Translate this page into:
Renal Collecting System Anatomy in Living Kidney Donors by Computed Tomographic Urography: Protocol Accuracy Compared to Intravenous Pyelographic and Surgical Findings
Address for correspondence: Dr. Reza Piri, Student's Research Committee, Tabriz University of Medical Sciences, Daneshgah Street, Tabriz, Eastern Azerbaijan, Iran. E-mail: dr.reza.piri@gmail.com
Dr. Mohammad Naghavi-Behzad, Medical Philosophy and History Research Center, Tabriz University of Medical Science, Daneshgah Street, Tabriz, Eastern Azerbaijan, Iran. E-mail: dr.naghavii@gmail.com
-
Received: ,
Accepted: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Objectives:
To evaluate the accuracy of triple-bolus computed tomography urography (CTU) as a surrogate of intravenous pyelography (IVP) for determining the anatomy of the urinary collecting system in living kidney donors.
Materials and Methods:
In an analytic descriptive cross-sectional study, 36 healthy kidney donors were recruited during 12 months. Preoperative IVP and CTU were utilized to evaluate kidneys’ anatomy; major and minor calyces and variation were used as anatomical indices to compare the accuracy of CTU and IVP; the images were then compared to surgical findings.
Results:
Thirty-six kidney donors (92% male; mean age: 28 ± 6 years) were enrolled in this study. The kappa coefficient value was significant and almost perfect for the CTU and IVP findings in detecting the pattern of calyces (kappa coefficient 0.92, asymptotic 95% confidence interval 0.86–0.97). Anatomic variations or anomalies of the urinary collecting system included the bifid pelvis (5.6%), duplication (8.3%), and extra-renal pelvis (2.8%). Both the sensitivity and specificity of CTU in the detection of the anatomy and variations were 100%; the sensitivity and specificity of IVP were 83.3% and 100%, respectively.
Conclusions:
The triple-bolus preoperative CTU can be considered an alternative to IVP for assessing the anatomy of the urinary collecting system.
Keywords
Anatomy
computed tomography urography
donor
intravenous pyelography
kidney transplant

- Reza Piri

- Mohammad Naghavi-Behzad
INTRODUCTION
Kidney transplantation is considered the definitive treatment option for patients with end-stage renal disease.[1] In contrast to most countries, the main source of harvesting kidneys for transplantation in Iran is healthy living donors. The kidney's anatomical parameters, the renal vessels, and the urinary collecting system are major determining factors for identifying if a donor kidney is fit for transplantation; these parameters may also determine the surgical approach and overall prognosis. Therefore, preoperative protocol requires that all kidney donor candidates undergo complete abdominal imaging to visualize the kidney's anatomy, vasculature, and urinary collecting system as a part of routine initial evaluation. A wide range of anatomical variations have been reported for the urinary collecting system; recognizing these variants facilitates surgeons in choosing the optimal surgical approach (laparoscopic vs. open nephrectomy), appropriate positioning during surgery, and determining the endourologic procedure that will minimize potential complications.[234]
Unenhanced computed tomography (CT) was initially established as the primary method of evaluation for nephrolithiasis. Currently, contrast-enhanced CT is recognized as the most appropriate imaging modality for visualizing renal masses and parenchymal abnormalities.[567] Although intravenous pyelography (IVP) is considered one of the preoperative evaluation methods of the urinary collecting system, CT with contrast has not replaced IVP for imaging the urothelium. The lower spatial resolution of CT makes it difficult to acquire images containing the intrarenal collecting system and ureters that are both completely opacified simultaneously.[89101112] Introduction of multi-detector technology for CT scan (MDCT) has provided images with thin collimation of the kidneys, ureters, and bladder during a single breath-hold.[13] In addition, MDCT angiography (MDCTA) is widely accepted as adequate for studying the renal vascular anatomy. Studies have demonstrated that the anatomic characteristics and anomalies of the urinary collecting system and ureters can be clearly assessed during the latent or excretion phase of MDCTA (i.e. CT urography [CTU]).[1415] Timed scanning and reconstruction gives the opportunity to opacify the urinary collecting system and carry out CTU in the meanwhile with angiography. These series of images let radiologists accurately assess the spatial anatomy of the urinary collecting system, anomalies, and variations as well as evaluate the vascular anatomy during a single CT study.[161718] This type of CTU, which is performed using multiple and timed contrast injections accompanied by the delayed scan, may serve as an excellent alternative to IVP for investigating the anatomy of vessels and urinary collecting system simultaneously. This modality avoids exposing kidney donors to unnecessary clinical workup.
