AMLs associated with TS are frequently bilateral and multiple. As AMLs enlarge, the tortuous vessels within can form aneurysms that are susceptible to rupture;[36] up to 60% of AMLs larger than 4 cm bleed spontaneously. Symptoms rarely occur in lesions smaller than 4 cm; in contrast, more than 80% of AMLs larger than 4 cm are symptomatic.[37] Clinical manifestations and symptoms include potentially life-threatening hemorrhage, recurrent flank pain, and mass effect.
Interventional technique
Selective renal artery embolization, which spares the normal renal parenchyma, has become an increasingly popular treatment option for patients with AMLs larger than 4 cm. It has been shown to be effective in both preventing hemorrhage and controlling symptoms.[38] Published embolization techniques have utilized ethanol with or without ethiodized oil, coils with or without gelatin sponge, tris-acryl gelatin microspheres, and/or polyvinyl alcohol particles.[36383940] Safe injection of ethanol with or without ethiodized oil may require balloon occlusion of the renal artery to avert non-target distribution of the liquid agent; analogously, simultaneous balloon occlusion of the renal vein may prevent systemic washout of the injected ethanol. Notably, if renal venous balloon occlusion technique is utilized, renal arterial occlusion should always be performed in conjunction, as injection of alcohol without inflow control can significantly increase hemorrhagic complications. Embolization with particles is typically pursued in a selective fashion via a microcatheter positioned in a tumor feeding branch, with care taken to avoid non-target embolization of the injected material [Figure 4].
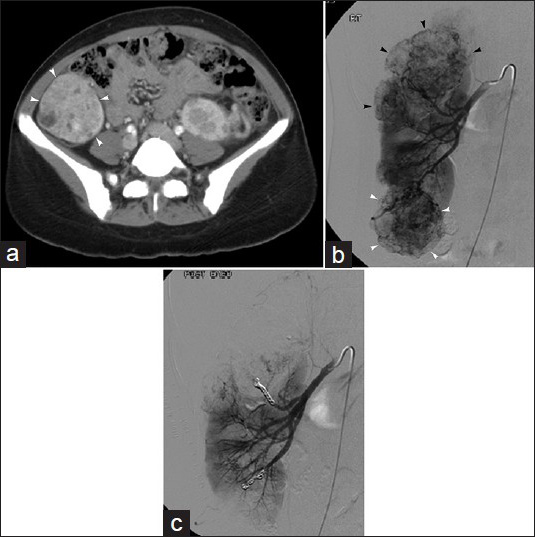
- 32-year-old asymptomatic woman with incidental discovery of renal angiomyolipoma. (a) Contrast-enhanced axial CT image in tuberous sclerosis patient demonstrates 5.8 cm right lower pole renal AML (arrow). (b) Right renal arteriogram displays multiple renal masses (arrowheads), consistent with the patient's known AMLs, including lower pole mass (white arrowheads). (c) Post-embolization right renal arteriogram shows no significant residual flow to embolized renal AMLs; in this case, particles and metallic coils were used.
Procedure clinical outcomes
Technical success rates in case series of selective renal artery embolization for AMLs range from 73 to 100%.[36383940] Clinical success following AML embolization is based on multiple factors. Kothary et al., described tumor recurrence in 32% of patients, all in patients with TS. Repeat embolization was required in six lesions, and the median time to recurrence was 79 months.[38] Lee et al., described tumor recurrence in two (18%) patients with incompletely embolized tumors.[36] Villalta et al., reported technically successful treatment of 72 AMLs, 10 of which required repeat embolization.[39] Chick et al., had a combined clinical and radiological success rate of 85% in 34 treated patients.
