Translate this page into:
Role of Endovascular Treatment in Pediatric Cerebral Aneurysms: A Series of Two Case Reports
Address for correspondence: Dr. Anand Alurkar, Department of Neurointervetion, King Edward Memorial Hospital, Pune-411011, India. E-mail: anandalurkar@gmail.com
-
Received: ,
Accepted: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Aneurysms in the pediatric age group are rare and have preponderance for the posterior circulation. These aneurysms are more commonly large, giant, and complex. We present two case reports of saccular aneurysms in pediatric patients who were treated successfully by endovascular technique.
Keywords
Aneurysms
coiling
endovascular
INTRODUCTION

Pediatric cerebral aneurysms are rare. According to a recent review by Rao et al.,[1] these aneurysms constitute 2.9% of all cerebral aneurysms. Incidence of these aneurysms in pediatric patients is more in the posterior circulation than in the anterior, when compared to the aneurysms in adults.[2] They tend to be large and more complex-shaped. A male preponderance is seen in the pediatric group.[3] Lasjunias et al.,[3] in a report on 20 pediatric aneurysm cases, suggested that these aneurysms must be the expression of various vessel wall dysfunctions, producing transient or permanent failure to repair a partial insult. However, the etiopathogenesis of these aneurysms still remains unclear. We present two case reports of non-giant aneurysms of the anterior circulation in the pediatric group, treated successfully by endovascular technique at our institute.
CASE REPORTS
Case 1
An 8-year-old female child presented with a complaint of persistent headache for 7 days (Grade I sub-arachnoid hemorrhage (SAH) (Hunt and Hess scale)). On clinical examination, there was no neurological deficit. Computerized tomography (CT) scan revealed Fischer Grade-3 SAH in the prepontine cistern and right sylvian fissure. Pre-procedure routine detailed work was done along with special investigations including antineutrophilic antibody (ANA), antineutrophilic cytoplasmic antibody (ANCA), and antiphospholipid antibody (APLA). Detailed screening and imaging were done to rule out any associated conditions, such as Autosomal dominant polycystic kidney disease (ADPKD), fibro muscular dysplasia, co-arctation of aorta, Ehlers syndrome, and Marfans syndrome.
Digital Subtraction Angiography (DSA) showed the presence of A1saccular aneurysm on the right side [Figure 1 ab]. Endovascular treatment was planned after analyzing the morphology of the aneurysm and the flow hemodynamics of the aneurysm. The treatment was decided after discussion with the neurosurgeon and neurointensivist. High-risk informed consent was obtained from the parents after detailed explanation of the risks versus benefits of the treatment procedure.

- Case 1: (a) Computerized tomography (CT) scan shows subarachnoid hemorrhage (SAH) (arrow) in the prepontine and sylvian fissures and (b) pre-procedure digital subtraction angiogram reveals saccular A1 aneurysm (arrow) on the right side
Under general anesthesia, right 5F common femoral was accessed. Standard heparinization (100 units/kg as bolus at the beginning of the procedure followed by 20 units/kg every hour) was given to maintain the activated clotting time of 250 s. Guiding catheter (5F Envoy, cordis) with Nimodipine infusion was placed in the Internal Carotid Artery (ICA). Microcatheter (Echelon-10, eV3) over microwire (Expedion 14, eV3) was navigated into the sac of the aneurysm [Figure 1 c, d, and e]. The aneurysm was densely packed with platinum coils. Post-procedure angiogram showed near total exclusion of the aneurysm from the circulation and excellent filling of all branches of the right ICA, anterior cerebral artery, and middle cerebral artery. Postprocedure, the patient was extubated in the cathlab and kept under observation for 3 days in the neurointensive care unit. There were no intra-, peri-, or post-procedural complications and the child was discharged on Day 7 post operation. Clinical follow-up after 1 month of the child showed absolutely normal results. Control angiogram done after 2 years showed stable occlusion of the aneurysm with coil mass in situ.