The aim of this study was to determine the accuracy of the triple-bolus CTU and IVP for assessing the urinary collecting system anatomy in kidney donors by comparing the results to surgical findings as the reference. This study is novel as the triple-bolus CTU is compared to IVP and surgical findings performed in healthy living donors.
MATERIALS AND METHODS
Study design and population
In this cross-sectional study, 53 healthy kidney donors were referred to the kidney transplant division of Imam Reza (P.B.U.H) Educational-Medical Center (January 2011 through January 2013) and were enrolled for imaging evaluation. Inclusion criteria included: Age 18–65 years, having normal renal function, coming through ethical and official processes of kidney donation, and filling a written consent to take part in the study. Exclusion criteria included any contraindication for open laparotomy (e.g., severe heart failure), impaired renal function, having renal artery aneurysms, morbid obesity, and patient's preference. Of 53 potential candidates, 17 were excluded because 2 had poor image quality on IVP, one with duplicated left renal artery and vein, one due to multiple significant calculus, two had various large cysts, other one had pelvic kidney, and remaining nine declined to participate in the study. The study included 36 patients who underwent both preoperative triple-bolus CTU and IVP, had no prior disease, and normal kidney function on routine preoperative testing.
No unnecessary interventions were performed and no additional expenditures were imposed on the patients. All participants signed a written consent and were given the option of free withdrawal from the study at any given time. The study protocol was approved by the Ethics Committee of Tabriz University of Medical Sciences (TUMS), which was in compliance with the Declaration of Helsinki.
The anatomy of the urinary collecting system on the triple-bolus CTU, IVP, and surgery (gross anatomy) were compared in the enrolled participants.
Computed tomography scan technique
As described in literature, two modified image series were acquired:[18] First, unenhanced CT images were obtained followed by a series of contrast-enhanced images obtained after triple-bolus injections of contrast media. As a preparation, patients were asked to drink water, 500 ml during a period of 30 min till the study and 150 ml 15 s before starting the study; any diuretics was avoided. A 64 multi-slice CT scanner (Somatom Sensation 64; Siemens, Erlangen, Germany) was used to scan all the patients. The nonenhanced scout image from the diaphragm to the pubic symphysis was obtained with 70 mAs. A total of 120 mL of iopromide 300 mg/mL (Ultravist 300, Schering, Germany) was given using an automatic power injector (Medrad, USA) while it was split into three bolus injections; contrast-enhanced scanning was then conducted. The first injection (30 mL at rate of 2 mL/s) was administered to opacify and reveal the urinary tract. The second bolus (40 mL at rate of 2 mL/s) was administered 5 min after withdrawal of the first bolus, to show the renal medulla and veins. The third bolus (50 mL at rate of 3 mL/s) was injected 1 min after withdrawal of the second bolus, for enhancing the renal cortex and arteries. Immediately after completion of the last bolus injection, a single contrast-enhanced series of images from the diaphragm to the pubic symphysis were acquired to evaluate the anatomy of the urinary collecting system.
