Translate this page into:
The many faces of cryptogenic organizing pneumonia (COP)

*Corresponding Author: Christopher Kloth, Department of Diagnostic and Interventional Radiology, University Hospital Ulm, Ulm, Germany. C.Kloth@gmx.de
-
Received: ,
Accepted: ,
How to cite this article: Kloth C, Thaiss WM, Beer M, Bösmüller H, Baumgartner K, Fritz J, et al. The many faces of cryptogenic organizing pneumonia (COP). J Clin Imaging Sci 2022;12:29.
Abstract
Organizing pneumonia (OP) is interstitial pneumonia with an acute or subacute clinical course and a histological pattern compatible with acute lung injury. It usually arises after a recent infection of the peripheral bronchial system, whereby is called secondary OP. However, often OP arises with no recognizable cause for which it is called cryptogenic organizing pneumonia (COP). From a radiological standpoint, COP exhibits different imaging appearances and this review makes radiologists and clinicians familiar with the entire spectrum of expected disease findings. Successful interpretation of imaging is pertinent for a rapid diagnosis of COP and knowledge of the entire spectrum of imaging findings in COP is mandatory.
Keywords
Organizing pneumonia
Interstitial pneumonia
Computed tomography
INTRODUCTION
Organizing pneumonia (OP) is interstitial pneumonia with an acute or subacute clinical course and a histological pattern compatible with acute lung injury. It usually arises after a recent infection of the peripheral bronchial system, whereby is called secondary OP. When it arises with no recognizable cause, it is called cryptogenic organizing pneumonia (COP). Until the year 2000, the name idiopathic bronchiolitis obliterans with organizing pneumonia (BOOP) was used synonymously.[1] For a better differentiation from the bronchiolitis obliterans syndrome, which is a known, frequent, pulmonary complication of allogeneic stem cell transplantation sometimes accompanying or preceding BOOP, the term COP was preferred in part because this pathology more commonly occurs in other clinical settings. For practical purposes, differentiation between primary COP without specific etiology and secondary OP is taken. The latter may be medically induced, infectious, or associated with vasculitis or malignancy.[2,3] An overview is given in Table 1.
| Classification of organizing pneumonia (OP) based on aetiologies |
