Translate this page into:
Radiological Imaging Features of Fasciola hepatica Infection – A Pictorial Review
Address for correspondence: Dr. Abdurrahim Dusak, 601 Elmwood Avenue, 14642, Rochester, NY, US. E-mail: adusak@yahoo.com
-
Received: ,
Accepted: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Fascioliasis refers to a zoonosis caused by Fasciola hepatica, a trematode infecting herbivores, but also occurs in humans who ingest the metacercaria found in fresh water plants. Infection in humans is common in developing countries and is also not uncommon in Europe. Diagnosis of this infection is difficult, as the history and symptoms are nonspecific and stool analysis for eggs is negative until the disease is in an advanced state by when the parasite has reached the biliary system. The clinical course consists of two phases; first a hepatic parenchymal phase in which immature larvae invade the liver parenchyma, followed by a ductal phase characterized by the excretion of larvae into the bile ducts. Parenchymal Phase: Ultrasonography (US) findings are nonspecific in this early phase. Computerized tomography (CT) may demonstrate subcapsular low attenuation regions in the liver. Magnetic Resonance imaging (MRI) can also be utilized to establish liver parenchymal involvement, and is better than CT in characterizing hemorrhagic lesions, as well as identifying more lesions relative to CT. Ductal Phase: US examination is most useful at this stage, with its ability to demonstrate the live movement of the worms within the dilated ducts. A CT demonstrates dilated central biliary ducts with periportal tracking, whereas, mild ductal dilatation is poorly appreciated under MRI. Therefore, familiarity with the multimodality imaging features of fascioliasis, in combination with an available confirmatory enzyme-linked immunoassay, would be most helpful for early diagnosis.
Keywords
Fascioliasis
Fasciola hepatica
radiological imaging features
amoebic abscess
hydatid disease
pyogenic abscess
INTRODUCTION

Fascioliasis is a food-borne hepatic trematode zoonosis, caused by Fasciola hepatica (F. hepatica) and Fasciola gigantica. Parasites infect herbivores, but also occur in humans who ingest the metacercaria found in fresh water plants. Human infection is common in developing countries and is also not uncommon in Europe.[1]
Diagnosis of this infection is difficult, as the history and symptoms are nonspecific. Stool analysis for eggs is negative until the parasite has reached the biliary system. Therefore, familiarity with the multimodality imaging features of fascioliasis, in combination with an available confirmatory enzyme-linked immunesorbent assay (ELISA), would be most helpful for early diagnosis.[2]
We discuss the imaging techniques for evaluating F. hepatica infection, during both its parenchymal and ductal phases. We demonstrate the imaging characteristics suggestive of fascioliasis, as well as other disease entities that can mimic F. hepatica infection.
Life cycle of Fasciola hepatica
F. hepatica is a flat, leaf-shaped hermaphroditic parasite, and needs two hosts to complete its life cycle [Figure 1]. The definitive host range includes herbivorous mammals, including humans. Intermediate hosts are freshwater snails.[1]
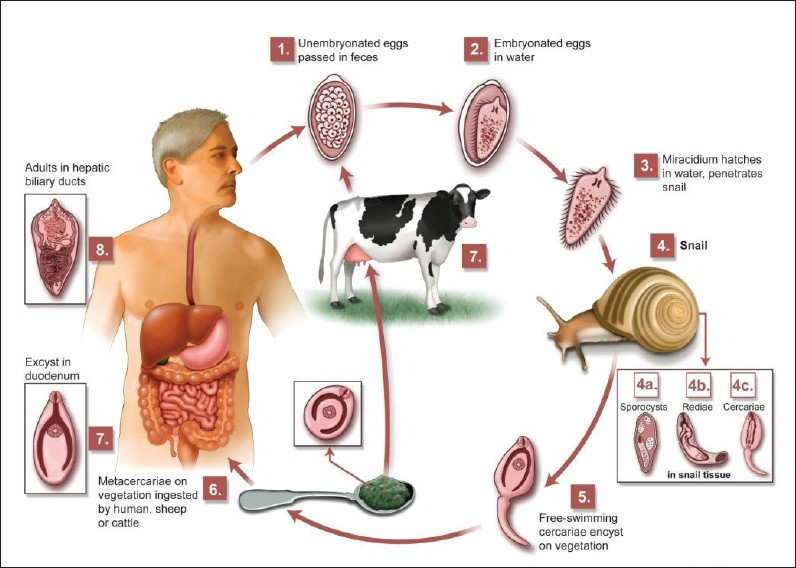
- Life cycle of F. hepatica.