Intravenous pyelography technique
Standard IVP technique was used for IVP imaging.[19] Following a preliminary kidney, ureter, and bladder radiograph, a 100 mL IV bolus of ionic contrast material (Imagopaque, Nycomed Imaging AS; Oslo, Norway) was given followed by radiographs at 1, 5, 10, 15, and 20 min, beside postvoid and delayed films, if indicated. The patients were asked to wear a gown and to lie on a couch before contrast injection. The patients were asked to remain on the couch between each X-ray. The designated radiologic technician injected the contrast media; the technician was well-trained in recognizing the signs and symptoms of an adverse reaction and in monitoring the candidates for any potential side-effect. The supervising physician was available to react promptly should the patient exhibit any adverse signs or symptoms. There was at least 1-week interval between CTU and IVP. Kidney length on IVP was estimated by measuring the distances between the outermost upper and lower renal poles boarders on the films. Bipolar length was used for all individuals.
Surgery method
The surgical procedures were carried out by two skilled and well-experienced surgeons using the open laparotomy technique (A.Z. urologist with 23 years and A.B. transplant surgeon with 25 years of renal transplantation experience). Donors were positioned in the right lateral position and a longitudinal incision, close to 7 cm, was made at the left flank. The incision was extended to the retroperitoneal space using the muscle-splitting technique. Retractors were then used to expand the retroperitoneal space. Linear stapling devices were used to separate the renal artery and vein; the kidney was then extracted. Incision sites were sutured intracutaneously. Anatomy of vessels, kidney, and collecting system was grossly analyzed. After removal, the kidneys were clamped before anastomosis, and length, width, thickness, and weight were measured using sterilized Vernier callipers and excluding as much perirenal fat as possible. The blades of the callipers were positioned to touch the outmost points of the upper and lower poles of the kidney lightly. The accuracy of the measurement was ±1 mm.
Images analysis
Two readers (radiologists with 5 years of experience in abdominal imaging) independently assessed CT scan and IVP images, and any disagreements were resolved by the radiologists by consensus. The axial, coronal, and sagittal multiplanar reformations were reconstructed and studied using a dedicated workstation (Leonardo Workstation, Syngo Circulation, Siemens Medical Solutions). Readers of IVP and CT scan images were blinded to the images, which were coded randomly.
The number of major and minor calices in the upper, middle, and lower poles, distribution pattern of calices in the upper, middle, and lower poles in CTU were compared to IVP as the reference to show the qualitative accuracy of the triple-bolus CTU. In addition, longitudinal diameter of the transplanted kidney, types of anomalies and variations (such as bifid pelvis, complete duplication, partial duplication, extrarenal pelvis, malrotated kidney, ectopic kidney, and hump like kidney) in CTU and IVP were assessed in comparison to surgical findings as the standard reference. Diagnostic value of CTU and IVP for differentiating of urinary collecting system anomalies was recorded.
Statistical analysis
Obtained data are presented as mean ± standard deviation, distribution, and percentage (%). Statistical Package for the Social Sciences™ version 15 (SPSS Ltd., Chicago, IL, USA) was used for statistical analysis. Linear correlation of variants was investigated by Pearson correlation coefficient (r). Level of compatibility was determined using Kappa coefficient.[10] The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated and reported for both methods.
RESULTS
A total of 36 live kidney donors were investigated (33 were male and 3 were female). The mean age was 28 ± 6 years (minimum age was 20 years and maximum age was 48 years). Figure 1 shows the modified CTU method, used so that all arteries, renal parenchyma, and collecting system could be enhanced with maximum detail.