|---|
| Primary cryptogenic organizing pneumonia (COP) • without specific etiology. |
| Secondary organizing pneumonia • association with bacterial infections • association with virus (HIV, influenza) • association with noxes (cigarettes, ammoniac, radiation). |
| Connective tissue disease-related organizing pneumonia • association with rheumatoid arthritis, dermatomyositis. |
| Association with other pulmonary diseases, e.g., lung cancer |
| IIP with OP DAD- or OP-pattern in case of a acute UIP |
OP: Organizing pneumonia, COP: Cryptogenic organizing pneumonia, HIV: Human immunodeficiency virus, IIP: Idiopathic interstitial pneumonia, DAD: Diffuse alveolar damage, UIP: Usual interstitial pneumonia
From a radiological standpoint, COP exhibits different imaging appearances and this review makes radiologists cognizant of the entire spectrum of expected disease findings.
Imaging features
High-resolution computed tomography (HRCT) shows a variety of changes in the COP. Typically, the COP is sharply delineated from the surrounding parenchyma with a lobular pattern next to the bronchovascular structures. This is an important imaging clue to differentiate it from other infectious processes, e.g., bronchopneumonia, which shows a fluctuating or blurred border surrounding normal lung parenchyma. In COP, usually, no signs of parenchymal destruction are found. Bi-pulmonary, spot-shaped infiltrates with rounded or flat consolidations and accompanying ground-glass opacities (GGO) are common [Figures 1 and 2]. Parenchymal consolidation is the most frequent imaging finding (80-95%), often accompanied by an air bronchogram [Figure 3].[3] Parenchymal consolidations most commonly change their extent and localization throughout the course of the disease. In the distribution, the peripheral and basal regions are frequently involved, especially along with the bronchial vascular bundles with lobular patterns [Figure 4]. Lower lung lobes predominance helps to differentiate COP from chronic eosinophilic pneumonia, which is usually encountered in the upper lung zones.
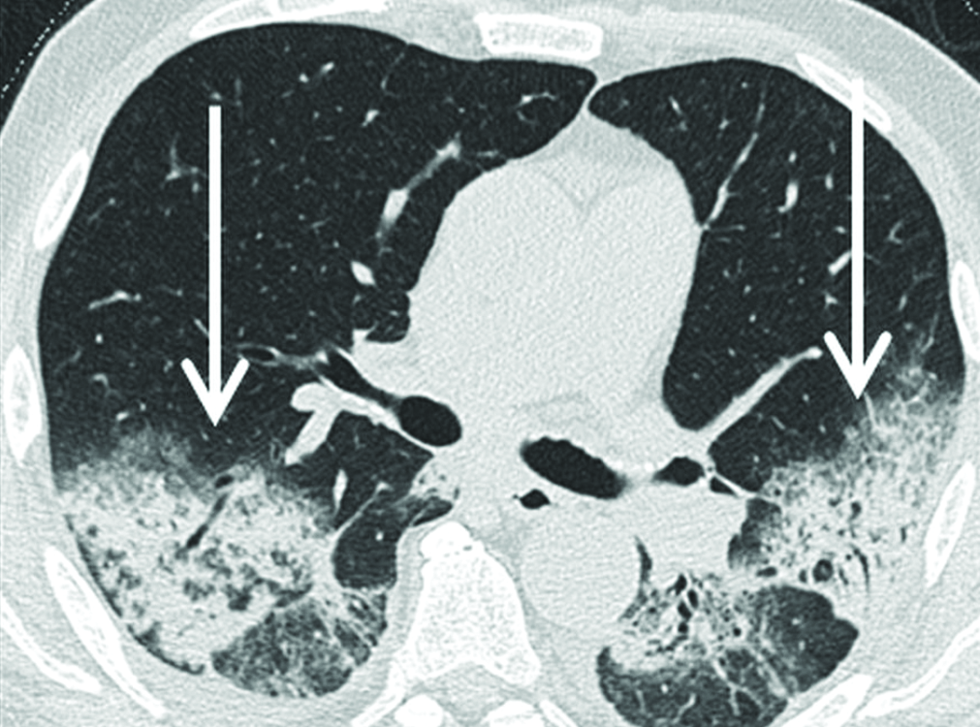
- A 79-year-old male patient with COP showing large areas of symmetrical parenchymal consolidation in both lower lobes with associated ground glass opacities (arrows) and already detectable bronchiectasis in high resolution computed tomography.