Complications reported following AML embolization include post-embolization syndrome – a self-limited constellation of abdominal pain, fever, and leukocytosis commonly seen after visceral organ embolization – as well as renal abscess, access site complications, and acute respiratory distress.[36383940]
RENAL CELL CARCINOMA
Epidemiology and pathophysiology
Renal cell carcinoma (RCC) is the most common renal malignancy, accounting for 3.5% of all malignancies. RCC can remain occult for most of its course; more than 50% of patients with RCC present with locally advanced or metastatic disease.[41] When symptomatic, RCC may present with hematuria, flank pain, retroperitoneal hemorrhage, mass effect, and other constitutional symptoms associated with malignancy.[41]
Interventional technique
Although nephrectomy remains an essential component of the multimodal treatment of RCC, there may also be a role for preoperative renal artery embolization.[42] Reports of preoperative renal artery embolization for RCC have described the use of agents such as ethanol, coils, gelatin sponge, tris-acryl gelatin microspheres, polyvinyl alcohol particles, and various combinations of these embolic agents [Figure 5]; autologous tissue has also been employed. Similar to AMLs, safe injection of liquid sclerosants or embolic particles may require balloon occlusion or selective injection via a microcatheter. Renal artery embolization may also have a limited role in the palliative setting;[43] a recent report described radioembolization with yttrium-90 glass microspheres for palliative treatment of RCC in an 87-year-old woman with a 14.7 × 11.1 cm left renal mass, in which the mass remained stable in size and the patient reported decreased pain and hematuria during the first year following treatment.[44]
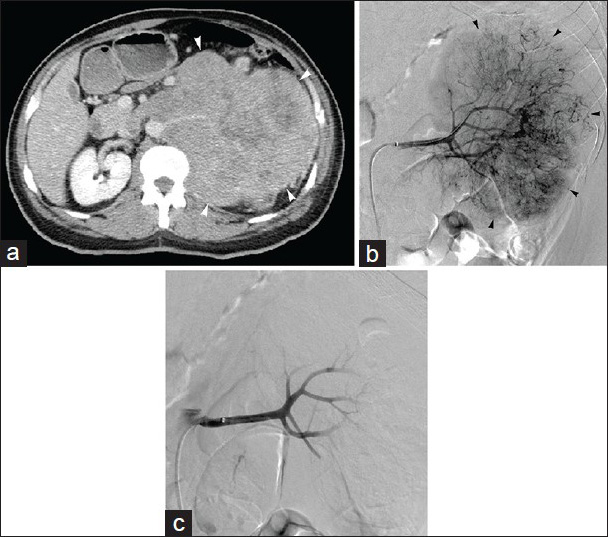
- 48-year-old man with flank pain found to have renal cell carcinoma. (a) Contrast-enhanced axial CT image demonstrates 19 cm left renal mass (arrowheads); preoperative embolization requested prior to resection. (b) Left renal arteriogram displays hypervascular tumor (arrowheads) with abundant neovascularity. (c) Post-embolization left renal arteriogram following particle embolization shows no significant residual flow to tumor, with pruning of distal vasculature; metallic coils placed in distal renal artery after particle devascularization (not shown).
Procedure clinical outcomes
Technical success rates for preoperative renal artery embolization for RCC range from 79 to 100%.[42454647] Many RCCs are hypervascular, and preoperative embolization of large, hypervascular tumors – particularly those with venous extension – may facilitate nephrectomy through decreased operative blood loss, ease of dissection secondary to the development of edema in tissue planes, and decreased operative time.[42454748] However, in one report of RCC patients with inferior vena cava thrombus, the opposite was true.[46] The embolization cohort in this study demonstrated statistically significant increases in perioperative blood requirement, operative time, and postoperative complications; notably, the embolization group had a greater rate of retro-hepatic or supra-diaphragmatic inferior vena cava thrombus extension (67% vs 48%, P = 0.032), which could contribute to the worse clinical outcomes. The impact of preoperative embolization on survival remains unknown, although Zielinski et al., reported a possible benefit in their retrospective series.[47]
The most common complications reported following preoperative renal artery embolization for RCC include post-embolization syndrome, non-target embolization, access site complications, and back pain.[42454647] Despite the increasing popularity of this intervention, however, data regarding its efficacy are limited, warranting further evaluation in large prospective studies.
