Translate this page into:
Primary Hyperparathyroidism: Imaging to Pathology
Address for correspondence: Dr. Sara Piciucchi, Department of Radiology, Morgagni Pierantoni Hospital, Via Forlanini 34, Forlì, Italy. E-mail: s.piciucchi@alice.it, piciucchi.sara@gmail.com
-
Received: ,
Accepted: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
The aim of this review is to describe the multimodal imaging (ultrasound, magnetic resonance, computed tomography, and nuclear medicine) of primary hyperparathyroidism and its correlation to the pathological findings. In the last decades, imaging science has progressed a great deal. Accurate preoperative localization of the involved glands is essential for surgical success.
Keywords
Calcinosis
parathormone
primary hyperparathyroidism
INTRODUCTION
Hyperparathyroidism (HPT) is a clinical disorder, which occurs due to the increased activity of the parathyroid glands, resulting either from an intrinsic abnormal change that alters the excretion of the parathyroid hormone (primary or tertiary hyperparathyroidism) or from an extrinsic abnormal change affecting the calcium homeostasis stimulating production of the parathyroid hormone (secondary hyperparathyroidism).
Primary hyperparathyroidism (P-HTP) is the third most common endocrine disorder, after diabetes and thyroid dysfunction, with the highest incidence in postmenopausal women.
The estimated prevalence of P-HPT in the general population is one to two cases/1,000 inhabitants, with a 2-3 : 1 female-to-male ratio.
The incidence generally increases with advancing age and it is higher among postmenopausal women (older than 50 years). It is currently estimated to be approximately 30 : 100,000 / year, although it is thought to be rising as a result of the introduction of automated methods for serum calcium determination. Primary hyperparathyroidism may present in sporadic (90%) or familial (10%) forms. The familial forms include: Multiple endocrine neoplasia (MEN 1 and MEN 2) and isolated familial hyperparathyroidism associated with jaw tumors (HPT-jaw tumor syndrome). In MEN I the endocrine glands most affected are: Parathyroid glands, pancreas, and pituitary glands. Almost everyone who inherits MEN 1 develops an overactivity of the parathyroid glands at some stage of their life, with a consequent HPT. MEN II is a defect in a gene called RET that causes tumors of the thyroid gland or more than one of the parathyroid and adrenal glands.
Parathyroid glands derive from the third and fouth pharyngeal pouches and are generally four in number. Two are behind the superior third of the two thyroid lobes (upper parathyroid glands) and two localized behind the inferior third of thyroid gland. Localization of the parathyroid glands can be really variable, especially the site of the lower glands, due to the longer pathway and difficult migration process.
Actually lower parathyroid glands can be intrathyroidal, within the thyrothimic ligament, within the thymus, and in the mediastinum. Migration can also fail and glands can be seen high in the neck.
Ionized calcium in the plasma is closely regulated and ranges from 1 – 1 mmol / L to 1 – 3 mmol / L. Precise control of ionized calcium is needed, to ensure optimum function of the physiological processes, particularly cell signaling, neural function, muscular function, and bone metabolism.
The parathyroid hormone has a major biological function in maintaining ionized calcium and phosphate within the reference range, by stimulating a specific receptor-mediated response in cells throughout the body.
If there is a decrease in the circulating ionized calcium, the parathyroid hormone increases. The hormone has three major functions that help to restore the normal circulating ionized calcium via the calcium-sensing receptor located on the surface of the chief cells.[1]
The term primary hyperparathyroidism (P-HPT) refers to the inappropriate or unregulated overproduction of the parathyroid hormone (PTH). It leads to abnormal calcium homeostasis. High levels of PTH lead to increased renal resorption of calcium, phosphaturia, and increased synthesis of 1,25-dihydroxyvitamin D3, which increase interstitial calcium resorption and increased resorption of the bone.[2]
Single gland adenoma is the most common cause (75 – 85%). Multigland adenoma arises in a substantial proportion (two glands in 2 – 12%; three glands in < 1 – 2%, and four or more in < 1 – 15%). Parathyroid carcinoma is rare (1%).[3] Few cases of apparent P-HPT may result from the paraneoplastic production of PTH by a non-parathyroid tumor.[45]
The aim of this article is to provide a review of P-HPT, including the pathology and approaches to its diagnosis and treatment.
Parathyroid adenoma
Parathyroid adenomas are benign neoplasms composed of chief cells, oncocytic cells, or transitional oncocytic cells, with frequent admixtures of these cell types.[6]
Most adenomas occur in normally situated glands, but they may also occur from near the carotid bifurcation and pericardial sac to the mediastinum and retroesophageal space. Additional locations include the vagus nerve, soft tissue at the angle of the jaw, and the thyroid gland. The average size of adenomas in patients without significant bone disease is approximately 1 g, with many less than 0.5 g.
Usually, microadenomas weigh less than 0.1 g. In general, larger adenomas are associated with higher levels of calcium and PTH and are more likely to be symptomatic.
Foci of cystic change are particularly common in large adenomas.
Microadenomas are typically non-encapsulated, whereas, larger adenomas are often separated from the adjacent rim of the normocellular parathyroid gland by a fibrous capsule. The cells of adenomas are arranged in cords, nests, sheets, and follicles, and frequently have a palisade arrangement around the blood vessels[7] [Figures 1 and 2].
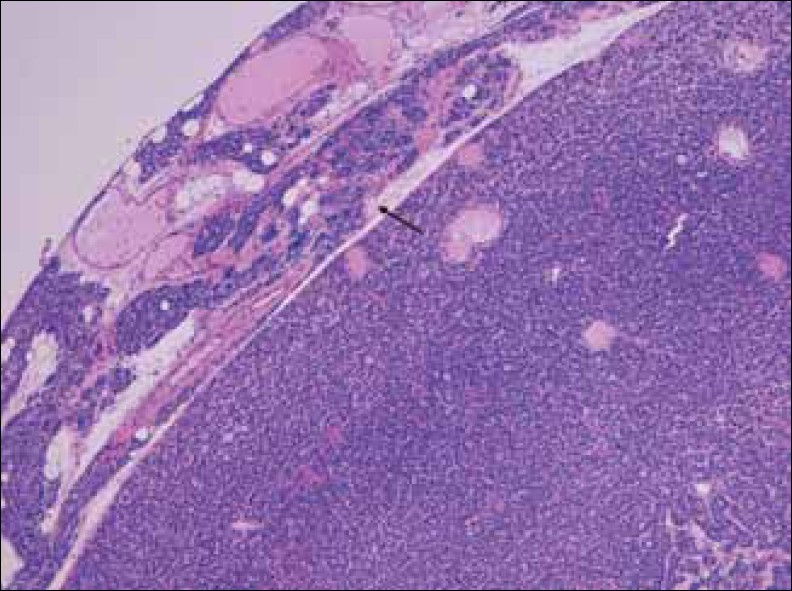
- Hematoxylin-Eosin stain at 10x shows enlarged parathyroid gland composed mainly of chief cells, without stromal fat, and a rim of normal parathyroid tissue (arrow).