Fasciola flukes live in the hepatic bile ducts of their definitive hosts and eggs pass out with the host's feces. When the eggs come into contact with water, ciliated miracidia hatch and subsequently infect an intermediate freshwater host, a snail. Free cercaria leave the snail, attach to aquatic plants (watercress, water lettuce, mint, parsley), and develop into metacercarial cysts. After ingestion of aquatic plants by humans, the metacercariae excyst in the duodenum and migrate through the intestine wall into the peritoneum, and via the Glisson's capsule into the liver. The larvae then migrate into the hepatic bile duct, mature into adult flukes, and begin laying eggs. The period between infection and maturation to egg laying, in humans, is approximately three to four months. Adult flukes can live for 10 years.[3]
Epidemiology
F. hepatica has been found in prehistoric human populations of the Stone Age, living 5000 years ago, at the end of the Mesolithic period and in the Neolithic age, a period marked by the domestication of animals and the development of agriculture. F. hepatica has been found in people who lived in the Bronze Age and Middle Ages, as well as, in ancient Europeans of the Gallo-Roman period. Interestingly, liver fluke eggs have been found in many palaeoparasitological studies performed in Europe (France, Netherlands, Denmark, Germany, Austria, Poland, Switzerland), but never in coprolites from the New World. This clearly indicate that fascioliasis in the Americas has been relatively recently introduced as a consequence immigration. Taking these data into account, the present situation may be cataloged as re-emerging or emerging, depending on the geographical area in question.[3]
Endemic areas
The geographic distribution of F. hepatica parallels the distribution of the specific freshwater snail, Lymnaea. Human infections are common in developing countries and are not uncommon in Europe. Major health problems are encountered in Andea (Bolivia, Peru, Chile, and Ecuador), Caribbea (Cuba), Northern Africa (Egypt), Western Europe (Portugal, France, and Spain) and the Caspia (Iran and neighboring areas). F. hepatica is usually seen in Europe, South America, and Oceania. Both F. hepatica and F. gigantica can be found in many areas of Africa and Asia.[4]
Prevalence
The prevalence of fascioliasis in Portugal, Egypt, and Peru, has been reported to be 3%, 7%, and 9% respectively. The highest prevalence has been reported in Bolivia, with 72% of stools analyzed and 100% serology tests showing positive for the infection. In hyperendemic areas, prevalence of zoonosis is significantly higher in females.[3]
PATHOPHYSIOLOGY
The parasitic infection in humans begins when water plants, like watercress, containing larva are ingested. The larva (metacercaria) excyst in the stomach and penetrate the duodenal wall, escape into the peritoneal cavity, and then penetrate the Glissons capsule, entering the liver parenchyma. In the liver, the flukes slowly migrate randomly through the hepatic parenchyma making multiple small holes and cavities, causing inflammation, abscess formations, hemorrhage, necrosis, granulation, and fibrosis, until they reach the larger bile duct and penetrate into the lumen, which is their permanent residence. Young flukes within the hepatic parenchyma measure few millimeters, while adult flukes within the bile ducts measure 20 – 40 mm in length and 8 – 13 mm in width. The hepatic stage lasts two to several months and the parasite may persist for a decade or more in the bile ducts. Hepatic parenchymal calcification may be observed rarely, in cases with chronic disease.[4]
Pathomicroscopy of hepatic fascioliasis demonstrates parasitic granuloma with necrosis, surrounded by heavy mononuclear and eosinophilic infiltration. The surrounding liver parenchyma show fibrosis with inflammation [Figure 2].[5]
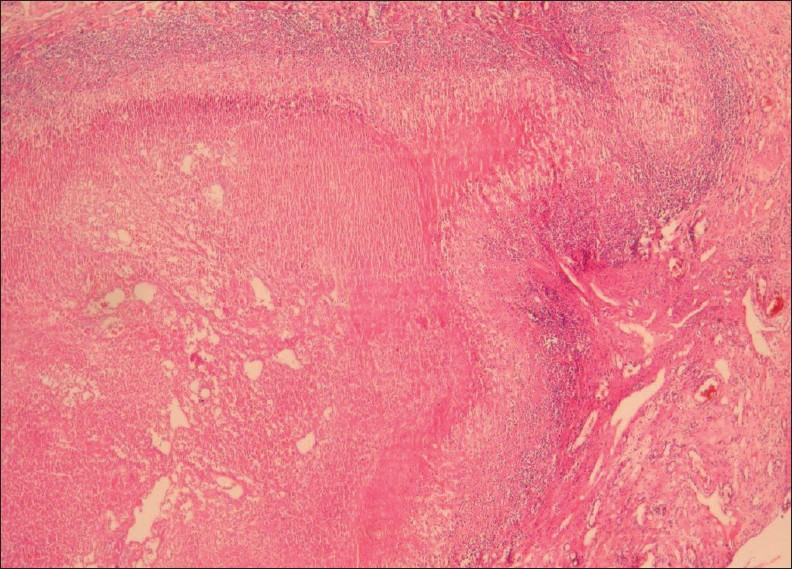
- A 38-year-old female with hepatic fascioliasis: Photomicrograph of a liver specimen shows parasitic granuloma with necrosis surrounded by heavy mononuclear and eosinophilic infiltration. Surrounding liver parenchyma shows fibrosis with inflammation (H and E, 40×).
DIAGNOSIS
Direct parasitological techniques, indirect immunological tests, and imaging techniques are presently used for diagnosis of fascioliasis in humans.
Direct parasitological techniques
Traditionally, diagnosis of F. hepatica infection has relied on detecting the presence of eggs in the stool, although this method is unreliable and complicated. At 10 weeks after infection, the fluke eggs can be detected in the stool [Figure 3]. Adult flukes and eggs may also be found elsewhere, such as, in the duodenal fluid, duodenal and biliary aspirates, and surgical (laparotomy, cholecystectomy, sphincterotomy) or biopsy specimens.[3]
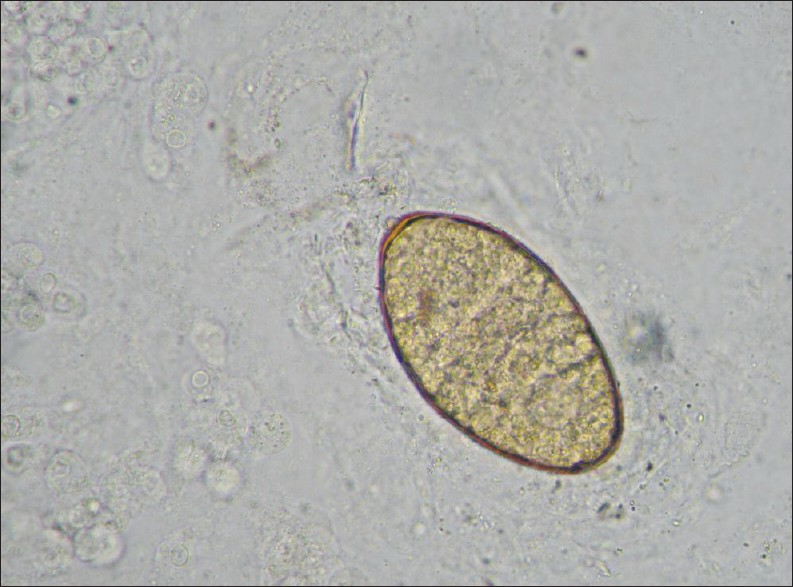
- High power (400×) view of an F. hepatica egg in a stool sample.