- A 58-year-old woman with COP shows spotty bilateral ground glass opacity (GGO) areas (arrows) as well as focal pulmonary parenchymal consolidation in high resolution computed tomography. The apical location of these COP-related changes is not typical and therefore difficult to differentiate from lobular pneumonia. Halo sign can be seen (asterix), which is defined as a ground glass opacity surrounding a pulmonary nodule or mass. Ground glass opacities represents hemorrhages around the central nodule.

- A 56-year-old patient shows large nodular COP (arrow) in the middle lobe in high resolution computed tomography. In the ipsilateral lower lobe, close to the costal pleura, another, smaller nodular parenchymal consolidations can be seen. These findings cannot be reliably differentiated from tumor, vasculitis or focal infection.
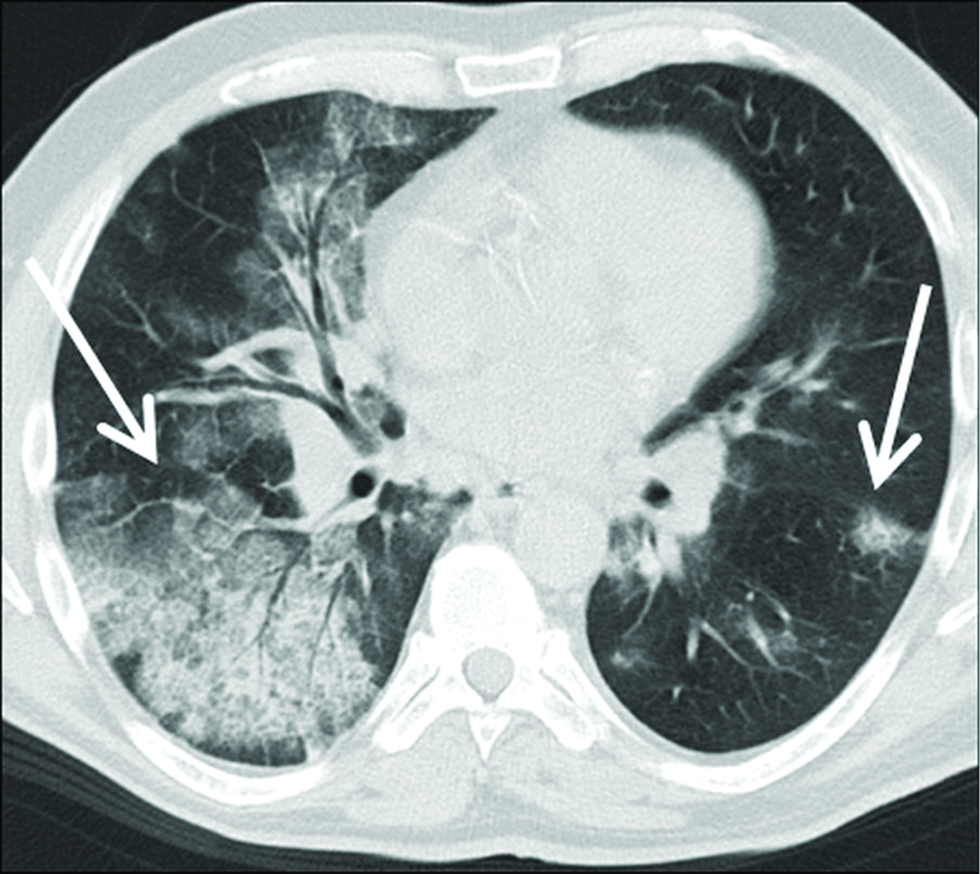
- A 56-year-old patient with COP extensive areas of asymmetrically distributed areas of GGO, crazy paving and in part parenchymal consolidation with air bronchogram (arrows) are seen on axial chest-HRCT images. Of note, these lung changes showed very little dynamic over time.
Other common imaging findings include ground-glass opacity occurring in 60-90% and reflecting alveolar inflammation followed by perilobular opacities (55%) [Figure 5].[3]

- Extensive, bilateral but asymmetrical crazy-paving pattern of both upper lobes (arrow). The subpleural parenchyma regions are speared, which is frequent in COP.
The perilobular region is defined by localization in the lobule’s periphery, i.e., in neighboring the interlobular septa. Differentiation toward an interlobular septal thickening can occasionally be difficult. The perilobular pattern exists of bowed or polygonal opacities.
Margins of these were poorly defined around the interlobular septa. An abutment of the pleural surface is normal. Perilobular pattern regularly accompanied by consolidations and/or GGO in the same lung zone. The ring-shaped appearance is usually groundbreaking here, but this sign can only be found in about 20% of cases with COP [Figure 6]. In addition, bronchial wall thickening and pleural effusions are found in up to 20% of cases, but they are nonspecific [Figure 7]. If there is the initial evidence of a reticular pattern, a poorer response to steroids and a possible change in pulmonary fibrosis can be expected.[4] The tendency to fibrosis is often associated with consolidation as a primary manifestation of the disease.[3]