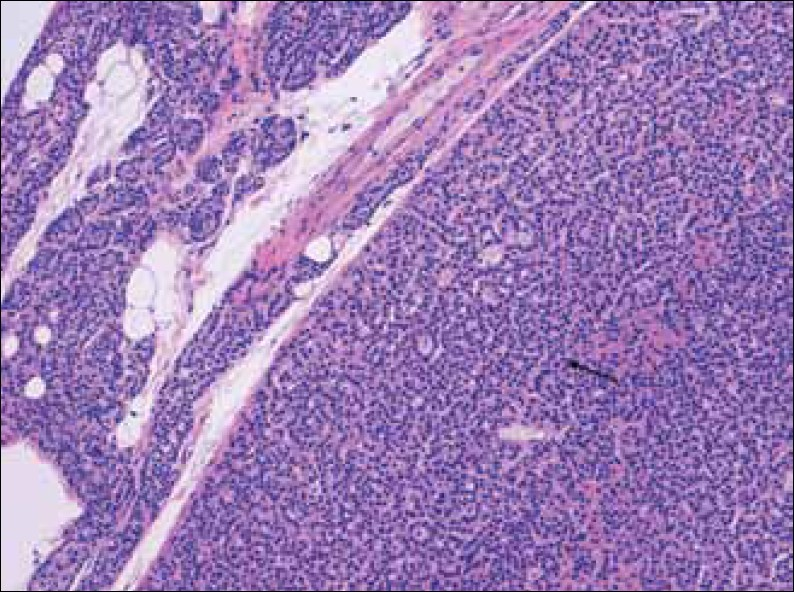
- Hematoxylin-Eosin stain at 20x shows enlarged parathyroid gland composed mainly of chief cells, without stromal fat (arrow), and a rim of normal parathyroid tissue.
Parathyroid hyperplasia
Chief cell hyperplasia is currently defined as an absolute increase in the parenchymal cell mass, which occurs as a result of the proliferation of chief cells, oncocytes, and transitional oncocytes in multiple parathyroid glands. The enlargement of the glands is symmetric in approximately 50% of the cases[89] [Figure 3].
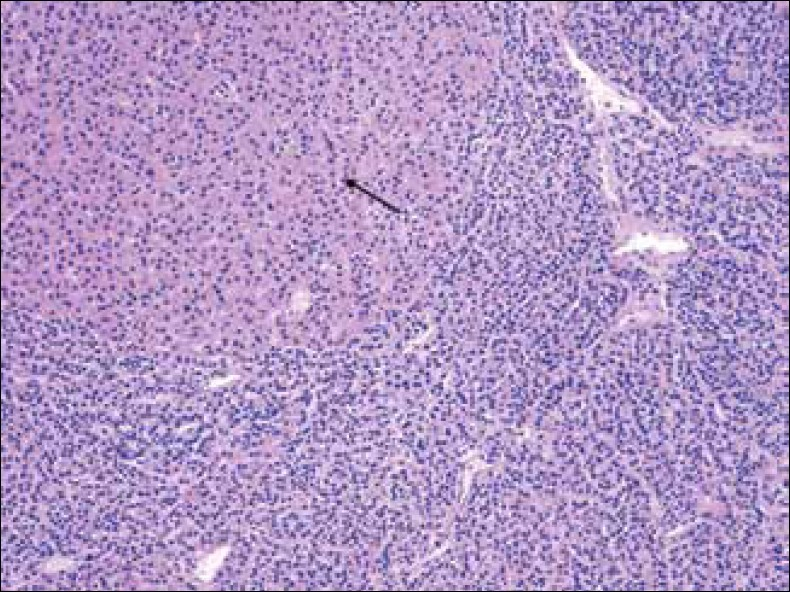
- Hematoxylin-Eosin stain at 20x shows parathyroid gland with chief cell hyperplasia and oncocytic cells (arrow).
Heritable hyperparathyroidism syndrome
Familial HPT is a clinically and genetically heterogeneous group of disorders that includes multiple endocrine neoplasia MEN Type 1, MEN Type 2, neonatal severe hyperparathyroidism, hyperparathyroidism–jaw tumor (HPT-JT) syndrome, familial isolated hyperparathyroidism, and autosomal dominant mild hyperparathyroidism or familial hypercalcemia with hypercalciuria.
MEN 1 is inherited as an autosomal dominant trait, characterized by the development of multiglandular parathyroid tumors, gastroenteropancreatic neuroendocrine tumors, and pituitary adenomas.
Parathyroid disease occurs in more than 90% of patients, gastroenteropancreatic tumors in 60%, and pituitary tumors in 30%.[10]
Parathyroid carcinoma
Parathyroid carcinoma is an uncommon tumor, which most often appears as a large mass that is densely adherent to the surrounding soft tissues or the thyroid gland. The diagnosis of malignancy should be restricted to those cases that show invasion of the adjacent soft tissues or thyroid gland, blood vessels, or perineural spaces, and to those tumors with documented metastases.[11]
The diagnosis of parathyroid carcinoma is challenging. Fibrous band formation within the tumor is common, but by itself, this feature is insufficient for the diagnosis of malignancy.
Atypical parathyroid adenoma
Atypical adenomas of the parathyroid glands represent a controversial entity. These tumors have some of the features of parathyroid carcinomas, but they lack the unequivocal evidence of invasive growth (peritumoral vascular invasion, perineural invasion, or invasion of the adjacent soft tissues or thyroid).