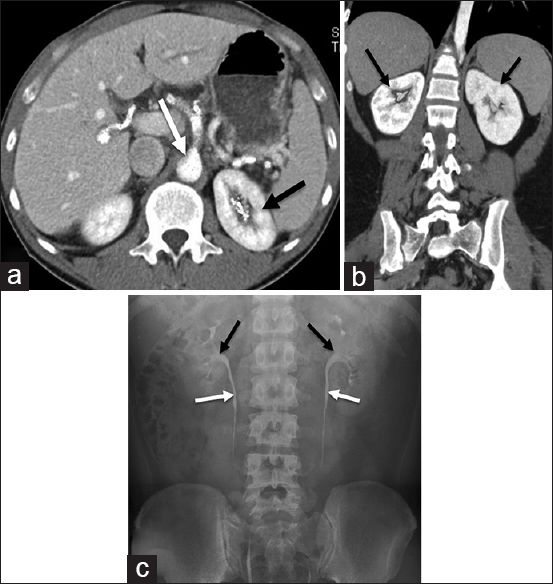
- Triple-bolus computed tomography urography in transverse plane (a) demonstrates renal parenchyma enhanced on both sides including contrast media excreted into left pelvicalyceal system (black arrow pointing to left kidney and contrast media excreted into left pelvicalyceal system). Intravenous contrast is seen in the aorta and subsequent hepatic arteries (white arrow points to the aorta). (b) Triple-bolus computed tomography urography in coronal plane demonstrates enhanced renal parenchyma on both sides including intravenous contrast in aorta; contrast media is excreted into pelvicalyceal systems on both sides (black arrows point to kidneys and contrast media excreted into pelvicalyceal system). (c) A normal intravenous pyelography shows enhanced pelvicalyceal systems with no dilation in subsequent ureters (black arrows point to enhanced pelvicalyceal systems and white arrows point to enhanced ureters).
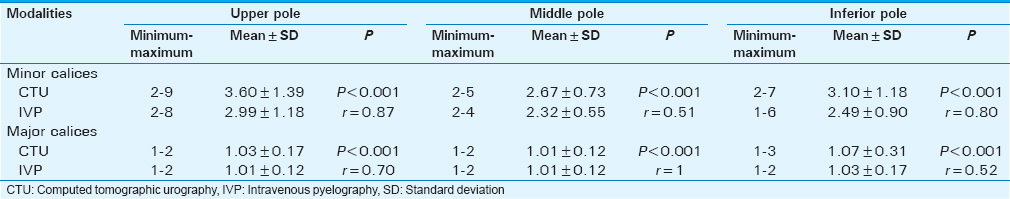
The number of minor and major calices in the upper, middle, and lower poles is shown in Tables 1 and 2. Caliceal distribution pattern on the triple-bolus CTU and IVP images is summarized in Table 2; the kappa coefficient value was significant and almost perfect for the triple-bolus CTU and IVP findings in the detection of calyceal patterns (kappa coefficient 0.92, asymptotic 95% confidence interval is 0.86–0.97).

The length of the harvested kidneys was measured using three methods [Table 3]. A positive and significant direct linear correlation was shown between the triple-bolus CTU and IVP (r = +0.62, P < 0.001). The results also demonstrated a positive and significant linear correlation when comparing the triple-bolus CTU with surgical findings (r = +0.77, P < 0.001) and when IVP was compared to surgical findings (r = +0.65, P < 0.001).

The variations and anomalies observed in the kidney and urinary collecting system during the triple-bolus CTU, IVP, and surgery are summarized in Table 4.

The frequency of urinary collecting system anomalies (based on the result of surgery) was 1 (2.8%) extrarenal pelvis, 2 (5.6%) full duplication, 1 (2.8%) partial duplication, and 2 (5.6%) bifid pelvis.
The triple-bolus CTU demonstrated sensitivity, specificity, PPV, and NPV of 100% for detecting urinary collecting system anomalies.
The sensitivity, specificity, PPV, and NPV of IVP in detecting urinary collecting system anomalies were all 100%, except for imaging an extrarenal pelvis in which case it demonstrated 83.3% sensitivity, 100% specificity, 100% PPV, and 96.8% NPV.
DISCUSSION
The accuracy of the triple-bolus CTU was significant and almost perfect with regard to both major and minor anatomical details of the urinary collecting system, compared to IVP. Common variations/anomalies of the urinary collecting system were the bifid pelvis, duplication, and extrarenal pelvis; the sensitivity and specificity of the triple-bolus CTU were 100%, whereas the sensitivity and specificity of IVP were lower close to 83.3% and 100%, respectively.