- A 74-year-old patient with focal consolidation in the left upper lobe (arrow) located directly beneath the pleura pathologically diagnosed as COP.
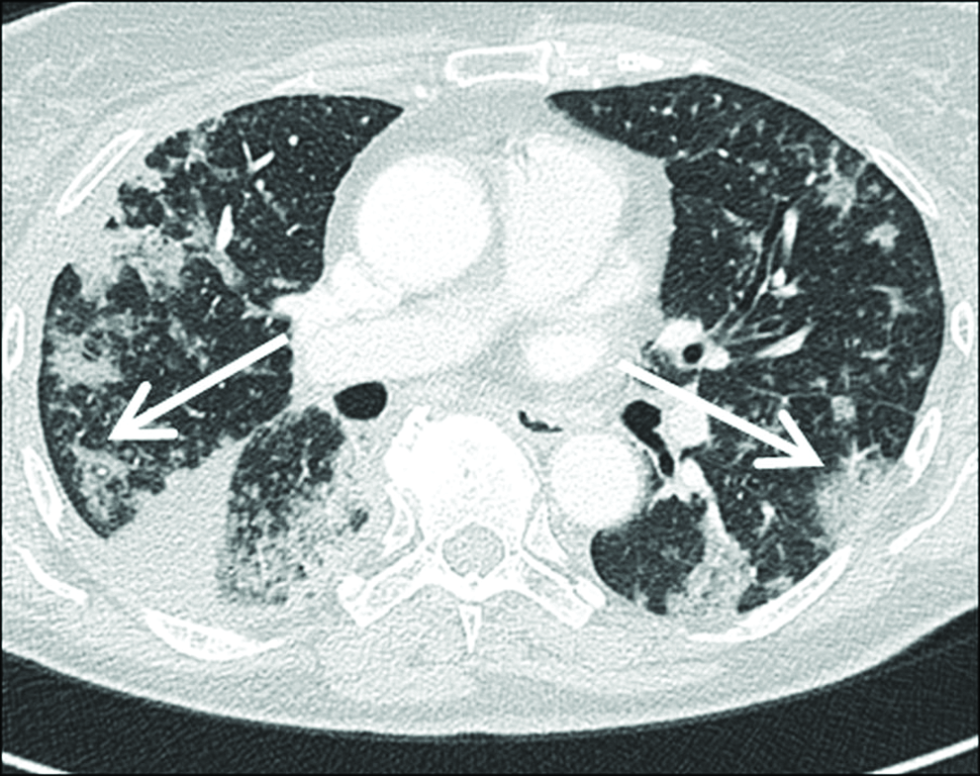
- A 80-year-old patient with confirmed COP with multiple areas of parenchymal consolidation (arrows). Fine nodules could also suggest the presence of sarcoidosis (sarcoid galaxy sign). The peripheral location suggests also the diagnosis of chronic eosinophilic pneumonia (inversed lung edema sign). A small amount of pleural effusion is encapsulated basally on the right side (asterix).
Radial bands of consolidation extending toward the pleura can be observed in COP.[5] A characteristic of parenchymal involvement comprises a migratory appearance with partly disappearing and partly newly appearing consolidations affecting different areas of the lungs. This wax and wane phenomenon suggests this diagnosis and reduces the list of differentials.[5] Other etiologies experiencing also a migratory appearance are eosinophilic pneumonia, alveolar hemorrhages and vasculitis-related lung involvement.
According to the literature, the symmetrical bilateral pattern of involvement is the most common, but one-sided manifestations have also been described [Figures 8-15].[6] Another imaging finding is the inverted halo sign. This is a ground-glass opacity area surrounded by a peripheral consolidation (min. 2 mm in thickness), which has been described also in COP, as well as in infectious pneumonia (e.g., tuberculosis, mycoses), sarcoidosis known synonymously also under the name “atoll sign” [Figures 2].[6] In some cases, solitary alveolar opacities or even multiple pulmonary nodules are the predominant patterns, however less common [Figures 12]. With multiple pulmonary nodules, the diameter is frequently over 5 mm diameter of each nodule without zonal predominance.

- A 30-year-old male patient with COP with subpleural, posterobasal areas of parenchymal consolidation (arrows). Note pronounced parenchymal retraction symptoms with volume reduction of affected lung areas and even areas of calcification. There is subpleural honey-combing suggesting fibrosis.

- A 67-year-old patient with confirmed COP presenting with asymmetric bilateral involvement pattern. Note, extensive ground glass opacities and parenchymal consolidations (arrows) explaining the poor respiratory function.