Indirect immunological techniques (serological examination)
Serological, intradermal, and stool antigen detection tests have been used for the diagnosis of human infection. Plasma levels of IgG directed against F. hepatica increase in two weeks after infection.[6] Indirect immunological techniques have an advantage of being applicable during all stages of the disease, and have also recently proved to be useful for monitoring post-treatment evolution. Enzyme-linked immunosorbent assay (ELISA) is a biochemical technique mainly used to detect the presence of antifluke antibodies in the serum.[1]
Biochemistry
Serum aspartate aminotransferase (AST), alanine aminotransferase (ALT), and lactate dehydrogenase (LDH) levels significantly increase four weeks post infection. Values do not differ significantly from the baseline, 11 and 15 weeks after infection, respectively. Gamma-glutamyl transpeptidase (GGT) activity increases nine weeks post infection and remains significantly elevated at 18 weeks post infection.[2]
Non-invasive diagnostic imaging techniques
Non-invasive methods for the diagnosis of fascioliasis include Ultrasonography (US), Computerized tomography (CT), and Magnetic Resonance imaging (MRI). Radiological findings can often demonstrate characteristic changes, and thereby, assist in the diagnosis of fascioliasis. It is important to differentiate fascioliasis lesions from other focal liver lesions by imaging.
Ultrasonography
Parenchymal phase
Ultrasonography (US) findings are nonspecific in this early phase. US features of F. hepatica lesions show a large variability until eight weeks post infection. The parenchymal phase US findings include focal hypoechoic or hyperechoic lesions or diffuse involvement of the liver [Figure 4]. In serious infection hypoechoic lesions with irregular distribution in the liver parenchyma can be observed. US findings in cases with mild infection can present with a diffuse increase in the echogenicity of the liver.[2]
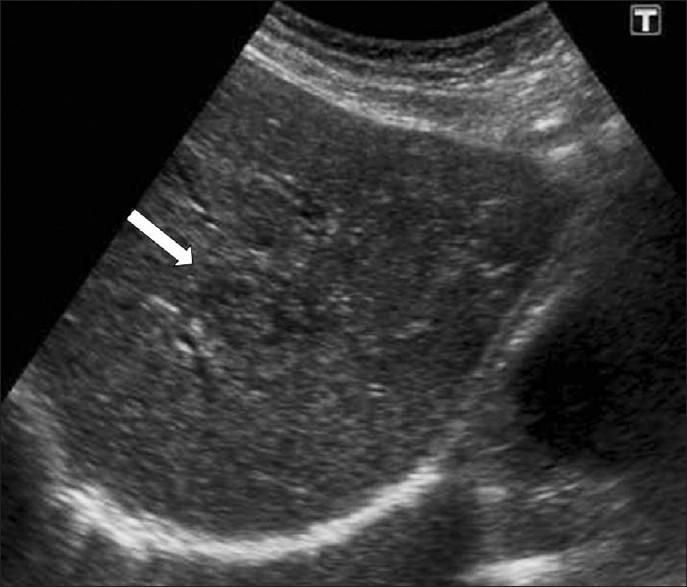
- Parenchymal phase of fascioliasis. US shows a parenchymal focal lesion with a halo around it within the liver (arrow).
Ductal phase
Parenchymal lesions regress with the beginning of the ductal phase, but in some cases hyperechoic lesions of the parenchymal phase may co-exist with the ductal phase. Ductal ectasia may be observed after eight weeks, with the duct wall thickening. Ductal dilatation appears as thin hypoechoic lines parallel to the portal areas at the beginning of the ductal phase, followed by an increase in biliary dilatation and tortuousness of the bile ducts after the twelfth week post infection [Figure 5]. Sometimes US can demonstrate mobile fluke in the dilated bile ducts [Figure 6] and gallbladder.[5]
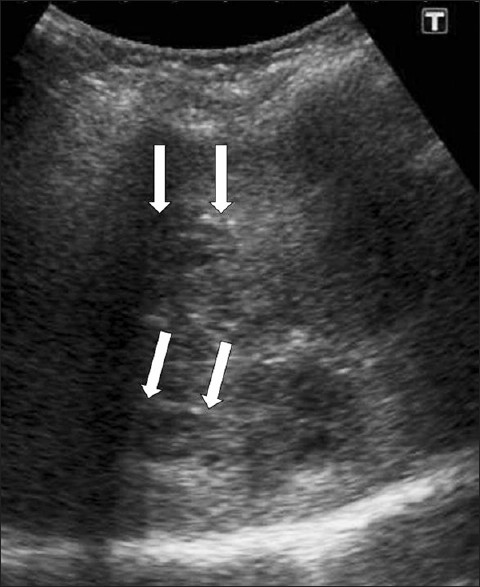
- Periportal tracking. US shows linear hypoechogenicity (arrows) along the intrahepatic bile ducts.
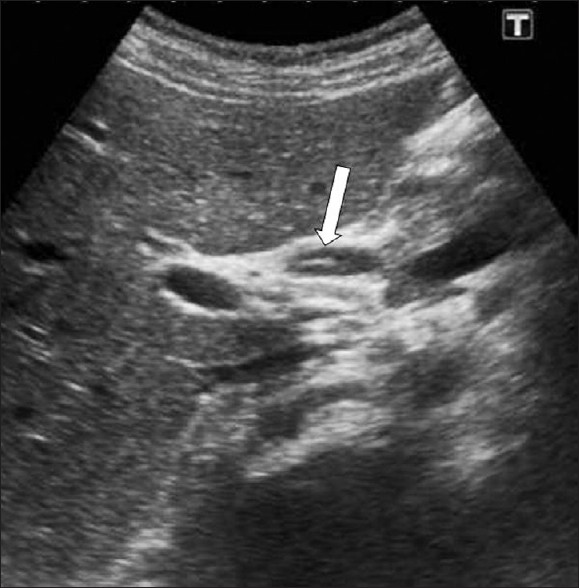
-
F. hepatica worms. US demonstrates a linear echogenic material (arrow) within the dilated common hepatic duct representing a dead F. hepatica worm.