CATHETER-BASED RENAL ARTERIAL SYMPATHECTOMY
Epidemiology and pathophysiology
The origins of hypertension are complex and multifactorial, but there has been a shift in thinking toward unification under the “neuroadrenergic hypothesis,” which proposes that excess sympathetic tone is fundamental to the development of multiple complex interactions that promote and reinforce the development of hypertension. It follows that excess sympathetic tone is an excellent target for the treatment of hypertension. Transluminal ablation of renal artery sympathetic nerves – which encircle the artery within the adventitia – is one mechanism to address this problem.[2] In patients with resistant hypertension refractory to three or more antihypertensive medications, sympathectomy may represent the last line of therapy.
Interventional technique
A catheter-based method of ablating renal artery sympathetic nerves for blood pressure control has recently been developed as another method of blocking a dysfunctional sympathetic nervous system in patients with resistant hypertension.[49] Catheter-based renal arterial sympathectomy is achieved by transluminal ablation of the renal sympathetic nerves using thermal ablative technology. Currently, a dedicated radiofrequency catheter system (Symplicity; Medtronic, Minneapolis, MN, USA) is available in Europe and Australia and is under investigation in the United States.[50] Additional catheter-based systems have also been used for this procedure,[51] and other thermal technologies (e.g. microwave) may be available in the future.[2]
Procedure clinical outcomes
Technical success of catheter-based renal arterial sympathectomy in the Symplicity (Medtronic) hypertension (HTN)-1 and HTN-2 trials was 98-100%.[4952] Patients in Symplicity HTN-1 trial experienced a significant decrease in systemic blood pressure averaging −20/−10 mm Hg from their baseline average of 177/101 mm Hg.[49] Follow-up from the same investigators in a larger cohort suggested durable results out to 24 months.[53] The renal denervation group in the Symplicity HTN-2 randomized controlled trial experienced a significant decrease in systemic blood pressure of −32/−12 mm Hg from their baseline of 178/96 mm Hg after 6 months.[52] After 12 months, this blood pressure reduction remained similar.[54] Reported complications of this procedure include renal artery dissection, access site complications (e.g. femoral artery pseudoaneurysm), post-procedural hypotension, and transient intra-procedural bradycardia.[495255]
Although the results of renal denervation have been promising in early trials, the procedure has not been shown to obviate the need for continued pharmacologic management in patients,[2] and the recent failure of the Symplicity HTN-3 trial to meet its primary efficacy endpoint may temper enthusiasm for this approach.[56] Nevertheless, renal artery denervation represents a promising development in the management of hypertension, and the indications for catheter-based arterial sympathectomy may eventually extend beyond resistant hypertension to include patients with obesity, diabetes, and dyslipidemia.[2]
CONCLUSION
There are an increasing number of catheter-based interventions available to IRs for the treatment of a wide variety of kidney diseases, including renal vascular pathologies, renovascular and essential hypertension, and renal neoplasms. Familiarity with disease epidemiology, interventional procedures aimed at managing these conditions, and therapeutic outcomes will help practicing IRs appropriately treat patients with renal ailments.
Available FREE in open access from: http://www.clinicalimagingscience.org/text.asp?2015/5/1/5/150448
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- Society of Interventional Radiology (SIR). Quality improvement guidelines for angiography, angioplasty, and stent placement for the diagnosis and treatment of renal artery stenosis in adults. J Vasc Interv Radiol. 2010;21:421-30.
- [Google Scholar]
- Catheter-based arterial sympathectomy: Hypertension and beyond. J Vasc Interv Radiol. 2012;23:1125-34.
- [Google Scholar]
- Management of renovascular disease: A review of renal artery stenting in ten studies. QJM. 1999;92:159-67.
- [Google Scholar]
- Stents for atherosclerotic renovascular disease. J Vasc Interv Radiol. 1999;10:689-705.
- [Google Scholar]
- CORAL Investigators. Stenting and medical therapy for atherosclerotic renal-artery stenosis. N Engl J Med. 2014;370:13-22.
- [Google Scholar]
- Predictors of cure of hypertension in fibromuscular renovascular disease. Am J Kidney Dis. 1996;28:334-8.
- [Google Scholar]
- Endovascular treatment of visceral and renal artery aneurysms. J Vasc Interv Radiol. 2011;22:1246-53.