Parathyroid multimodality imaging
The primary purpose of imaging HPT is to accurately localize the disease gland and identify patients who are suitable for minimal invasive surgery (solitary adenoma).
Sonography and 99mTc-sestamibi scintigraphy are the dominant imaging techniques for the preoperative location of parathyroid adenomas.
Numerous studies comparing these techniques suggest similar sensitivities and specificities for solitary adenoma detection. When both studies are carried out preoperatively, the findings improve the accuracy with which the location of the lesion is identified.[12–14]
Contrast-enhanced CT and magnetic resonance imaging (MRI) can also effectively locate parathyroid adenomas, but they are less commonly used preoperatively for identifying the location of the lesion and are more commonly used in the setting of failed parathyroidectomy, for the detection of suspected ectopic — often mediastinal — glands.
Sonography
The patient should be scanned supine, with a pillow beneath the shoulders to slightly hyperextend the neck. Gray-scale imaging should be performed with a high-frequency linear transducer. The study should include longitudinal images extending from the carotid artery to the midline and transverse images extending from the hyoid bone superiorly to the thoracic inlet inferiorly. Scanning at 12 – 15 MHz is possible, with currently available higher level platforms.
Having the patient swallow under real-time observation may help to show the inferior glands located deep, in relation to the clavicles.[15]
Normal parathyroid glands have sizes of about 5 × 3 × 1 mm and are usually not seen on routine sonography. The histology of a normal parathyroid gland — chief cells, fibrovascular stroma, and adipocytes — may account for the isoechogenicity of the gland, relative to the adjacent thyroid. Diagnosis of hyperplasia is difficult by sonography, because the gland volume is much less than the parathyroid adenoma. A reactive lymph node can be mistaken for parathyroid adenoma or hyperplasia. On multivariate analysis, body mass index (BMI), gland size, and gland volume are the statistically significant independent factors predicting detection by both ultrasound (US) and methoxy isobutyl isonitrile (MIBI) in primary HP. The sensitivity of US is better for a single gland disease than for a multigland disease in primary HP.[16] Color Doppler (CD-US) can be a useful modality for accurate diagnosis of the parathyroid lesion and differentiation of parathyroid adenoma from other cervical pathologies.[17] The overall sensitivity, specificity, and accuracy of CD-US in the correct diagnosis of parathyroid adenoma have been 97%, 100%, and 98.6%, respectively. The sensitivity and specificity of ultrasonography in the detection of parathyroid adenoma and differentiating it from other cervical masses has reached up to 97% and 100%, respectively, by combining the Color Doppler technique with the grayscale evaluations of the parathyroid adenoma.
Parathyroid adenomas are nearly always homogeneously hypoechoic to the overlying thyroid gland on gray-scale imaging. They are commonly detected using gray-scale imaging alone, when they are larger than 5 mm in diameter.
They are usually oval or bean-shaped, but larger adenomas can be multilobulated. Color and power Doppler imaging commonly show a characteristic extrathyroidal feeding vessel (typically a branch off the inferior thyroidal artery), which enters the parathyroid gland at one of the poles. Internal vascularity is also commonly seen in a peripheral distribution.
The artery feeding the adenoma tends to branch around the periphery of the gland before penetrating deeper, resulting in a characteristic arc or rim of vascularity [Figure 4].
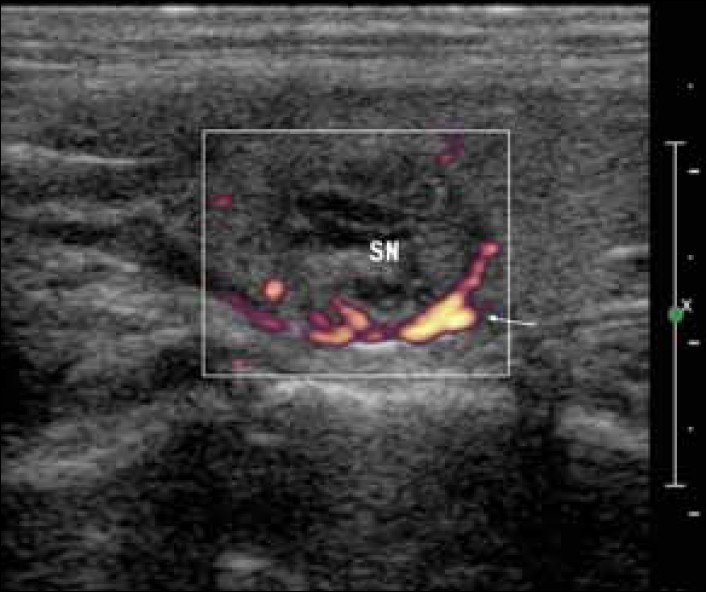
- Longitudinal ultrasound image of a 3-cm oval-shaped, multiloculated nodule in the upper parathyroid. Power-Doppler shows peripheral pattern of vascularity (white arrow).
In addition, Color Doppler sonography of the overlying thyroid gland may show an area of asymmetric hypervascularity, which may help to locate an underlying adenoma.[18–20]
The cervical ultrasound can give very precise anatomic localization, but it is not sensitive, because it cannot always detect glands deep in the neck or in ectopic localizations.
An inherent advantage of the ultrasound is that it includes an evaluation of the thyroid gland. Routine use of the ultrasound will, therefore, increase recognition of the concurrent thyroid pathology.
Scintigraphy
Scintigraphy represents one of the most common imaging modalities.
Multiple radiotracers have been described for the detection of the parathyroid lesions. Thallium, sestamibi, and tetrofosmin are the three most widely used.
In 1983, Ferlin et al., introduced the use of Thallium (201Tl) and the pertechnetate-99mTc (99mTc - technetium-pertechnetate) subtraction method.[21] The thyroid tissue takes up both tracers. The abnormal parathyroid tissue, such as, parathyroid adenomas, hyperplastic parathyroid glands, and parathyroid carcinoma, take up 201Tl, but not 99mTc pertechnetate. Separate 201Tl and 99mTc pertechnetate images are obtained in a single session, without moving the patient. Then, the 99mTc-pertechnetate image is subtracted from the 201Tl image by computer processing. Any remaining activity usually indicates abnormal parathyroid tissue.