Computed tomography
Parenchymal phase
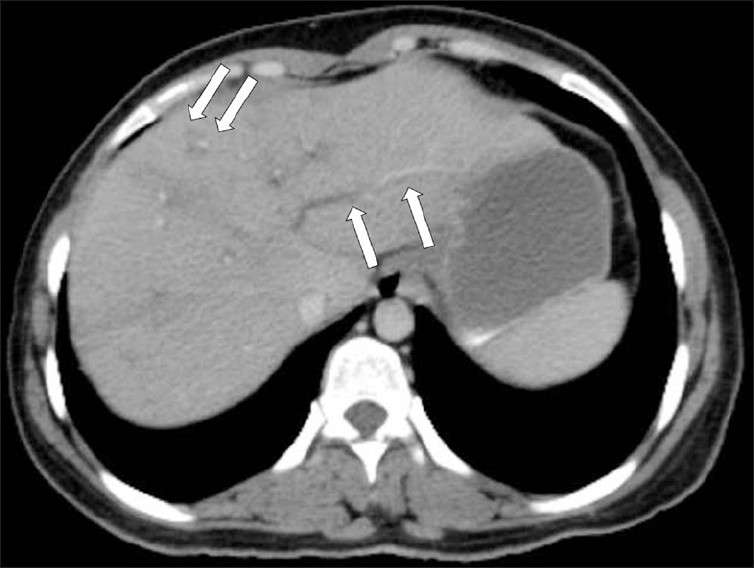
Computed tomography (CT) findings in the parenchymal phase of hepatic fascioliasis include multiple, small, round, or oval clustered hypodense lesions, with peripheral contrast enhancement [Figure 7]. Hypodense nodular lesions arise in the subcapsular area in the first weeks after ingestion of the metacercariae, progressing to tortuous clustered lesions by six weeks. In addition the CT may demonstrate subcapsular, low-attenuation regions in the liver. Focal liver capsule thickening and enhancement can be demonstrated on CT, secondary to penetration of the parasites into the Glisson capsule [Figure 8].[2]
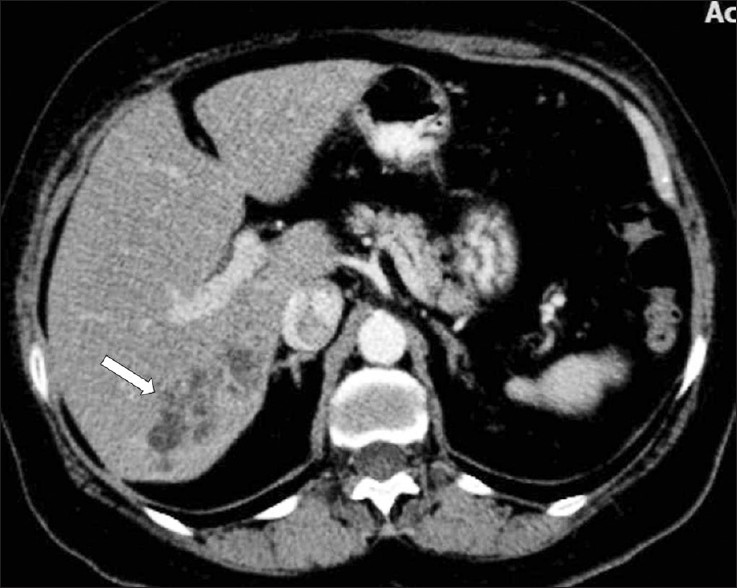
- A contrast-enhanced CT scan demonstrates multiple, round, clustered, hypodense lesions, with peripheral contrast enhancement in the liver (arrow).
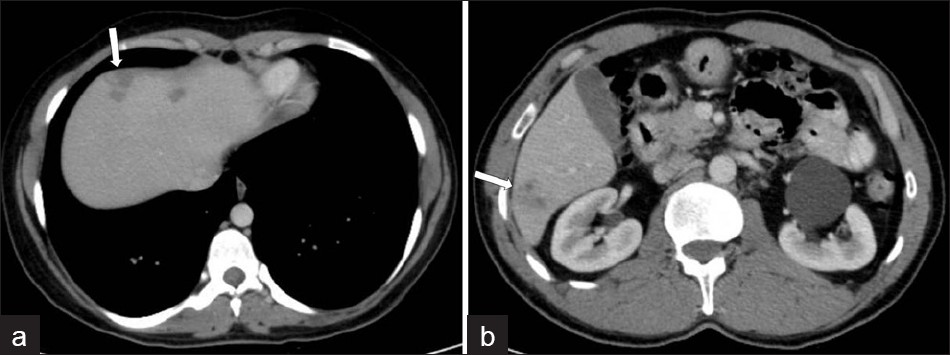
- Infiltration of the liver in the early parenchymal phase. Contrastenhanced CT scan shows irregular low attenuation lesions (arrows) in the subcapsular portion of the (a) left and (b) right hepatic lobes (two different patients).
Ductal phase
The extent of parenchymal lesions regresses rapidly eight weeks after infection. CT demonstrates dilated biliary ducts with periportal tracking [Figure 9]. Low attenuation develops progressively in the periportal areas, making them indistinguishable from ductal dilatation, 10 weeks post infection. In CT scans, residual parenchymal liver calcification may rarely be seen in the chronic phase [Figure 10]. Worms can rarely be observed in the bile.[4]
- A contrast-enhanced CT scan shows low attenuation tracks (arrows) along the portal triads.
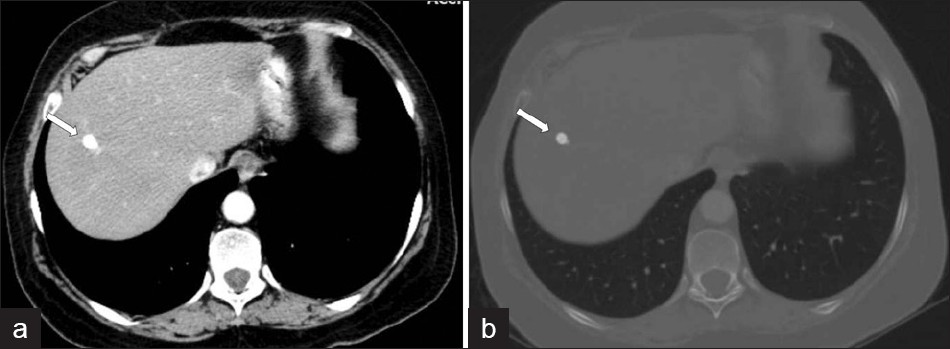
- A CT scan shows (a, b) residual parenchymal calcification (arrow) after treatment, in a patient with F. hepatica.