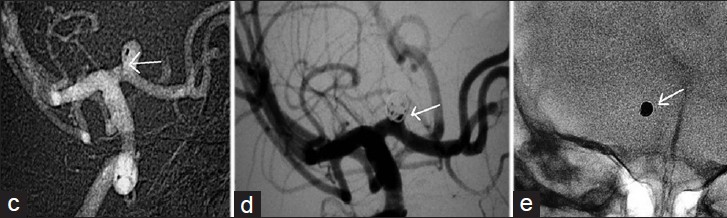
- (c) Microcatheter is shown positioned in the sac of the aneurysm (arrow) and (d) the post-procedure angiogram shows exclusion of the aneurysm (arrow) from the circulation with (e) the coil mass in the aneurysm (arrow)
Case 2
A 11-year-old male child presented with 2 episodes of generalized seizures and severe headache for 2 days. On clinical examination, there was no focal neurological deficit (Grade I SAH-Hunt and Hess scale).
CT scan [Figures 2a] showed hemorrhage in the left frontal lobe with intraventricular extension (Fischer Grade IV). Detailed work up was done as described in Case 1. DSA was done subsequently which revealed a bilobed ICA bifurcation aneurysm on the left side [Figure 2b]. There was no vasospasm seen on the angiogram. Endovascular treatment (Balloon-assisted coiling) was planned after discussion with the team of neurosurgeon and neurointensivist. High-risk informed consent was obtained after detailed explanation of the risks versus benefits to the parents.
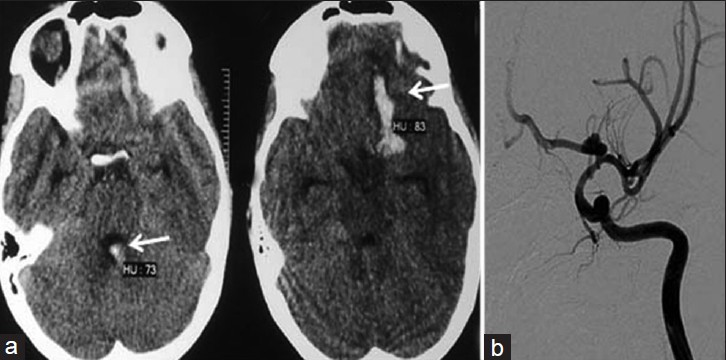
- Case 2: (a) Computerized tomography (CT) scan shows bleeding in the left frontal region with intraventricular extension. (b) Pre-procedural angiogram reveals internal carotid artery (ICA) bifurcation aneurysm on the left side.
Under general anesthesia, right 5F common femoral was accessed. Standard heparinization (100 units/kg as bolus at the beginning of the procedure followed by 20 units/kg every hour) was given to maintain the activated clotting time of 250 s. Guiding catheter (6F Envoy, cordis) with Nimodipine infusion was placed in the left ICA. Hyper glide occlusion balloon (3 mm ×10 mm, eV3) was placed across the neck of the aneurysm. Microcatheter (Echelon-10, eV3) over microwire (Neuroscout-10, codman) was navigated into the sac of the aneurysm. Subsequently, we started packing the aneurysm with the platinum coils using ‘Balloon remodeling technique’. After placement of the second coil and balloon deflation, a small loop of a previously detached coil was seen projecting into the parent vessel [Figure 2 c–f] on the check angiogram.
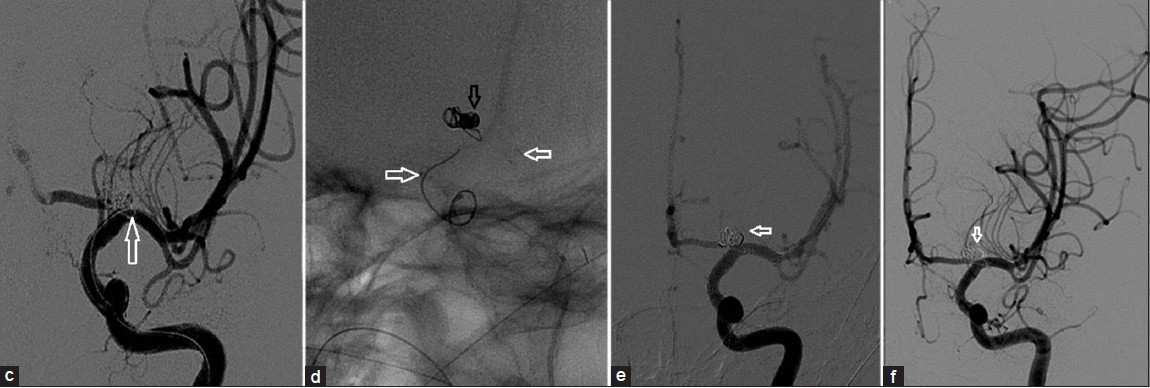
- (c) Magnified image shows the coil loop in the internal carotid artery (ICA) (arrow) (d) Enterprise stent(white arrows) deployed from M1 to supraclinoid ICA with secured coil mass (black arrow) in the aneurysm sac. (e) Postprocedure angiogram shows complete exclusion of aneurysm (arrow) from circulation. (f) Follow-up angiogram reveals stable occlusion of the aneurysm
In order to secure the aneurysm and prevent untoward effects of the prolapsed coil loop into the parent vessel, we deployed an 4 mm ×22 mm ‘Enterprise’ self-expanding stent (Codman, Johnson and Johnson) across the neck of the aneurysm from the right supraclinoid ICA to M1. Thereafter, the aneurysm was densely packed with further coils. The patient was loaded with antiplatelet drugs before stent deployement through the nasogastric tube (clopidogrel, 225 mg and aspirin, 150 mg) and was also started on infusion of Tirofiban which was continued for 4 hours (till the effect of oral antiplatelet agents took over). Post-procedure angiogram showed complete exclusion of the aneurysm from the circulation. Patient was kept under observation for 3 days in the neurointensive care unit. There were no peri- or post-procedural complications and the patient was discharged on day 7 after operation on double antiplatelet regimen (aspirin,50mg and clopidogrel,75mg). Clinical follow-up of the child after 1 month showed normal results. On the 6-month follow-up, neurological examination was normal and the patient was continued on only aspirin, 50 mg.