Many centers report different sensitivities of detection for primary adenomas with this procedure: In a review of the literature, based on 14 studies, with a total of 317 patients with primary hyperparathyroidism, who had been operated upon, Hauty et al.,[22] found a sensitivity in the detection of parathyroid adenomas of 82%, a diagnostic accuracy of 78%, and a positive predictive value of 94%. On the contrary, Sandrock et al.,[23] showed a lower sensitivity of this procedure, with a correct detection of 30 of 71 adenomas (42%) and 18 of 56 hyperplastic glands (32%). This technetium-pertechnetate subtraction method was the first widely accepted method for radionuclide imaging of the parathyroid glands until the early 1990s, when Coakley et al.,[24] first recommended the use of 99mTc sestamibi for parathyroid imaging. Then, in 1995, Ishibashi et al., [25] found that 99mTc-tetrofosmin might be useful in parathyroid imaging because its imaging characteristics were similar to those of 99 mTc-sestamibi. Both 99mTc-sestamibi and 99mTc-tetrofosmin, as lipophilic cationic complexes, were actively taken up by the cell, and indicated cellular viability. However, the uptake mechanism of 99mTc-tetrofosmin depended on both the cell membrane potential and the mitochondrial potential, while 99 mTc-sestamibi uptake depended primarily on the mitochondrial potential.[26] This might be one explanation for the clinical observation that the thyroid washout rates were slower for 99 mTc-tetrofosmin than for 99 mTc-sestamibi. On the other hand, the washout from parathyroid adenomas had also been shown to be slower for 99 mTc -tetrofosmin than for 99 mTc –sestamibi, in individual patients.
Comparisons of the diagnostic accuracy of the two 99 mTc- labeled agents have shown mixed results. In fact several studies have shown that 99 mTc-tetrofosmin performs at least as well as 99 mTc-sestamibi[27–29] , but others have found discordant results, with 99 mTc-sestamibi performing better.[30–32] All studies have shown that the differential washout of 99 mTc-tetrofosmin from the thyroid gland is slower than that of 99 mTc-sestamibi, resulting in lower adenoma-to-thyroid / background ratios. For this reason, 99 mTc-sestamibi is, at this moment, the radiopharmaceutical of choice.
Furthermore, there is still a debate not only on the choice of the radiopharmaceutical, but also on how to perform the diagnostic procedure. Several authors have presented different techniques and imaging protocols for 99 mTc-sestamibi parathyroid imaging, including subtraction scintigraphy with 123I or 99 mTc-pertechnetate and dual-phase scintigraphy with early and delayed images. Parathyroid scintigraphy subtraction technique (dual-tracer) with 99 mTc-sestamibi and 123I or 99 mTc provides the subtraction of the 123I or 99 mTc thyroid image acquired first, from the 99 mTc-sestamibi image acquired after the injection of a dose of 200 – 400 MBq of 99 mTc-sestamibi[33] [Figure 5].
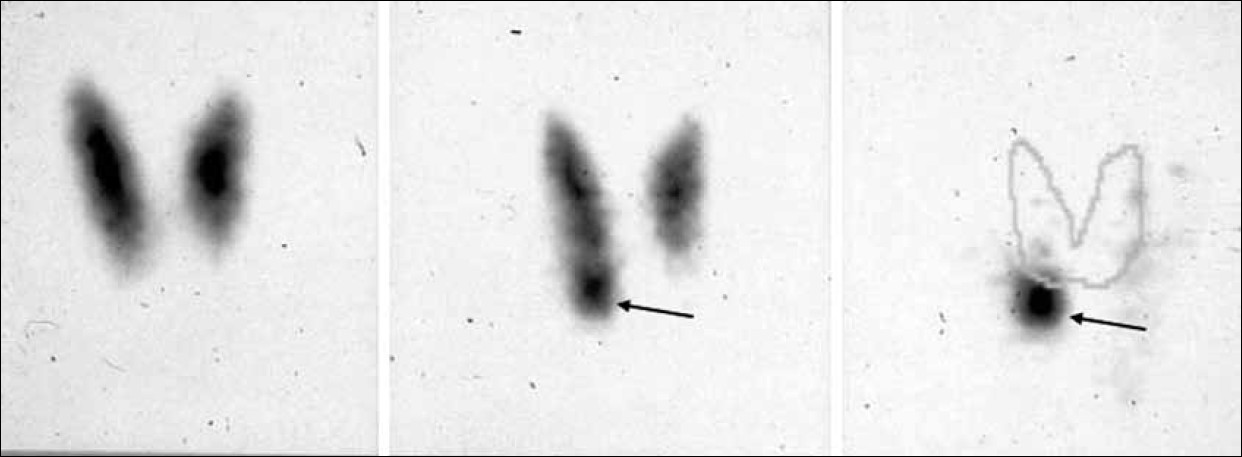
- Example of parathyroid scintigraphy according to the dual-tracer protocol (99m-Tc-pertechnetate and 99mTc-sestamibi). (On the left) the 99 mTc-pertechnetate scan shows a normal thyroid gland. (In the center) the 99mTc-sestamibi scan suggests the presence of adenoma of the lower right parathyroid glaand (arrow), better outlined when subtracting the 99mTc-pertechnetate scan from the summation scan (arrow).
As there are some disadvantages of using 123I for thyroid imaging such as its high costs, its limited availability, and the overall length of the procedure (four to five hours) 99 mTc -pertechnetate has been widely used as a thyroid imaging agent for subtraction techniques,[34–36] with same results, in terms of diagnostic accuracy.[37] However, the need for absolute neck immobility to avoid motion artifacts when using sequential dual-nuclide acquisition presents a considerable methodological challenge.