Kidney imaging is an important part of the overall management of patients with kidney diseases, whether it is used for assessing disease burden, determining pelvicalyceal anatomy, or planning the therapeutic approach. In the current study, the triple-bolus CTU and IVP demonstrated a significant concordance in determining anatomical features. When utilized for studying anomalies/variants of the urinary collecting system, the sensitivity and specificity of the triple-bolus CTU were both 100% when compared to the findings during surgery; the sensitivity and specificity of IVP for detecting anomalies/variants were 83.3% and 100%, respectively. This finding is supported by the literature wherein CT has been demonstrated to be superior to IVP in diagnosing and evaluating renal anomalies.[10202122]
Heneghan et al., compared the CTU and IVP methods of imaging including 50 cases that had undergone compression of the CTU to visualize the urinary collecting system.[10] Excretory phase images were additionally acquired through the kidneys, 3 min after injection. In the study, the diagnostic power of CTU in showing opacifications of the pelvicalyceal system was reported equal to or higher than IVP up to the middle parts of the ureters.
Another study evaluated 65 patients with suspicion of renal anomalies and pathologies using multi-detector CTU compared to intraoperative findings as the reference. Nephrographic, unenhanced, compression, and excretory-phase images through the abdomen and pelvis were obtained. In the study, 5 cases of congenital anomalities, 18 cases of calices/papillary anomalies, and 30 cases of pelvic anomalies were found; all CTU findings were in complete concordance with surgery findings.[22]
Prospective evaluation of 77 living kidney donors to demonstrate the anatomy of the urinary collecting system and ureters was performed by vascular and excretory phases of CTU in comparison to the surgical findings. The results of CTA and CTU were then compared to the surgical findings. Renal anomalies included only two cases of malrotation without obstruction. In the study, the findings of CTA and CTU in investigating transplanted kidney arteries and the structure of the urinary collecting system and ureters demonstrated significant correlation with intraoperative findings.[23]
Numerous studies have shown that CTA and CTU have a similar accuracy for the evaluation of anatomical features of the kidney and collecting system when compared to intraoperative findings.[24252627] The results of this study are also in accordance with the findings of the mentioned studies.
While some argue that using CTU for preoperative evaluation of kidney donors is unnecessary due to the adequacy of CT scanogram and screen-film radiography, it is important to note that CT scanogram has limitations. These limitations include its low spatial contrast power (<1 line in mm) and CT scanogram's utility of screen-film radiography which requires rapid transport of the patient to the urography ward after using contrast material, consequently creating complexity during preoperative evaluation.[2328] Furthermore, through technical improvements, especially using very high-contrast devices to run CTU studies, it is possible to study the smallest anomalies in the urinary collecting system. The ability to reformat obtained images and create three-dimensional (3D) and more tangible results has substantially improved the diagnostic power of this modality.[102129]
To summarize the findings of prior investigations, the advantages of CTU over IVP in investigating the renal and urinary collecting system are 4-fold: The ability to complete scanning of the entire body during only one breathing stage; simultaneous evaluation of the vascular system, renal parenchyma, and urothelium; increased diagnostic sensitivity in detecting small lesions; and providing needed information for creating 3D images.[25]
However, further studies are needed in this field to determine the best protocol. Factors that may affect the results include the effect of the patient's position during imaging, fullness versus emptiness of the bladder, type of contrast utilized, using adjunctive drugs during imaging, and type and method of assistive maneuvers during imaging (e.g., simultaneous pressure on the stomach, etc.).[10212330] Furthermore, the voltage can result in different image qualities in this modality.
