Translate this page into:
Preoperative embolization of hypervascular spinal tumors: Two case reports

*Corresponding author: Dr. Nguyen Minh Duc, Department of Radiology, Pham Ngoc Thach University of Medicine, Ho Chi Minh, Vietnam. bsnguyenminhduc@pnt.edu.vn
-
Received: ,
Accepted: ,
How to cite this article: Binh NT, Hoa TQ, Linh LT, My TTT, Anh PQ, Duc NM. Preoperative embolization of hypervascular spinal tumors: Two case reports. J Clin Imaging Sci 2022;12:21.
Abstract
The performance of preoperative embolization on a spinal tumor can be a useful adjunctive procedure that minimizes blood loss and complications, particularly for both metastatic and non-metastatic hypervascular tumors. We discuss two cases of hypervascular spinal tumors that were successfully treated with preoperative embolization and surgery. The first patient was an 18-year-old man who presented with cervical and shoulder pain for two months without paralysis or weakness. Magnetic resonance imaging revealed a tumor located in the D2 posterior vertebral arch that extended into the spinal canal and compressed the spinal cord. The second patient was a 68-year-old man with back pain that radiated to the legs for ten days. Magnetic resonance imaging revealed a mass in the L4 vertebral body. Both patients received tumor embolization and surgery. After surgery, neither patient experienced any major complications. Histological examination revealed osteoblastoma in the first patient and plasmacytoma in the second patient.
Keywords
Hypervascular tumor
Spinal tumor
Preoperative embolization
Angiography
INTRODUCTION
The surgical resection of spinal tumors is necessary to reduce pain, improve neurological function, maintain spinal stability, and provide local tumor control. However, the surgical resection of hypervascular tumors is associated with high risks of intraoperative blood loss and complications.[1] Preoperative embolization (PE) is a useful adjunctive procedure for improving surgical outcomes and reducing complications in patients with hypervascular spinal tumors.[2] First reported by Benati et al.,[3] PE not only reduces intraoperative blood loss and facilitates complete tumor resection but also reduces the mass effect and relieves spinal cord compression.[4] We report two cases that highlight how patients with hypervascular tumors can benefit from PE.
CASE REPORTS
Case 1
An 18-year-old man was hospitalized at our clinic for left-sided neck stiffness and shoulder pain that lasted for approximately two months. The patient had no symptoms of weakness, paralysis, loss of sensation, or numbness, and his medical history was normal. Spinal magnetic resonance imaging (MRI) revealed a mass arising from the left posterior vertebral arch of T2 and extending into the vertebral body of T2 [Figure 1]. The mass had ill-defined borders, appeared hypointense on T1-weighted (T1W) images, and hyperintense on T2-weighted (T2W) and short tau inversion recovery (STIR) images. The tumor extended into paraspinal tissue and the spinal canal, causing spinal cord compression [Figure 1]. This tumor was markedly enhanced on T1W with a contrast agent. A computed tomography (CT) image showed that the tumor was mostly lytic, with a rim of sclerosis and small areas of internal calcification [Figure 2]. These imaging features suggested that the tumor was likely an osteoblastoma or osteosarcoma. No additional lesions were identified on the chest or abdominal CT scans. We recognized this was a highly vascular tumor and decided to perform PE instead of obtaining a biopsy before surgery due to the increased risk of bleeding into the spinal canal. Angiography indicated that the supply vessels arose from the left thyrocervical trunk and radiculomedullary artery. The selective embolization of the feeding arteries was performed using 250-micron embozene microspheres, resulting in the successful occlusion of all branches supplying the tumor while conserving the other branches of the left thyrocervical trunk [Figure 3]. Post-intervention, the patient was stable without complications. Thirty-six hours after embolization, the patient underwent posterior decompression with total laminectomy of T1–T3 and resection of the left transverse process and pedicle of T2. The tumor had expanded into the surrounding tissue, preventing complete tumor removal. Pathology results indicated that the tumor was an osteoblastoma. One month after surgery, the patient was stable without neurological deficits or bleeding.