A further problem with subtraction techniques using either 123I or 99 mTc-pertechnetate is the insufficient thyroid uptake, both in patients with a suppressed thyroid, due to iodine contamination, and in those receiving thyroxine treatment.[3839] Taillefer et al., in 1992, introduced the use of 99 mTc-sestamibi as a single agent in a dual-phase technique.[40] The double-phase technique relies on a more rapid washout of 99 mTc-sestamibi from the thyroid tissue than from the abnormal parathyroid glands [Figure 6]. The dual-phase method is usually performed 10 – 30 minutes and 90 – 180 minutes post injection.[38–40] Ninety minutes post injection is sufficient for preoperative parathyroid imaging.[4142] The thyroid pathology, which can have increased sestamibi uptake and retention, may be mistaken for an abnormal parathyroid gland in the clinical setting of hyperparathyroidism. Correlation with anatomic imaging and the use of subtraction techniques are important in these cases. The use of various imaging techniques for 99 mTc sestamibi parathyroid scintigraphy may be the likely reason for its wide range of diagnostic performances in the literature. In fact, the sensitivity of 99Tc-sestamibi parthyroid scintigraphy for detecting and localizing a single adenoma in patients with primary hyperparathyroidism ranges from 54 to 96%,[254035–44] while the specificity, calculated only in a few studies, ranges from 83 to 99%.[44] Sensitivities were lower for detecting double adenomas (30%) or parathyroid hyperplasia (45%). The metanalysis of Ruda et al.,[45] included 96 studies using 99 mTc-sestamibi scintigraphy, between 1995 and 2003, in the setting of primary hyperparathyroidism. The calculated sensitivity for the detection of solitary adenomas was 88% (95% confidence interval, 87 – 89%). Similar to sonography, sensitivities for the detection of hyperplasia and double adenomas were low, calculated at 44% (95% confidence interval, 41 – 48%) and 30% (95% confidence interval, 2 – 62%), respectively.
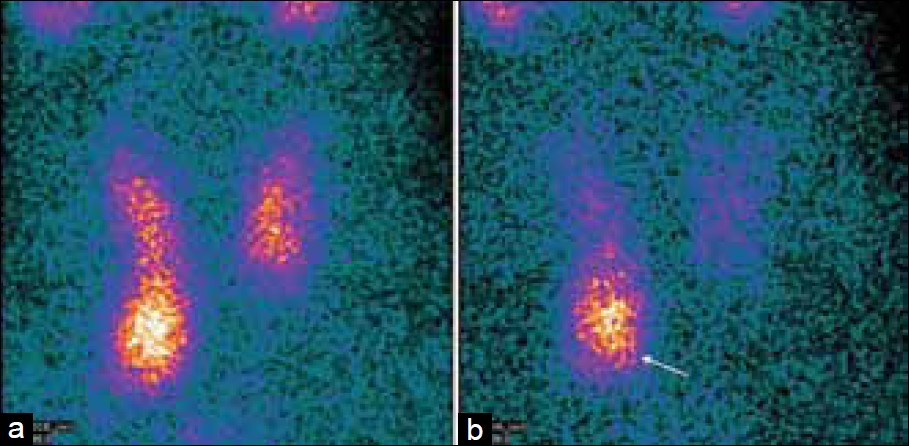
- Differential wash-out of 99mTc-Sestamibi between the normal thyroid gland and parathyroid adenoma. Early images (a) demonstrate the physiological uptake of 99mTc-sestamibi throughout the thyroid gland, which washes out the delayed images. (b) There is a persistent focus on the uptake, inferior to the right lower pole of the thyroid gland, on the early and delayed images (white arrow), which represents a parathyroid adenoma.
The use of single photon emission tomography (SPECT), in association with the planar scintigraphy [Figure 7] has been shown to offer increased sensitivity and provides a more precise localization of the abnormal parathyroid glands, with a better anatomical demarcation of the ectopic lesions.[44–46] A SPECT study should be acquired immediately following early planar acquisitions (to avoid false-negative results due to parathyroid adenomas with rapid washout),[4748] with the patient in the same position. A few studies have directly compared planar with SPECT imaging for the detection of parathyroid adenomas. These studies have demonstrated a higher sensitivity of SPECT for detecting and localizing parathyroid adenomas compared to planar imaging.[454849] The false-negative rate is reported to be lower for SPECT, and SPECT detects small parathyroid adenomas immediately posterior to the thyroid gland better than planar imaging.
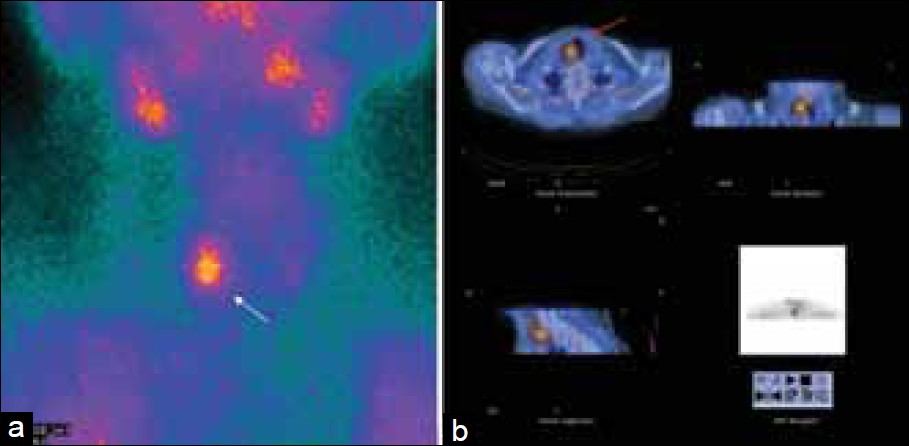
- This figure demonstrates the importance of SPECT / CT for determining the relationship of the parathyroid adenomas with the adjacent structures. (a) Patient with previous thyroidectomy, a focus of uptake is seen in the right cervical area (white arrow). (b) SPECT / CT images localize this focus (red arrow) to the right paratracheal region. At surgery, this was determined to be a true right inferior parathyroid adenoma.
The introduction of hybrid SPECT / CT cameras has improved the sensitivity of parathyroid scintigraphy in the localization of adenomas [Figure 7],[4950] especially in patients with ectopic glands.[46]
Lavely and colleagues[47] performed a comprehensive study of 99 mTc-sestamibi parathyroid scintigraphy in 110 patients with primary hyperparathyroidism. The patients underwent planar, SPECT, and SPECT / CT imaging, early and delayed, after injection of 99mTc-sestamibi. A total of 19 different combinations of imaging techniques were evaluated for each patient. Early SPECT / CT with any delayed imaging emerged as the best methodology for preoperative parathyroid adenoma localization (sensitivity approximately 73%, specificity approximately 99%, accuracy approximately 86%, positive predictive value 86 – 91%, and negative predictive value approximately 98%).