Besides CTU, the emergence of new technologies such as magnetic resonance imaging has resulted in newer imaging methods to examine the urinary tract. Although numerous artifacts in magnetic resonance urography has hampered this method, initial results are promising for evaluating the urothelium.[31] Furthermore, comparison between CTU and magnetic resonance urography has shown that CTU is the superior method for detecting urologic pathologies. However, it has also been concluded that combining these two methods can yield a higher accuracy for detecting urologic pathologies.[32]
Though there are many advantages of CTU, this modality also has some disadvantages including radiation exposure. Many efforts have been made to reduce the radiation dose for donor CTU by modifying scan protocols, improving iteratively reconstruct algorithms, or utilizing dual energy CT scans, combining unenhanced and excretory phases with a normal-dose corticomedullary phase, or using a split-bolus dual-phase protocol that utilizes furosemide.[33343536] In the present study, using triple-bolus CTU was the main method to decrease the level of exposure to radiation, in which three different phases are shown through a single image using CTU. Although different methods are proposed to increase enhancement induced by contrast media, such as diuresis, in our study, diuresis was not done.[93738]
CONCLUSION
The triple-bolus CTU can evaluate the anatomy of the kidney and urinary collecting system in detail with a similar accuracy compared to surgical confirmation and IVP, respectively. Application of this modality resulted in achieving two main goals, reduced radiation exposure and increased extent of anatomy enhanced by contrast media; future attempts should focus on further minimizing the radiation exposure, as the dose of contrast already decreased to 120 ml in this method. Furthermore, radiologists can successfully consider using imaging protocols that are patient-specific and tailored to answering the clinical question.
Financial support and sponsorship
This study was supported by Tabriz University of Medical Sciences.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The scientific guarantor of this publication is Dr. Mohammad Kazem Tarzamni. We would also like to show our gratitude to Dr. Aboulfazl Bouhlouli, TUMS and Dr. Javad Rashid, TUMS, for sharing their pearls of wisdom with us during the course of this research. The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article. This study has received funding by TUMS. One of the authors has significant statistical expertise. Institutional Review Board (TUMS Research Department) approval was obtained. Written informed consent was obtained from all subjects (patients) in this study.
Available FREE in open access from: http://www.clinicalimagingscience.org/text.asp?2016/6/1/1/175079
REFERENCES
- Inferior pole collecting system anatomy: Its probable role in extracorporeal shock wave lithotripsy. J Urol. 1992;147:322-4.
- [Google Scholar]
- Assessment of 100 live potential renal donors for laparoscopic nephrectomy with multi-detector row helical CT. Radiology. 2005;237:973-80.
- [Google Scholar]
- Utility of 16-multidetector CT angiography in the preoperative evaluation of vascular and ureteral anatomy of donor nephrectomy. Afr J Urol. 2013;19:7-12.
- [Google Scholar]
- Comprehensive comparative study of computed tomography-based estimates of split renal function for potential renal donors: Modified ellipsoid method and other CT-based methods. J Comput Assist Tomogr. 2012;36:323-9.
- [Google Scholar]
- Evaluation of radiation-induced xerostomia in patients with nasopharyngeal carcinomas. J Dent Res Dent Clin Dent Prospects. 2007;1:65.
- [Google Scholar]
- Multidetector CT angiography for preoperative evaluation of living laparoscopic kidney donors. AJR Am J Roentgenol. 2003;180:1633-8.
- [Google Scholar]
- Pre-operative assessment of living renal transplant donors with state-of-the-art imaging modalities: Computed tomography angiography versus magnetic resonance angiography in 118 patients. World J Urol. 2013;31:983-90.
- [Google Scholar]
- CT urography: A comparison of strategies for upper urinary tract opacification. Eur Radiol. 2007;17:1262-6.
- [Google Scholar]
- CT urography: Definition, indications and techniques. A guideline for clinical practice. Eur Radiol. 2008;18:4-17.
- [Google Scholar]
- Compression CT urography: A comparison with IVU in the opacification of the collecting system and ureters. J Comput Assist Tomogr. 2001;25:343-7.
- [Google Scholar]
- Acute kidney injury in a patient with metabolic syndrome. BioImpacts: BI. 2015;5:155.
- [Google Scholar]
- Combined vascular-excretory phase MDCT angiography in the preoperative evaluation of renal donors. AJR Am J Roentgenol. 2010;194:145-50.
- [Google Scholar]
- Optimization of multi-detector row CT urography: Effect of compression, saline administration, and prolongation of acquisition delay. Radiology. 2005;235:116-23.
- [Google Scholar]
- Surgically relevant normal and variant renal parenchymal and vascular anatomy in preoperative 16-MDCT evaluation of potential laparoscopic renal donors. AJR Am J Roentgenol. 2007;188:105-14.