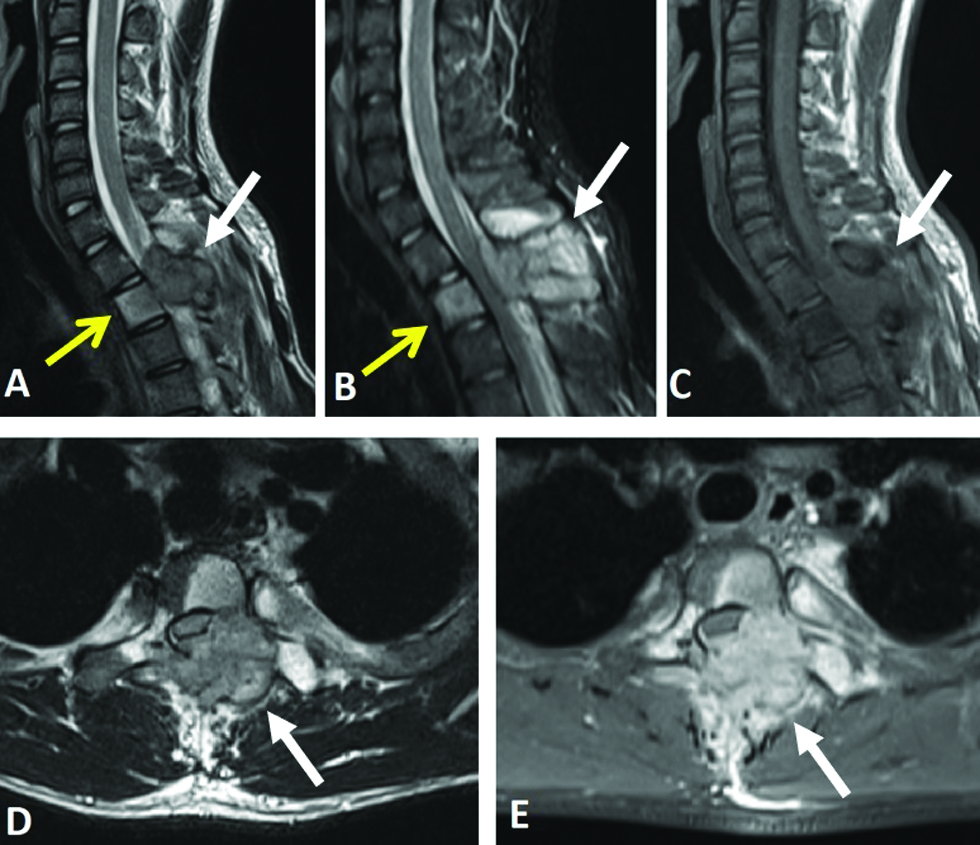
- An 18-year-old man suffering from left-sided neck stiffness and shoulder pain underwent spinal magnetic resonance imaging. Magnetic resonance images of the tumor. (A and B) Sagittal T2-weighted and short tau inversion recovery images indicated that the lesion on the left posterior vertebral arch of T2 (white arrows) was hyperintense compared with the bone marrow extending to the T2 vertebral body (yellow arrows). (C) The lesion was hypointense on axial T1-weighted images. (D) The mass compressed the spinal cord (arrow). (E) The mass was markedly enhanced on axial T1-weighted with contrast agent and extended to the paraspinal tissue (arrow).