Follow-up angiogram done after 1 year showed stable occlusion of the aneurysm and normal patency of the stented artery. The patient is being monitored to determine any long term effects of the treatment.
DISCUSSION
Pediatric aneurysms constitute 2.9% of all cerebral aneurysms[1] with common location being the posterior circulation and with higher incidence of large and giant complex aneurysm. The ICA bifurcation is the commonest location in the anterior circulation.[4] During the past decade, there has been dramatic improvement in endovascular techniques that are increasingly being used in treatment of pediatric age group patients.[5] Large and giant aneurysms are more common in the pediatric age group. Both our cases were saccular aneurysms in the anterior circulation with Grade I SAH on presentation. Xianli et al.,[5] in their series, reported the use of coils in all saccular aneurysms. In Case 1, coiling of the saccularA1 aneurysm was done with dense packing and exclusion of the aneurysm from the circulation.
Liang et al.,[6] in their series of 24 pediatric patients, concluded that this age group differed in many ways from adults. Both microsurgical approaches and endovascular treatment were effective in these cases. In two patients with fusiform giant aneurysms of their series, they used balloon-mounted stents to treat the aneurysm and one of the patients with basilar trunk aneurysm succumbed to bleeding on the 23rd day post operation. In one patient in the series, a 10-year-old female child with basilar apexsaccular aneurysm, stent (Neuroform, Boston Scientific) assisted coiling was done with successful outcome (Glasgow outcome scale = 5).
In Case 2, we used a self-expanding stent as a ‘bail out’ measure (Enterprise, Johnson and Johnson) to secure the neck of the aneurysm and to achieve dense packing of the aneurysm with coils, we did not encounter any untoward effects. However, a close long-term follow-up has been planned for this patient to look for any long-term adverse effects of the stent, if any.
CONCLUSION
Pediatric cerebral aneurysms are rare with varying clinical presentation and natural history compared to those encountered in adults. Endovascular method is an elegant and minimally invasive alternative treatment of choice in managing these aneurysms and has promising results. However, a larger case series is required to better evaluate the safety and efficiency of the treatment method.
Available FREE in open access from: http://www.clinicalimagingscience.org/text.asp?2012/2/1/75/104308
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- Pediatric aneurysms and vein of Galen malformations. J Pediatr Neurosci. 2011;6:S109-17.
- [Google Scholar]
- Aneurysmal disease in children. Review of 20 cases with intracranial arterial localisations. Interv Neuroradiol. 1997;3:215-29.
- [Google Scholar]
- Pediatric intracranial aneurysms-clinical characteristics and outcome of surgical treatment. Childs Nerv Syst. 2007;23:327-33.
- [Google Scholar]
- Endovascular treatment for cerebral perforating artery aneurysms. Neurol Res. 2011;33:553-7.
- [Google Scholar]
- The clinical features and treatment of pediatric intracranial aneurysm. Childs Nerv Syst. 2009;25:317-24.
- [Google Scholar]