Neumann and colleagues[49] showed that the sensitivity (approximately 70%) for localizing an adenoma did not improve with SPECT / CT, but anatomic localization significantly improved the specificity (96% for SPECT / CT vs. 48% for SPECT alone, P < 0.006).
This innovation might improve the relatively poor results obtained in the detection of multiglandular hyperplasia.
Positron emission tomography
In the last decade, the possible role of positron emission tomography (PET) in the detection of parathyroid adenomas or hyperplasia has been evaluated, with variable success.
In particular, the role of 2-[18 F]-fluoro-2-deoxy-D-glucose (FDG)-PET remains uncertain. A few studies over the past two decades have yielded mixed sensitivities. In fact, although in one study sensitivity was excellent (higher sensitivity compared to 99 mTc sestamibi, but low specificity), the results were unsatisfactory in another study.[50]
In recent times, the use of labeled amino acid 11C-methionine for the detection of parathyroid adenomas has been proposed.
11C-methionine uptake in the parathyroid tissue reflects several phenomena, including protein synthesis and transmembrane amino acid transport. In addition, a correlation between the amount of uptake and the biochemical activity of hyperparathyroidism has been shown.
Several articles have described the high sensitivity of 11C-methionine to localize the abnormal parathyroid glands.[51–61] In a majority of these studies, 11C-methionine has proved to be superior to 99 mTc-sestamibi or other conventional imaging techniques, with sensitivities around 85% and excellent specificity. A recent study has confirmed the diagnostic value of imaging in patients scheduled for reoperative parathyroid surgery.[60] Other studies have shown the benefit of using 11C-L-methionine in patients with nonprimary hyperparathyroidism and hypercalcemia, when conventional 99 mTc-sestamibi imaging has been non-localizing.[62]
Costs and availability have to be kept in mind, restricting 11C-methionine only to a highly preselected patient cohort and also to a special center having an in-house cyclotron and a Radiochemistry Unit.
99 mTc-sestamibi and sonography remain the first-line imaging procedures to be supplemented by 11C-methionine PET / CT in equivocal or negative cases.[63]
Computed tomography
Cross-sectional imaging using multidetector CT is primarily reserved for localization of suspected ectopic parathyroid adenomas and problem-solving, when scintigraphy or SPECT / CT demonstrates an equivocal abnormality. Thin-slice, contrast-enhanced CT images are acquired from the skull base to the mediastinum, and parathyroid adenomas are detected as enhancing soft tissue nodules.
Computed Tomography with contrast medium is an emerging technique for parathyroid adenoma localization, often as a second-line localization technique. A focused search in midline locations between the carotid sheaths from the hyoid to the carina, show the highest detection of adenomas. Ectopic adenomas are more frequently seen when CT with contrast medium is used as a second-line localization technique, when a prior ultrasound has been negative. An important example of the importance of a CT scan is represented by the retropharyngeal parathyroid adenoma. Actually it is not visible with an ultrasound, whereas, a contrast-enhanced CT scan offers precise preoperative localization.
Most parathyroid adenomas (92.7%) are hyperenhancing, a feature that distinguishes them from lymph nodes. Technical parameters important for optimal parathyroid imaging with CT include craniocaudal coverage from the oropharynx to the carina, and a short delay to imaging after contrast administration, is preferred.
In recent times, Beland et al., have described the application of a contrast-enhanced CT for occult parathyroid adenomas.[56] In 2006, four-dimensional computed tomography was introduced as an alternative to traditional imaging.
This contrast CT protocol formats multiplanar images illustrating the perfusion features over time. Using precontrast, postcontrast, and delayed images, the 4D- CT demonstrates the precise anatomic localization of abnormal parathyroid glands, characterized by the rapid uptake and washout.[58–60]
Magnetic resonance
Although less commonly used for preoperative localization than sonography and scintigraphy, an MRI provides a similar sensitivity to other techniques in the detection of the abnormal parathyroid tissue. More commonly, MRI is used in patients with persistent or recurrent hyperparathyroidism, in whom it has been seen to be effective in locating the remaining abnormal parathyroid tissue. Images of the neck are generally obtained with an anterior neck surface coil from the hyoid bone to the sternal notch.
Magnetic resonance acquisition sequences involve T1- and fat-suppressed T2-weighted sequences, preferably with electrocardiogram (ECG) gating, for imaging of the mediastinum.
Parathyroid adenomas have variable MR characteristics, but typically show intermediate-to-low signal intensity on T1-weighted images and high signal intensity on T2-weighted images.
The acquisition of gadolinium-enhanced T1-weighted images, with fat suppression, has not been shown to significantly increase the detection of adenomas when they exhibit T2 hyperintensity.
However, false-negative studies are most commonly associated with adenomas that are isointense on T1 and T2 sequences; the addition of contrast-enhanced images can increase the sensitivity in these cases.[58–60] Grayev et al.,[61] have identified an excellent adjunct for preoperative parathyroid planning in a 3 Tesla MR. Localization has significantly improved with the advent of fat-suppression techniques and the chemical shift.
Diagnostic workup
In the diagnosis and management of primary hyperparathyroidism, the preoperative approach combines the anatomic information of sonography and the physiological information of scintigraphy.[62–70]
This combination shows a high predictive value for the presence and location of solitary adenomas more accutarely than either technique alone. Actually, the ultrasound has the advantage of being more specific regarding the localization to the correct quadrant or side and in relation to the thyroid gland. Scintigraphy clearly has the advantage of detecting ectopic glands, particularly in the mediastinum.[62]
Important advancements in the field of parathyroid disease include, improvements in preoperative localization, the use of intraoperative parathyroid hormone monitoring, and the development of minimally invasive and videoscopic surgical techniques. Although, the cause of primary hyperparathyroidism is still poorly understood, surgical parathyroidectomy results in a long-term cure in greater than 95% of the cases.[63]
Surgery is indicated for all patients with symptomatic primary hyperparathyroidism. Asymptomatic individuals must also be surgically treated when patients are younger than 50 years, have severe hypercalcemia, markedly reduced creatinine clearance, and / or profound osteopenia (according to the NIH Consensus Conference 2002). A precise preoperative localization of parathyroid adenomas, using cervical ultrasound and Sestamibi scintigraphy, enables electing for minimally invasive parathyroidectomy in most of the cases.[64]
Available FREE in open access from: http://www.clinicalimagingscience.org/text.asp?2012/2/1/59/102053
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- The calcium-sensing receptor in normal physiology and pathophysiology: A review. Crit Rev Clin Lab Sci. 2005;42:35-70.