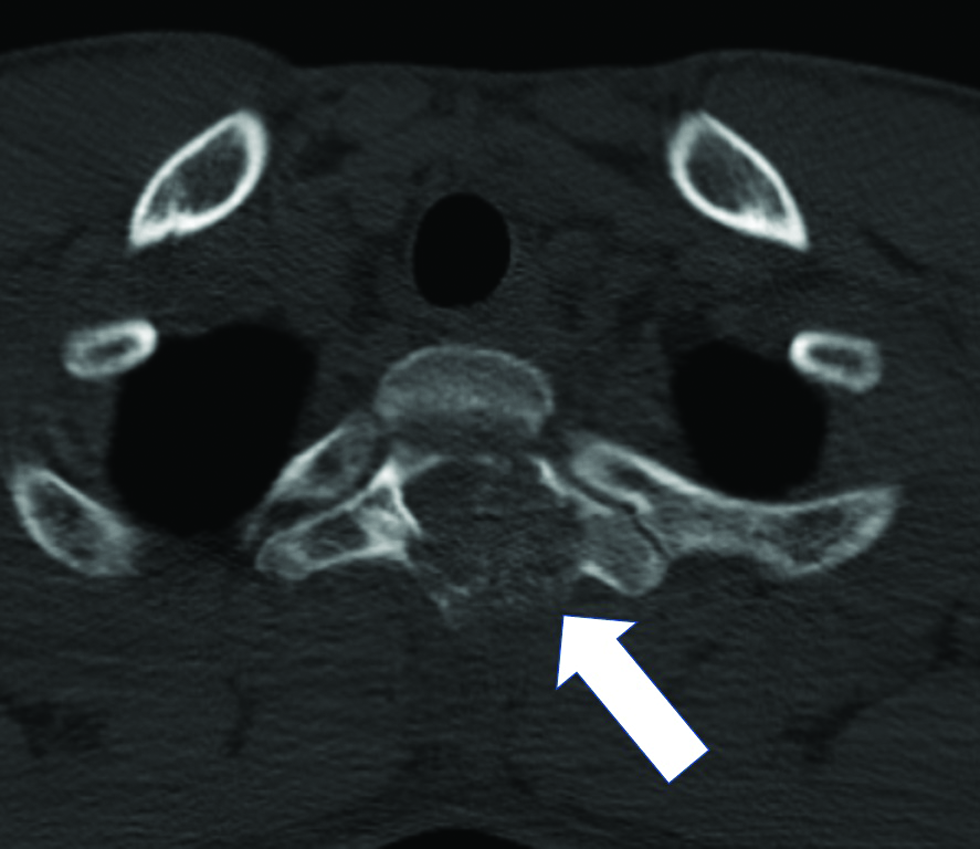
- An 18-year-old man suffering from left-sided neck stiffness and shoulder pain underwent computed tomography. Computed tomography scan revealing that the tumor was lytic with a rim of sclerosis and a small area of internal calcification (arrow).
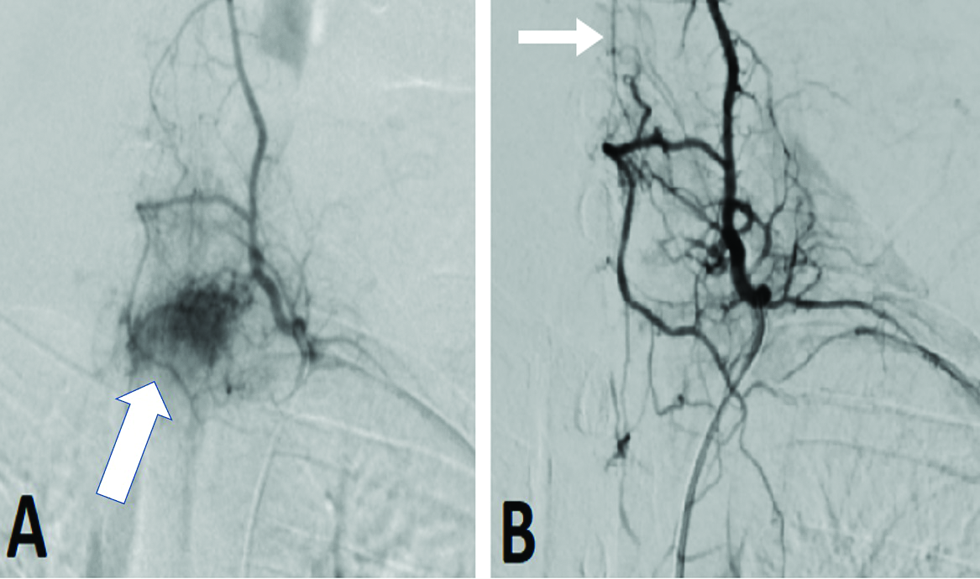
- An 18-year-old man suffering from left-sided neck stiffness and shoulder pain underwent digital subtraction angiography. (A) Selective angiogram demonstrated a hypervascular tumor with a feeding artery arising from the left thyrocervical trunk (arrow). (B) Postembolization angiogram showed complete devascularization, with no reflux into the radiculomedullary artery (arrow).