- Focal lung parenchyma consolidation with marginal ground glass in the case of acute stage COP (arrows). Both pneumonia and tumor are potential differentials.

- Typical appeareance with spotty alveolar opacities and reticular-linear interstitial bilateral opacies (arrow).

- Imaging demostrates perbronchial consolidation of the lower lobes with tree-in-bud by intrabronchiolar location (arrow).

- Asymmetric, left-sided confirmed COP presenting with patchy and linear peribronchial involvement pattern (arrow).

- Coronary CT images of a female patient with multiple smaller nodular parenchymal consolidations in the left lower lobe (arrow). It reflects COP of the nodular type. Differentiation from lobular pneumonia (bronchopneumonia) or vasculitis is challenging in this given case. Fine nodular appearance and grpound-glass-opacities were missing.

- A 55-year-old woman with multifocal appearance of COP in with parenchymal consolidation, volume reduction (right), and marginal ground glass opacity (arrows). Differential diagnosis includes MALT lymphomas as well as other lymphomas as well as bronchoalveolar carcinomas or nodular amyloidosis.
Differential diagnosis
COP typically affects people around 50-60 years, women and men equally, although smokers are more likely to be affected.[6,7] It presents with nonspecific symptoms similar to a flu infection. The development is usually subacute protracted for several weeks without improvement under antibiotics administered. Dyspnea, mild fever, and unproductive cough are commonly observed.
The diagnosis is made by the triad of suggestive imaging findings, the clinical picture compatible with pulmonary infection, but not responding to antibiotics, and the characteristic histological changes of organizing pneumonia. If possible, other causes of the condition must be excluded. Laboratory tests show mostly an increase in C-reactive protein (CRP) and leukocytes. The pulmonary function tests disclose a mild to moderate restrictive ventilation disorder with reduced diffusion capacity.[6] A bronchoalveolar lavage (BAL), a mixed-cell picture, comprising lymphocytosis with a reduced CD4/CD8 ratio is usually found.[8] The gold standard in the diagnosis is histological analysis obtained from transbronchial cryobiopsy or percutaneous CT-guided lung biopsy. In many cases, the diagnosis is set noninvasively, guided by the clinical findings, the evolution of imaging findings, and in particular by symptom improvement under steroids. The most important differential diagnosis is nonspecific interstitial pneumonia (NSIP) with overlapping OP. This pattern was mostly discovered in Sjogren syndrome, polymyositis/dermatomyositis, and other rheumatic diseases. Further relevant differential diagnoses are eosinophilic pneumonia and alveolar hemorrhages. In the case of prominent patchy consolidation areas, chronic eosinophilic pneumonia and other diseases of the vasculitic group (granulomatosis with polyangiitis, eosinophilic granulomatosis with polyangiitis) must also be considered. In the primary consolidating form, bronchial carcinoma and pulmonary lymphoma can also mimic COP.
A special form is the acute fibrinous organizing pneumonia (AFOP).[3] Fibrin balls, Masson bodies, as well as other inflammatory cells characterize histologically this in the alveoli, are visible.[3]
Histology
Pathologically, COP comprises buds of granulation tissue in the peripheral alveoli and bronchioles [Figure 16]. The buds of granulation tissue and inflammatory debris are also known as characteristic “Masson Bodies.”[5] They consist of exudate, fibrin, and fibroblasts. There is damage to the alveolar epithelium with impaired permeability and consecutive transgression of plasma proteins and inflammatory cells.[9] Activation of the coagulation cascade leads to fibrin deposits. A chronic alveolar wall inflammation is shown [Figure 17]. These changes are localized along the respiratory bronchioles and peribronchial accompanied only weakly by moderate interstitial inflammatory cell infiltrates consisting of lymphocytes and plasma cells. There is mild interstitial inflammation with the deposition of mononuclear cells. With time, fibroblasts and myofibroblasts occur, however, the natural structure of the alveolar spaces remains preserved.