- [Google Scholar]
- Renal artery aneurysm and arteriovenous fistula associated with fibromuscular dysplasia: Successful treatment with detachable coils. J Vasc Interv Radiol. 2009;20:1083-6.
- [Google Scholar]
- Visceral and renal artery aneurysms: A pictorial essay on endovascular therapy. Radiographics. 2006;26:1687-704.
- [Google Scholar]
- Renal artery aneurysms: A 35-year clinical experience with 252 aneurysms in 168 patients. Ann Surg. 2001;234:454-63.
- [Google Scholar]
- Therapy of renal artery aneurysms in New York State: Outcomes of patients undergoing open and endovascular repair. Ann Vasc Surg. 2009;23:194-200.
- [Google Scholar]
- Endovascular treatment of renal artery aneurysms and renal arteriovenous fistulas. J Vasc Surg. 2013;57:765-70.
- [Google Scholar]
- Endovascular treatment of renal aneurysms: A series of 18 cases. Eur J Radiol. 2012;81:3973-8.
- [Google Scholar]
- Endovascular treatment of eight renal artery aneurysms. Acta Radiol. 2012;53:430-4.
- [Google Scholar]
- Staged endovascular occlusion of giant idiopathic renal arteriovenous fistula with platinum microcoils and silk suture threads. J Vasc Interv Radiol. 2002;13:747-52.
- [Google Scholar]
- Post-biopsy arteriovenous fistula in transplant kidney: Treatment with superselective transcatheter embolisation. Eur J Radiol. 2012;81:e721-6.
- [Google Scholar]
- Transcatheter embolization of biopsy-related vascular injury in the transplant kidney: Immediate and long-term outcome. J Vasc Interv Radiol. 1998;9:1011-9.
- [Google Scholar]
- Transcatheter embolization of a high-flow renal arteriovenous fistula with use of a constrained wallstent to prevent coil migration. J Vasc Interv Radiol. 2006;17:363-7.
- [Google Scholar]
- The endovascular treatment of a renal arteriovenous fistula: Placement of a covered stent. J Vasc Surg. 2002;36:1066-8.
- [Google Scholar]
- Dual use of an amplatzer device in the transcatheter embolization of a large high-flow renal arteriovenous fistula. J Vasc Interv Radiol. 2007;18:671-6.
- [Google Scholar]
- High-flow renal arteriovenous fistula treated with the Amplatzer vascular plug: Implementation of an arterial and venous approach. Cardiovasc Intervent Radiol. 2009;32:543-7.
- [Google Scholar]
- Transcatheter embolization of a renal arteriovenous fistula complicated by an aneurysm of the feeding renal artery. Cardiovasc Intervent Radiol. 2008;31:415-7.
- [Google Scholar]
- Renal arteriovenous fistula with rapid blood flow successfully treated by transcatheter arterial embolization: Application of interlocking detachable coil as coil anchor. Cardiovasc Intervent Radiol. 2004;27:374-6.
- [Google Scholar]
- Catheter-directed thrombectomy and thrombolysis for acute renal vein thrombosis. J Vasc Interv Radiol. 2006;17:815-22.
- [Google Scholar]
- Clinical characteristics and long-term follow-up of patients with renal vein thrombosis. Am J Kidney Dis. 2008;51:224-32.
- [Google Scholar]
- Bilateral renal vein thrombosis secondary to membraneous glomerulonephritis: Successful treatment with thrombolytic therapy. Ann Vasc Surg. 2006;20:411-4.
- [Google Scholar]
- Thrombolytic therapy in thrombosis: A National Institutes of Health consensus development conference. Ann Intern Med. 1980;93:141-4.
- [Google Scholar]
- Successful treatment of acute transplant renal vein thrombosis with selective streptokinase infusion. Transplant Proc. 1991;23:2297-300.
- [Google Scholar]
- Selective low-dose streptokinase infusion in the treatment of acute transplant renal vein thrombosis. Cardiovasc Intervent Radiol. 1986;9:86-9.
- [Google Scholar]
- Acute renal vein thrombosis: Successful treatment with intraarterial urokinase. Radiology. 1988;169:681-2.