- [Google Scholar]
- Lower caliceal stone clearance after shock wave lithotripsy or ureteroscopy: The impact of lower pole radiographic anatomy. J Urol. 1998;159:676-82.
- [Google Scholar]
- Percutaneous Nephrolithotomy in patient with Renal calculi: balloon dilatation versus telescopic technique for tract dilatation. Medical Journal of Tabriz University of Medical Sciences and Health Services. 2014;36:6-9.
- [Google Scholar]
- Kidney and urinary tract imaging: Triple-bolus multidetector CT urography as a one-stop shop - Protocol design, opacification, and image quality analysis. Radiology. 2010;255:508-16.
- [Google Scholar]
- Intravenous urography: Technique and interpretation. Radiographics. 2001;21:799-821.
- [Google Scholar]
- Multiphasic helical CT of the kidney: Increased conspicuity for detection and characterization of small (<3-cm) renal masses. Radiology. 1997;202:211-7.
- [Google Scholar]
- Beam hardening artifacts: comparison between two cone beam computed tomography scanners. J Dent Res Dent Clin Dent Prospects. 2012;6:49.
- [Google Scholar]
- Urinary tract abnormalities: Initial experience with multi-detector row CT urography. Radiology. 2002;222:353-60.
- [Google Scholar]
- Living donor kidneys: Usefulness of multi-detector row CT for comprehensive evaluation. Radiology. 2003;229:869-76.
- [Google Scholar]
- Analysis of 64-row multidetector CT images for preoperative angiographic evaluation of potential living kidney donors. Radiologe. 2008;48:673-80.
- [Google Scholar]
- Multidetector CT urography: Comparison of two different scanning protocols for improved visualization of the urinary tract. J Comput Assist Tomogr. 2006;30:33-6.
- [Google Scholar]
- Multislice CT urography (MSCTU): Evaluation of a modified scan protocol for optimized opacification of the collecting system. Rofo. 2006;178:531-7.
- [Google Scholar]
- Multi-detector row CT in evaluation of 94 living renal donors by readers with varied experience. Radiology. 2005;235:905-10.
- [Google Scholar]
- Image quality and dose comparison among screen-film, computed, and CT scanned projection radiography: Applications to CT urography. Radiology. 2001;221:395-403.
- [Google Scholar]
- Influence of bladder distension on opacification of urinary collecting system during CT urography. Eur Radiol. 2008;18:1065-70.
- [Google Scholar]
- Contrast-enhanced magnetic resonance urography at 3T: Clinical feasibility. J Comput Assist Tomogr. 2013;37:29-36.
- [Google Scholar]
- Comparison of computed tomographic urography, magnetic resonance urography and the combination of diffusion weighted imaging in diagnosis of upper urinary tract cancer. Eur J Radiol. 2014;83:893-9.
- [Google Scholar]
- Experiences with the use of iteratively reconstructed dose-modified MDCT angiography examinations of living renal donors. J Comput Assist Tomogr. 2014;38:535-43.
- [Google Scholar]
- Comparison of accuracy of conventional periapical radiography and direct digital subtractions radiography with or without image enhancement in the diagnosis of density changes. J Dent Res Dent Clin Dent Prospects. 2012;6:54.
- [Google Scholar]
- Optimization of 64-MDCT urography: Effect of dual-phase imaging with furosemide on collecting system opacification and radiation dose. AJR Am J Roentgenol. 2011;197:W882-6.
- [Google Scholar]
- Targeted delayed scanning at CT urography: A worthwhile use of radiation? Radiology. 2012;265:143-50.
- [Google Scholar]
- Computed tomography urography technique, indications and limitations. Curr Opin Urol. 2007;17:56-64.
- [Google Scholar]
- Multi-detector row CT urography of normal urinary collecting system: Furosemide versus saline as adjunct to contrast medium. Radiology. 2006;240:749-55.
- [Google Scholar]