Magnetic resonance imaging
Parenchymal phase
Magnetic Resonance imaging (MRI) can also be utilized to establish liver parenchymal involvement. On T2 weighted (T2W) images, capsular hyperintensity can be demonstrated on the axial and coronal images, as a penetrating area of the parasite [Figure 11]. Early migration routes appear in patients as hypointense and hyperintense lines in the subcapsular area on T1W and T2W MR images, respectively. Parenchymal clustered lesions show hyperintensity on T2W and hypointensity on T1W images, with peripheral enhancement after contrast administration [Figure 12].[2]
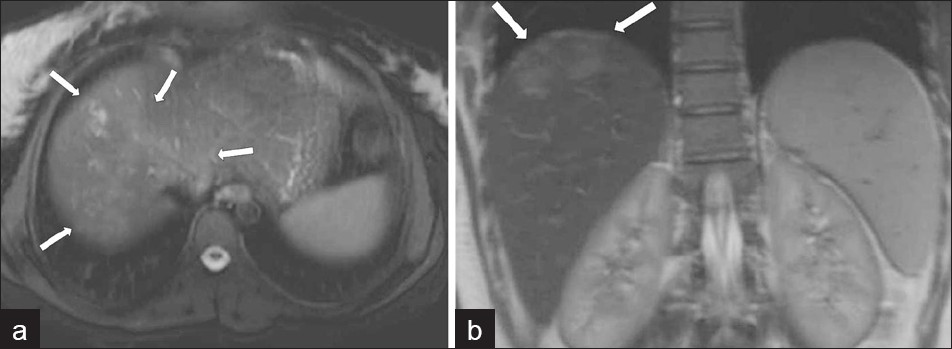
- (a) Axial and (b) coronal T2W MR images show more prominent serpigineous hyperintense lesions in the liver (arrows in a) and a hyperintense glisson capsule (arrows in b).
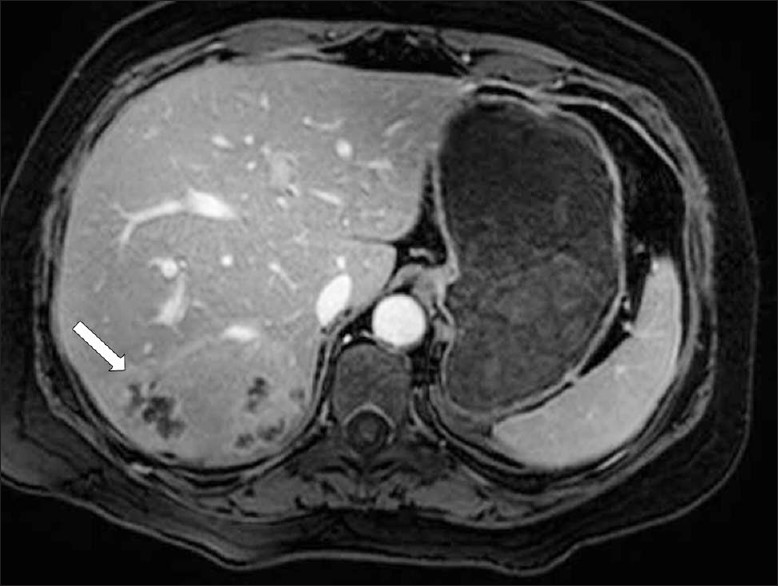
- A contrast-enhanced T1W MR image shows multiple, round, clustered hypodense lesions with peripheral contrast enhancement (arrow) in the liver (same patient in Figure 7)
Ductal phase
Mild ductal dilatation is poorly appreciated under MRI. Mild dilated bile ducts appear on T2W images as hyperintense areas parallel to hypointense lines, corresponding to the portal vessels [Figure 13]. Capsular and subscapsular fibrotic scars develop during the ductal phase and appear as an irregular heterogenity on the MRI. Intermediate signal-filling defects representing worms can be seen in the dilated ducts on the MRI [Figure 14].[6]
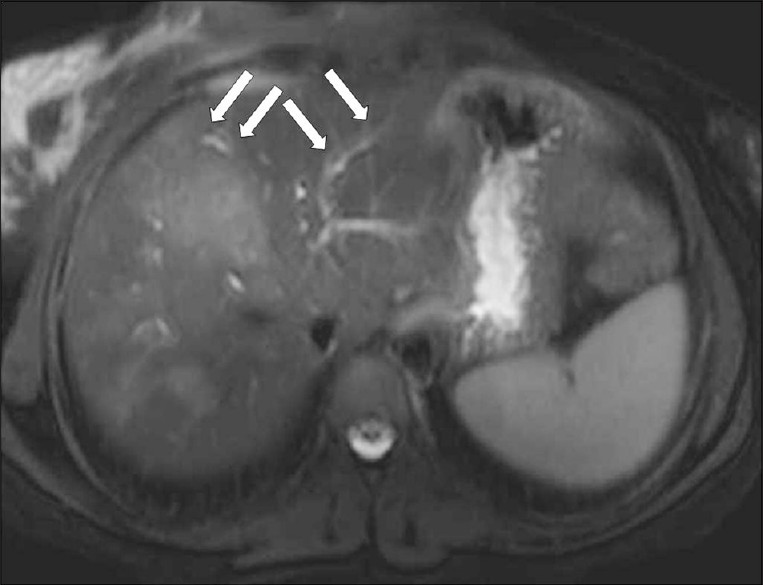
- A T2W axial MR image shows hyperintense tracks (arrows) along the portal veins.