- [Google Scholar]
- Kronenberg HM, Melmed S, Polonsky KS, eds. Williams Textbook of Endocrinology (10th ed). Philadelphia, Pa: Saunders; 2003. p. :1303-72.
- Hypercalcemia and ectopic secretion of parathyroid hormone by an ovarian carcinoma with rearrangement of the gene for parathyroid hormone. N Engl J Med. 1990;323:1324-8.
- [Google Scholar]
- Hypercalcemia of malignancy due to ectopic transactivation of the parathyroid hormone gene. J Clin Endocrinol Metab. 2006;91:580-3.
- [Google Scholar]
- 2004 Parathyroid adenoma. In: DeLellis RA, Lloyd RV, Heitz PU, eds. World Health Organization classification of tumors, pathology and genetics: Tumors of endocrine organs. Lyon France: IARC Press; 2004. p. :128-32.
- [Google Scholar]
- Parathyroid glands. In: Silverberg SG, DeLellis RA, Frable WJ, LiVolsi VA, Wick MR, eds. Silverberg; Principles and Practice of Surgical Pathology and Cytopathology (4th edition). Philadelphia Pa: Churchill Livingstone Elsevier; 2006. p. :2149-68.
- [Google Scholar]
- Tumors of the Parathyroid Gland. In: Atlas of Tumor Pathology. Washington, DC: Armed Forces Institute of Pathology; 1993.
- [Google Scholar]
- Multiple endocrine neoplasia type 1. In: DeLellis RA, Lloyd RV, Heitz PU, Eng C, eds. Pathology and Genetics of Tumors of Endocrine Organs. Lyon France: IARC Press; 2004. p. :218-27.
- [Google Scholar]
- Pre-operative localization of parathyroid adenomas: A comparison of power and colour Doppler ultrasonography with nuclear medicine scintigraphy. Clin Radiol. 2001;56:984-8.
- [Google Scholar]
- Parathyroid localization with high-resolution ultrasound and technetium Tc 99 m sestamibi. Arch Surg. 1999;134:824-30.
- [Google Scholar]
- Parathyroid glands: Combination of (99 m) Tc MIBI scintigraphy and US for demonstration of parathyroid glands and nodules. Radiology. 2000;214:393-402.
- [Google Scholar]
- AIUM practice guideline for the performance of a thyroid and parathyroid ultrasound examination. J Ultrasound Med. 2003;22:1126-30.
- [Google Scholar]
- Factors contributing to negative parathyroid locaization: An analysis of 1000 patients. Surgery. 2008;144:74-9.
- [Google Scholar]
- The role of Color Doppler Ultrasonography in the preoperative localization of parathyroid adenomas. Endocr J. 2012;59:375-82.
- [Google Scholar]
- Color Doppler sonography: An adjunctive technique in assessment of parathyroid adenomas. J Ultrasound Med. 1994;13:303-8.
- [Google Scholar]
- Use of color and power Doppler sonography to identify feeding arteries associated with parathyroid adenomas. AJR Am J Roentgenol. 1998;171:819-23.
- [Google Scholar]
- Sonography in primary hyperparathyroidism: Review with emphasis on scanning technique. J Ultrasound Med. 2002;21:539-52.
- [Google Scholar]
- New perspectives in localizing enlarged parathyroids by technetium- thallium subtraction scan. J Nucl Med. 1983;24:438-41.
- [Google Scholar]
- Technetium-thallium scintiscanning for localization of parathyroid adenomas and hyperplasia.A reappraisal. Am J Surg. 1987;153:479-86.
- [Google Scholar]
- 99Tcm sestamibi--a new agent for parathyroid imaging. Nucl Med Commun. 1989;10:791-4.
- [Google Scholar]
- Tc-99 m tetrofosmin. A new diagnostic tracer for parathyroid imaging. Clin Nucl Med. 1995;20:902-5.
- [Google Scholar]
- Uptake of technetium-99 m tetrofosmin, technetium-99 m MIBI and thallium-201 in tumor cell lines. J Nucl Med. 1996;37:551-6.
- [Google Scholar]
- Comparison of technetium-99 m-MIBI, technetium-99 m-tetrofosmin, ultrasound and MRI for localization of abnormal parathyroid glands. J Nucl Med. 1998;39:320-4.
- [Google Scholar]
- Technetium-99 m-tetrofosmin for parathyroid scintigraphy: Comparison to thallium-technetium scanning. J Nucl Med. 1998;39:1433-41.
- [Google Scholar]
- Technetium-99 m-tetrofosmin for parathyroid scintigraphy: A comparison with sestamibi. J Nucl Med. 1997;38:831-4.
- [Google Scholar]
- Technetium-99 m tetrofosmin for parathyroid scintigraphy: A direct comparison with (99 m)Tc-MIBI, (201)Tl, MRI and U S. Eur J Nucl Med. 2001;28:1817-27.
- [Google Scholar]
- Discordant results in Tc-99 m tetrofosmin and Tc-99 m sestamibi parathyroid scintigraphies. Arq Bras Endocrinol Metabol. 2007;51:1166-8.
- [Google Scholar]
- 99mTc-tetrofosmin or 99mTc-sestamibi for double-phase parathyroid scintigraphy? Eur J Nucl Med Mol Imaging. 2003;30:193-6.
- [Google Scholar]
- Differences between 99mTc-sestamibi and 99mTc-tetrofosmin uptake in thyroid and salivary glands: Comparison with 99mTc-pertechnetate in 86 subjects. Nucl Med Commun. 2003;24:321-6.
- [Google Scholar]
- Non equivalent results of tetrofosmin and sestamibi imaging of parathyroid tumors. Endocr Pract. 2006;12:179-82.