- Hematoxylin-eosin staining of a lung biopsy sample (×100 magnification): Poke-like mesenchymal proliferates with displacement of the preexisting alveolar architecture are found reflecting granulation tissue in the peripheral alveoli and bronchioles. Note, dilated bronchioles in the right part of the picture.

- Hematoxylin-eosin staining of a lung biopsy with a magnification of 200: Typical foam cell foci reflecting interstitial inflammation.
An intact basal membrane of the alveolar epithelium is held responsible for complete reversibility[9]. These can be associated with an interstitial inflammatory infiltrate, which is the reason why COP is classified as interstitial lung disease.[5] In the differential diagnosis, the organizing form of the “diffuse alveolar damage”(DAD) and the usual interstitial pneumonia (UIP) must be considered. Fibroblast proliferates can also be found here, but they are prescribed intraseptally and not intra-alveolarly.[10]
Therapy/prognosis
The classic therapy of COP is immunosuppression, mostly using steroids. This leads to a rapid resolution of the symptoms and regression of the imaging findings. Adaptation of the cortisol dose to the severity of the symptoms, as well as a tapering of the cortisone dosage, are recommended. The medication should be continued after the symptom-free period after the immediate decay. The recurrence rate varies in the literature and is described between 10 to 30% of cases, usually within three months after the start of steroid therapy.[2,7] Saito et al. have reported that cases accompanied by traction bronchiectasis had a higher risk for recurrence.[2] In the absence of response, supplemental immunosuppressive therapy may be showed.
Although COP is a disease generally associated with a good prognosis, most patients with COP present with fibrotic sequelae at follow-up CT. A greater extent of consolidation (>10% of total lung area), as well as the presence of bronchiectasis on initial chest CT, were associated with a higher risk of disease relapse.
Moreover, they have also shown that connective tissue disease-related OP presents with a greater burden of lung infiltrations on initial chest CT.
CONCLUSION
Successful interpretation of imaging is pertinent for a rapid diagnosis of COP, according to this knowledge of the entire spectrum of imaging findings in COP is mandatory.
Declaration of patient consent
Patient’s consent is not required as patient’s identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflict of interest
There are no conflicts of interest.
References
- American Thoracic Society-European Respiratory Society Classification of the Idiopathic Interstitial Pneumonias: Advances in Knowledge since 2002. Radiographics. 2015;35:1849-71.
- [CrossRef] [PubMed] [Google Scholar]
- Predictive factors for relapse of cryptogenic organizing pneumonia. BMC Pulm Med. 2019;19:10.
- [CrossRef] [PubMed] [Google Scholar]
- Radio-pathological correlation of organizing pneumonia (OP): A pictorial review. Br J Radiol. 2017;90:20160723.
- [CrossRef] [PubMed] [Google Scholar]
- Idiopathic bronchiolitis obliterans organizing pneumonia. Definition of characteristic clinical profiles in a series of 16 patients. Chest. 1989;96:999-1004.
- [CrossRef] [PubMed] [Google Scholar]
- Oranizing pneumonia: What is it? A conceptual approach and pictorial review. Diagn Interv Imaging. 2014;95:771-7.
- [CrossRef] [PubMed] [Google Scholar]
- ’Crazy-paving’ pattern: An exceptional presentation of cryptogenic organising pneumonia associated with chronic obstructive pulmonary disease. BMJ Case Rep. 2016;2016:bcr2016215445.
- [CrossRef] [PubMed] [Google Scholar]
- Cryptogenic organizing pneumonia. Characteristics of relapses in a series of 48 patients. The Groupe d’Etudes et de Recherche sur les Maladles “Orphelines” Pulmonaires (GERM”O”P) Am J Respir Crit Care Med. 2000;162:571-77.
- [CrossRef] [PubMed] [Google Scholar]
- Intra-alveolar fibrosis of idiopathic bronchiolitis obliterans-organizing pneumonia. Cell-matrix patterns. Am J Pathol. 1990;137:155-70.
- [PubMed] [Google Scholar]
- Kryptogen organisierende Pneumonie versus sekundäre organisierende Pneumonie. Pathologe. 2021;42:55-63.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]