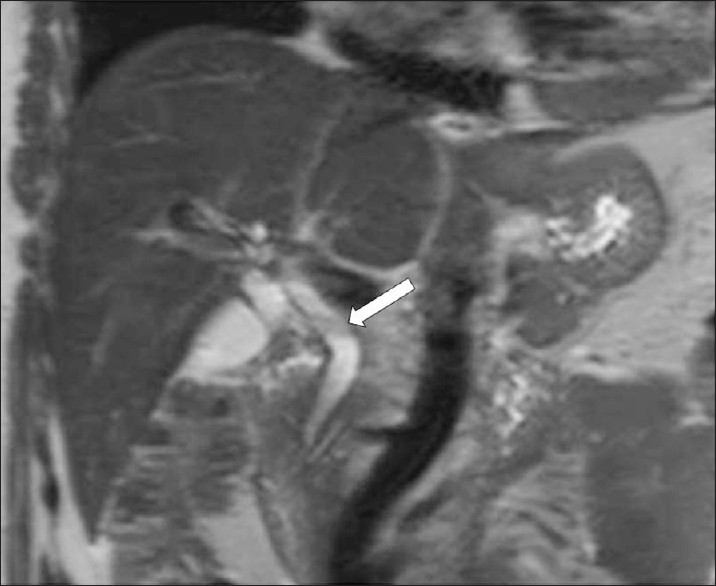
-
F. hepatica worms. T2W coronal MR image shows mild dilatation of the common bile duct, and a filling defect (arrow).
Complications of Fasciola hepatica infection
Ectopic localization of F. hepatica may occur during the transmigration of the parasite through the peritoneum or from the liver through the portal venous system. Unusual sites of presentation of F. hepatica include the pancreas, spleen, kidney, bowels, rarely spine, as well as the liver.[3]
Acute cholangitis and cholecystitis
The chronic stage of fascioliasis is characterized by recurrent episodes of biliary colic, angiocholitis, and cholecystitis. Inflammation of the bile ducts can be seen as dilatation with irregular wall thickness, and contrast enhancement of the duct walls on CT. US may demonstate Fasciola flukes in the bile ducts.[1]
Acute cholangitis, with hepatic abscess formation, may also show nodular hypoattenuating lesions and branch formations, corresponding to dilated bile ducts, but these branch formations are more central. Parenchymal invasion of F. hepatica may result in unilocular or multifocal abscess formations that appear as hypodense fluid collections, with the capsule formation mimicking pyogenic abscess, on CT. F. hepatica abscesses may have hyperdense content, possibly due to pus or hemorrhage. These lesions show a thick hypodense rim that results from edema, which shows minimal enhancement on contrast-enhanced CT [Figure 15]. The development of a chronic liver abscess appears to be extremely rare, but could develop because of prominent hepatic inflammation or an unusual chronic form of cholangitis in F. hepatica infection. Hepatic microabscesses caused by F. hepatica do not coalesce to form a large abscess cavity, unlike the individual lesions associated with pyogenic abscesses. They do not tend to increase in size and evolve slowly.[78]
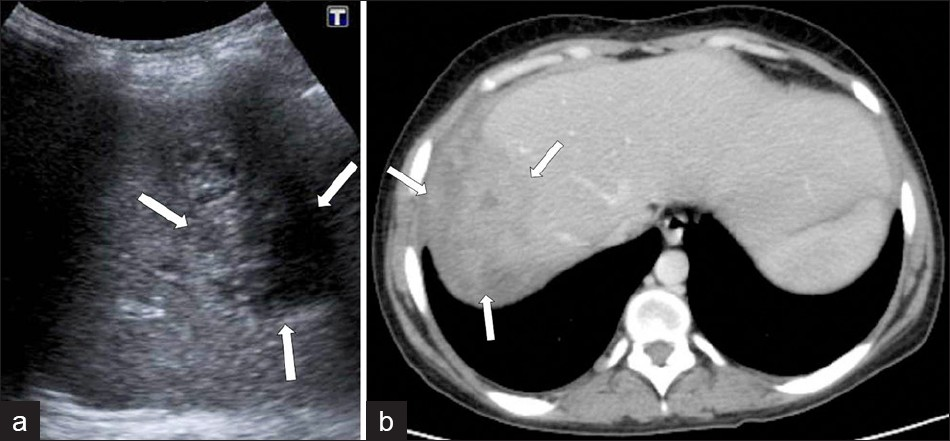
- Complicated parenchymal lesions: Abscess formation, (a) US demonstrates an irregular-shaped lesion containing heterogeneous anechoic areas (arrows). (b) A contrast-enhanced CT scan shows (same patient) an irregular heterogeneous lesion with irregular peripheral enhancement (arrows).
An organized abscess can mimic necrotic granuloma of the liver in fascioliasis. The possibility of hepatic fascioliasis should be included in the differential diagnosis of organized liver abscess in endemic areas.[4]
Percutaneous drainage of hepatic abscesses can be performed with CT or US guidance.[9]
Subcapsular hemorrhage and hematoma
Subcapsular hematoma in the liver is a rare complication of the hepatic migration of flukes during the hepatic phase [Figure 16]. The association of a subcapsular hematoma of the liver with eosinophilia suggests either polyarteritis nodosa or invasive hepatic fascioliasis. On rare occasions, the bleeding may be life-threatening because of its severity. The lesions may shrink and disappear or may apparently migrate toward the center of the liver, because the parasites migrate to the large bile ducts. Finally, calcifications may subsist.[1]
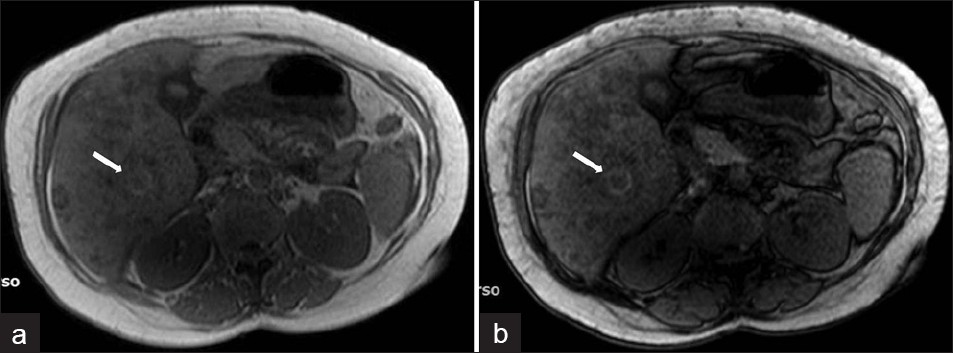
- Complicated parenchymal lesions: Hemorrhagic infarction: (a) T1W in-phase MR image shows rim-shaped hyperintense lesion (arrow). (b) T1W out-of-phase MR image shows rim-shaped hyperintensity without signal loss (arrow).