- [Google Scholar]
- Renal vein thrombolysis with selective simultaneous renal artery and renal vein infusions. J Vasc Interv Radiol. 1995;6:581-4.
- [Google Scholar]
- Successful thrombolytic therapy in two patients with renal vein thrombosis. Am J Med. 1984;77:1111-4.
- [Google Scholar]
- CIRSE and SIR Standards of Practice Committees. Quality improvement guidelines for percutaneous management of acute lower-extremity ischemia. J Vasc Interv Radiol. 2013;24:3-15.
- [Google Scholar]
- Rationale and design of the ATTRACT Study: A multicenter randomized trial to evaluate pharmacomechanical catheter-directed thrombolysis for the prevention of postthrombotic syndrome in patients with proximal deep vein thrombosis. Am Heart J. 2013;165:523-30. e3
- [Google Scholar]
- Embolization of renal angiomyolipomas: Short-term and long-term outcomes, complications, and tumor shrinkage. Cardiovasc Intervent Radiol. 2009;32:1171-8.
- [Google Scholar]
- Renal angiomyolipoma: Long-term results after arterial embolization. J Vasc Interv Radiol. 2005;16:45-50.
- [Google Scholar]
- Selective arterial embolization of angiomyolipomas: A comparison of smaller and larger embolic agents. J Urol. 2011;186:921-7.
- [Google Scholar]
- Long-term follow-up of the treatment of renal angiomyolipomas after selective arterial embolization with alcohol. BJU Int. 2010;105:390-4.
- [Google Scholar]
- Value of preoperative renal artery embolization in reducing blood transfusion requirements during nephrectomy for renal cell carcinoma. J Vasc Interv Radiol. 1993;4:727-31.
- [Google Scholar]
- Preoperative renal tumor embolization. A useful procedure? Acta Radiol. 1987;28:303-6.
- [Google Scholar]
- Radioembolization of renal cell carcinoma using yttrium-90 microspheres. J Vasc Interv Radiol. 2013;24:298-300.
- [Google Scholar]
- Renal artery embolization: Clinical indications and experience from over 100 cases. BJU Int. 2007;99:881-6.
- [Google Scholar]
- Utility of preoperative renal artery embolization for management of renal tumors with inferior vena caval thrombi. Urology. 2009;74:154-9.
- [Google Scholar]
- Comparison of preoperative embolization followed by radical nephrectomy with radical nephrectomy alone for renal cell carcinoma. Am J Clin Oncol. 2000;23:6-12.
- [Google Scholar]
- Preoperative angioinfarction of localized renal cell carcinoma using absolute ethanol. J Urol. 1985;133:21-4.
- [Google Scholar]
- Catheter-based renal sympathetic denervation for resistant hypertension: A multicentre safety and proof-of-principle cohort study. Lancet. 2009;373:1275-81.
- [Google Scholar]
- Catheter-based renal denervation for resistant hypertension: Rationale and design of the SYMPLICITY HTN-3 Trial. Clin Cardiol. 2012;35:528-35.
- [Google Scholar]
- Renal sympathetic denervation for treatment of resistant hypertension: A systematic review. J Clin Hypertens (Greenwich). 2013;15:75-84.
- [Google Scholar]
- Symplicity HTN-2 Investigators. Renal sympathetic denervation in patients with treatment-resistant hypertension (The Symplicity HTN-2 Trial): A randomised controlled trial. Lancet. 2010;376:1903-9.
- [Google Scholar]
- Symplicity HTN-1 Investigators. Catheter-based renal sympathetic denervation for resistant hypertension: Durability of blood pressure reduction out to 24 months. Hypertension. 2011;57:911-7.
- [Google Scholar]
- Symplicity HTN-2 Investigators. Renal sympathetic denervation for treatment of drug-resistant hypertension: One-year results from the Symplicity HTN-2 randomized, controlled trial. Circulation. 2012;126:2976-82.
- [Google Scholar]
- Medtronic. Medtronic Announces U.S. Renal Denervation Pivotal Trial Fails to Meet Primary Efficacy Endpoint While Meeting Primary Safety Endpoint. 2014
- [Google Scholar]