- [Google Scholar]
- Parathyroid imaging with technetium-99 m-sestamibi: Preoperative localization and tissue uptake studies. J Nucl Med. 1992;33:313-8.
- [Google Scholar]
- Localization of parathyroid enlargement: Experience with technetium-99 m MIBI and thallium-201 scintigraphy, ultrasonography and computerized tomography. Eur J Nucl Med. 1994;21:17-22.
- [Google Scholar]
- Preoperative imaging of abnormal thyroid glands in patients with hyperparathyroid disease using combination Tc-99 m pertechnetate and Tc-99 m sestamibi radionuclide scans. Ann Surg. 1994;5:568-72. discussion 572-3
- [Google Scholar]
- Visualization of a parathyroid adenoma with Tc-99 m MIBI in a case with iodine saturation and impaired thaUium uptake. Clin Nucl Med. 1993;18:214-6.
- [Google Scholar]
- Detection and localization of parathyroid adenomas in patients with hyperparathyroidism using a single radionuclide imaging procedure with technetium-99 m-sestamibi (double-phase study) J Nucl Med. 1992;33:1801-7.
- [Google Scholar]
- Single late phase sestamibi subtraction scintigraphy for parathyroid lesion localization. Eur J Nucl Med. 1996;23:1169.
- [Google Scholar]
- Rapid washout of technetium 99 m MIBI from a large parathyroid adenoma. J Nucl Med. 1995;36:241-3.
- [Google Scholar]
- Double phase parathyroid technetium-99 m-MIBI scintigraphy to identify functional autonomy in secondary hyperparathyroidism. J Nucl Med. 1996;37:565-9.
- [Google Scholar]
- Sestamibi parathyroid scintigraphy: A prospective study in 100 patients. J Nucl Med. 1995;36:202.
- [Google Scholar]
- Advantages of SPECT in technetium-99 m-sestamibi parathyroid scintigraphy. J Nucl Med. 1996;37:1773-8.
- [Google Scholar]
- Double-phase Tc-99 m sestamibi scintigraphy in the preoperative location of lesions causing hyperparathyroidism. Clin Nucl Med. 1998;23:291-7.
- [Google Scholar]
- Comparison of SPECT/CT, SPECT, and planar imaging with single and dual-phase (99m)Tc-sestamibi parathyroid scintigraphy. J Nucl Med. 2007;48(7):1084-9.
- [Google Scholar]
- Preoperative localization of parathyroid lesions.Value of 99mTc-MIBI tomography and factors influencing detection. Nuklearmedizin. 2008;47(4):158-62.
- [Google Scholar]
- Preoperative 123I/99mTc-sestamibi subtraction SPECT and SPECT/CT in primary hyperparathyroidism. J Nucl Med. 2008;49()(12):2012-7.
- [Google Scholar]
- PET and parathyroid L-[carbon-11]methionine accumulation in hyperparathyroidism. J Nucl Med. 1996;37:1766-70.
- [Google Scholar]
- [11C]methionine positron emission tomography for patients with persistent or recurrent hyperparathyroidism after surgery. Eur J Endocrinol. 1998;139:195-7.
- [Google Scholar]
- Localization of parathyroid adenomas using 11C-methionine positron emission tomography. Nucl Med Commun. 2005;26:133-6.
- [Google Scholar]
- 11C-methionine PET / CT in 99mTc-sestamibi negative hyperparathyroidism in patients with renal failure on chronic haemodialysis. Eur J Nucl Med Mol Imaging. 2006;33:453-9.
- [Google Scholar]
- Pre-operative localisation of hyperfunctional parathyroid tissue with 11C-methionine PET. Eur J Nucl Med Mol Imaging. 2004;31:1405-12.
- [Google Scholar]
- High success rate of parathyroid reoperation may be achieved with improved localization diagnosis. World J Surg. 2008;32:774-81. discussion 782-3
- [Google Scholar]
- Dynamic MDCT for localization of occult parathyroid adenomas in 26 patients with primary hyperparathyroidism. AJR Am J Roentgenol. 2011;196:61-5.
- [Google Scholar]
- Improved preoperative planning for directed parathyroidectomy with 4-dimensional computed tomography. Surgery. 2006;140:932-40. s discussion 940-1
- [Google Scholar]
- Preoperative contrast-enhanced MRI of the parathyroid glands in hyperparathyroidism. Invest Radiol. 2000;35:426-30.
- [Google Scholar]
- Comparison between MR imaging and 99mTc MIBI scintigraphy in the evaluation of recurrent or persistent hyperparathyroidism. Radiology. 2001;218:783-790.
- [Google Scholar]
- MR signal intensity of parathyroid adenomas: Correlation with histopathology. AJR Am J Roentgenol. 1989;153:873-6.
- [Google Scholar]
- Presurgical localization of parathyroid adenomas with magnetic resonance imaging at 3 T: An adjunct method to supplement traditional imaging. Ann Surg Oncol. 2012;19:981-9.
- [Google Scholar]
- Parathyroid imaging: Technique and role in the preoperative evaluation of primary hyperparathyroidism. AJR Am J Roentgenol. 2007;188:1706-15.
- [Google Scholar]
- Prospective evaluation of delayed technetium-99 m sestamibi SPECT scintigraphy for preoperative localization of primary hyperparathyroidism. Surgery. 2002;131:149-57.
- [Google Scholar]
- Parathyroid imaging using simultaneous double-window recording of technetium-99 m-sestamibi and iodine-123. J Nucl Med. 1998;39:1100-5.
- [Google Scholar]
- Parathyroid adenomas: Accurate detection and localization with Tc-99 m sestamibi SPECT. Radiology. 1996;201:85-91.
- [Google Scholar]
- Efficient parathyroidectomy guided by SPECT-MIBI and hormonal measurements. J Nucl Med. 1996;37:798-804.
- [Google Scholar]
- A systematic review of the diagnosis and treatment of primary hyperparathyroidism from 1995 to 2003. Otolaryngol Head Neck Surg. 2005;132:359-72.
- [Google Scholar]
- Comparison of FDGPET and sestamibi-SPECT in primary hyperparathyroidism. J Nucl Med. 1996;37:1809-15.
- [Google Scholar]