DIFFERENTIAL DIAGNOSIS
Fascioliasis presents with a wide clinical spectrum; therefore, a high index of suspicion is required to establish a correct diagnosis. Liver lesions, urticaria, and eosinophilia are the main clinical signs and symptoms of the infection, which are also common in some other infections.
The differential diagnosis includes hepatitis, cholecystitis, cholangitis, liver abscess, brucellosis, and primary and secondary hepatobiliary malignancies. Misdiagnosis or late diagnosis of fascioliasis is frequent and may lead to unnecessary surgical procedures such as cholecystectomy and hepatic segmentectomy. Furthermore, a delay in treatment may cause the patient to suffer longstanding biliary symptoms and face an increased risk of pigment gallstones. Treatment of advanced fascioliasis is more difficult and may necessiate invasive procedures such as endoscopic retrograde cholangiopancreatography (ERCP) or percutaneous abscess drainage. Therefore, appropriate imaging is important in the differential diagnosis.[8]
Amoebic liver abscess
Amoebiasis is caused by Entamoeba histolytica, and infection is acquired by ingesting food or water containing the protozoan. The adult trophozoite colonizes the large intestine that may result in amebic colitis. Extraintestinal infection occurs after the trophozoites enter the circulation. As the liver filters the portal circulation, extraintestinal manifestation of amoebiasis mostly occurs in the liver. An amoebic liver abscess can rupture into the abdominal cavity, resulting in generalized peritonitis. Rupture of an amoebic abscess into the biliary tract or into the pleural and pericardial cavity has been reported. Mortality from amoebiasis is mainly due to extraintestinal pathology, of which the amoebic liver abscess is the most common. Stool microscopy and stool antigen detection is not helpful for diagnosis; in fact less than 10% have identifiable E. histolytica in stool specimens.[6]
Under US, an amebic abscess appears as a hypoechoic lesion, with low-level internal echoes and absence of significant wall echoes. The lesion is typically oval or round and located near the liver dome. A contrast-enhanced CT demonstrates well-defined round lesions, with complex fluid attenuation values. A thick enhancing wall with peripheral edema is characteristic of the CT findings for amoebic abscess of the liver [Figure 17]. Another important imaging feature of the amoebic abscess of the liver is the presence of extrahepatic manifestations such as involvement of chest wall, pleural cavity, pericardium, and adjacent viscera, due to the extension of the amoebic liver abscess. Amebic abscesses have homogeneous low-signal intensity and high-signal intensity on T1W and T2W MR images, respectively.[10]
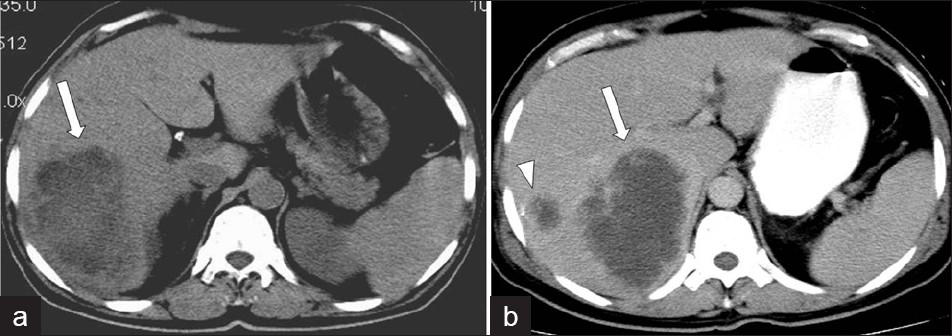
- Amebic liver abscess.(a) Non-contrast and (b) Contrast-enhanced CT scan demonstrates a large, lobulated, well-defined cystic mass in the right hepatic lobe, with enhanced thick wall (arrow), and a satellite cystic mass associated with the same imaging features.
Hydatid cyst disease
The hydatid cyst (HC) disease, which is caused by Echinococcus granulosus, is an endemic parasitosis in the sheep-raising areas of the world. The liver is the most common site of infection in adults. Diagnosis of HC is mainly based on imaging. However, serological tests can provide specific confirmation of HC both in the clinical and community screening studies. US findings of HC vary according to the type of HC and are summarized in Table 1.[8]

Under CT, a hydatid cyst usually appears as a well-defined, hypoattenuating lesion with a distinguishable wall. Coarse wall calcifications are present in 50% of the cases [Figure 18]. The hydatid cysts appears hypointense on T1W images and markedly hyperintense on T2W images; when present, the daughter cysts are hypointense relative to the matrix on both the T1W and T2W images. US and MR imaging may demonstrate thin-walled cysts with or without hydatid sand, or cysts with a multilayered wall, with or without detachment of a laminated membrane. Classic ultrasound features of the hydatid cyst on ultrasound include hydatid sand and water lily sign, secondary to detachment of the hydatid membrane.[10]
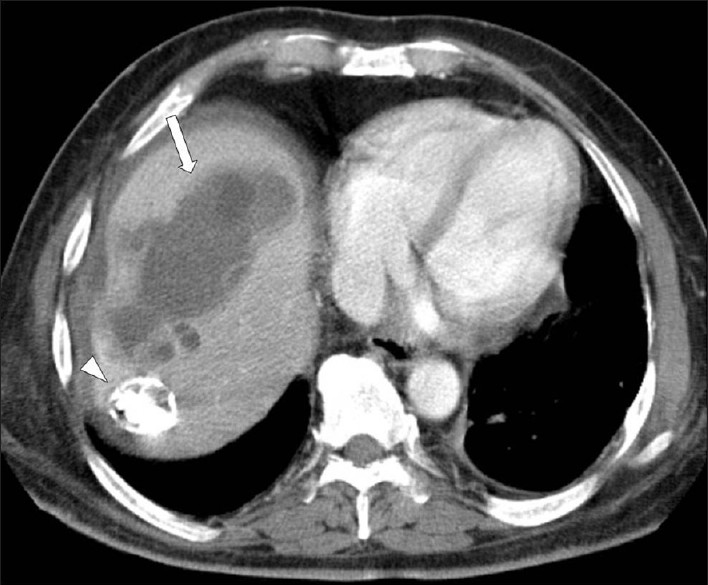
- A contrast-enhanced CT scan demonstrates a large, multilocular hydatid cyst in the right hepatic lobe, with multiple daughter cysts (arrow). Note a subtotally calcified lesion in the seventh segment of the liver (arrow head).
Pyogenic liver abscess
It is the most common type of visceral abscess. This is a serious, life-threatening condition, with a mortality rate of 6 to 14%. A pyogenic liver abscess usually arises secondary to a hematogenous spread, biliary tract disorder, and portal pyemia. The most common organism recovered from the pus of a liver abscess is K pneumonia, followed by E coli, and organisms in the Enterococcus, Burkholderia, Streptococcus, and Staphylococcus species. Negative serological tests strongly point to a pyogenic etiology in the setting of a liver abscess. Pyogenic microabscesses are usually multiple scattered lesions similar in distribution to fungal microabscesses in immunosuppressed patients. The types of pyogenic abscesses are multiple and can mimic fascioliasis, however, they do not involve the subcapsular areas of the liver.[9]
Under US, pyogenic microabscesses may present either as discrete hypoechoic nodules or ill-defined areas of distorted hepatic echogenicity. Under CT, they appear as multiple small, well-defined hypoattenuating lesions. The signal intensity of pyogenic abscesses on MR imaging varies depending on their protein content. Usually pyogenic abscesses appear hypointense on T1W and hyperintense on T2W images. Contrast-enhanced CT and MR often demonstrate rim enhancement [Figure 19]. Pyogenic abscesses may demonstrate the presence of intralesional air in some cases.[10]
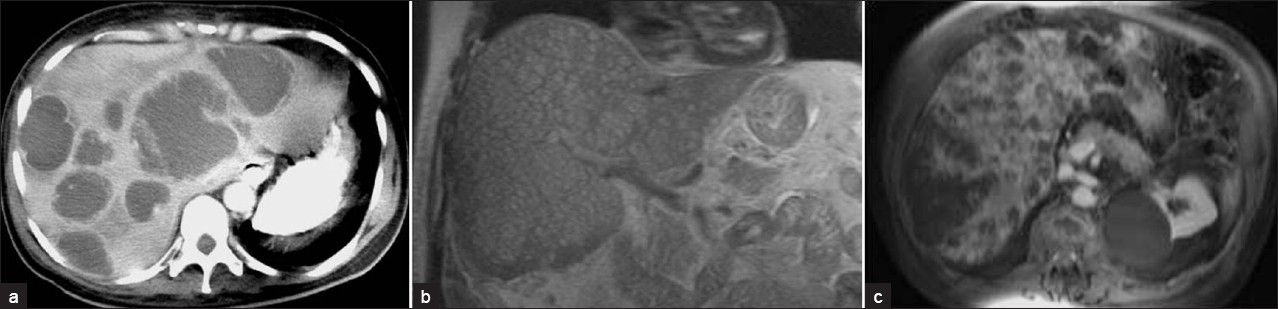
- Pyogenic multiple liver abscesses.(a) Contrast-enhanced CT scan shows multiple hypoattenuating lesions representing pyogenic abscesses scattered throughout the liver. These lesions demonstrate peripheral enhancement and surrounding edema. (b) Coronal MR T2W HASTE images and (c) contrast-enhanced MR images show multiple small hyperintense nodules representing pyogenic microabscesses disseminated in the liver, with peripheral contrast enhancement.
CONCLUSION
Imaging findings may help in a better understanding of the life cycle characteristics of the disease and describe the findings in which fascioliasis should be suspected, as well as other entities that may mimic F. hepatica infection. The role of US is limited in the parenchymal phase, and most useful in the ductal phase. CT and MR imaging may also help in reflecting the phase and activity of the disease. MR imaging can demonstrate the characteristic evolutionary pattern of fascioliasis that reflects the life cycle of F. hepatica in the early parenchymal phase, without using the contrast agent. Additionally, MR imaging may provide more details about the process of complicated lesions such as hemorrhagic lesions and abscess formation in fascioliasis. Early diagnosis, with demonstration of the exact phase of the disease would be helpful for appropriate treatment.
ACKNOWLEDGMENT
We gratefully acknowledge Dr. Shweta Bhatt for her constructive comments and critique during the preparation of this manuscript.
Source of Support: Nil
Conflict of Interest: We declare that we have no conflict of interest.
Available FREE in open access from: http://www.clinicalimagingscience.org/text.asp?2012/2/1/2/92372
REFERENCES
- Update on hepatobiliary flukes: Fascioliasis, opisthorchiasis and clonorchiasis. Curr Opin Infect Dis. 2008;21:523-30.
- [Google Scholar]
- Diagnostic imaging in sheep hepatic fascioliasis: Ultrasound, computer tomography and magnetic resonance findings. Parasitol Res. 2003;90:359-64.
- [Google Scholar]
- Fascioliasis and other plant-borne trematode zoonoses. Int J Parasitol. 2005;35:1255-78.
- [Google Scholar]
- Biliary parasitic diseases including clonorchiasis, opisthorchiasis and fascioliasis. Abdom Imaging. 2008;33:157-65.
- [Google Scholar]
- Experimental hepatobiliary fascioliasis in rabbits: A radiology-pathology correlation. Investig Radiol. 1999;34:99-108.
- [Google Scholar]
- Diagnostic imaging in the study of human hepatobiliary fascioliasis. Radiol Med. 2010;115:83-92.
- [Google Scholar]
- Hepatobiliary fascioliasis: sonographic and CT findings in 87 patients during the initial phase and long-term follow-up. AJR Am J Roentgenol. 2007;189:824-8.
- [Google Scholar]
- Hepatobiliary fascioliasis: Imaging characteristics with a new finding. Diagn Interv Radiol. 2009;15:247-51.
- [Google Scholar]
- CT appearance of pyogenic liver abscesses caused by Klebsiella pneumoniae. Radiology. 2011;260:129-38.
- [Google Scholar]
- The infected liver: Radiologic-pathologic correlation. Radiographics. 2004;24:937-55.
- [Google Scholar]