Case 2
A 68-year-old man with no remarkable medical history was admitted to our hospital with moderate back pain that radiated to the legs for ten days. The patient did not have any history of trauma. A spinal X-ray showed a degenerated lumbar spine. An MRI of the lumbar spine was performed, revealing that the vertebral body of the L4 was isointense on T1W and T2W but hyperintense on STIR [Figure 4]. The initial diagnosis was bone marrow edema. Therefore, we decided to perform a spinal CT. On CT, a well-defined osteolytic lesion could be observed without a sclerotic border and no evidence of internal mineralization [Figure 5]. No periosteal reaction or evidence of cortical breach or soft tissue mass was detected. Chest CT findings suggested a giant cell tumor of the vertebral body. We conducted a biopsy of the lesion, and the histopathological results revealed plasmacytoma. Blood test results, including complete blood count and liver and kidney function tests, were normal. Because this tumor was osteolytic and the risk of vertebral collapse was identified, this patient required spine fixation surgery. We performed angiography to assess tumor-feeding blood vessels before surgery. Selective angiography demonstrated a feeding vessel originating from the bilateral L4 segmental arteries [Figure 6]. Because the tumor was hypervascular, surgery was associated with a high risk of bleeding or hematoma development which would compress the spinal cord. We opted to perform tumor PE. Based on the tumor location, the tumor feeders were expected to arise from the branches of the L4 segmental arteries, and the embolization of the proximal and trunk L4 segmental arteries using glue (N-butyl cyanoacrylate mixed with lipiodol at a 1:2.5 ratio) was successful [Figure 6]. Two days later, the patient underwent tumor removal and spinal fixation surgery with cement augmentation. No post-surgery focal motor or sensory deficits were reported.
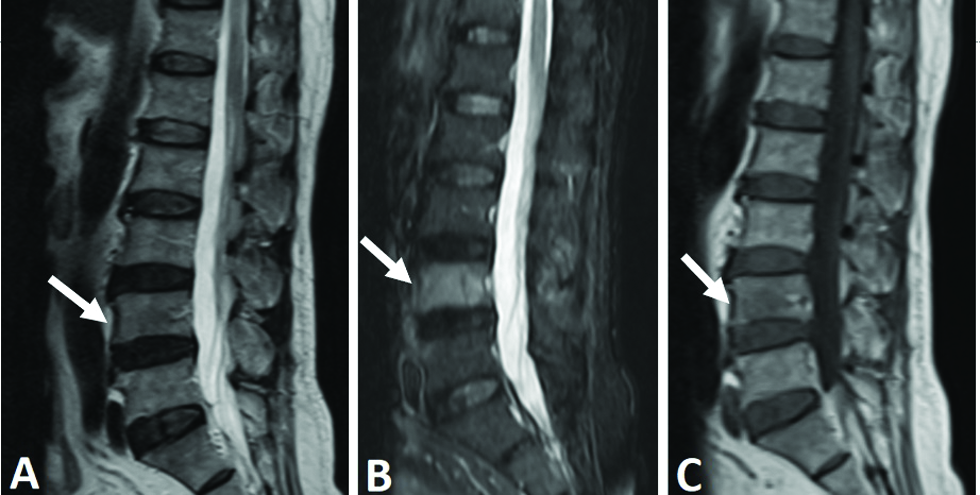
- A 68-year-old man with moderate back pain that radiated to the legs underwent spinal magnetic resonance imaging. The L4 vertebral body was isointense on sagittal T2-weighted (A) (arrow) and sagittal T1-weighted (C) (arrow) images but hyperintense on short tau inversion recovery (B) (arrow) images compared with the bone marrow.
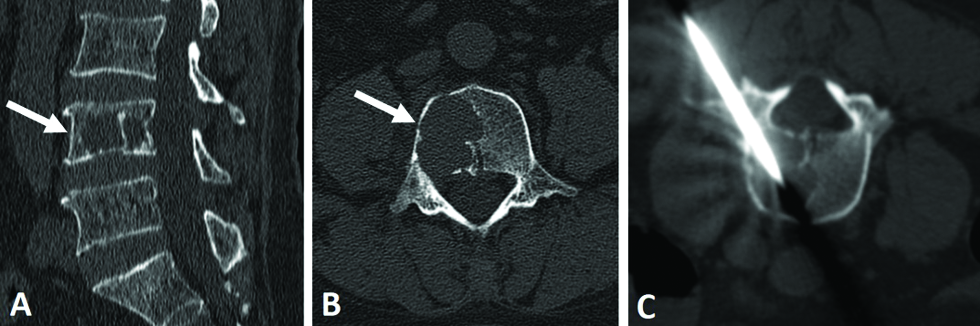
- A 68-year-old man with moderate back pain that radiated to the legs underwent computed tomography. (A and B) The lesion was well-defined and osteolytic, without sclerotic borders or internal calcification (arrow). (C) A CT-guided biopsy of the lesion was performed.
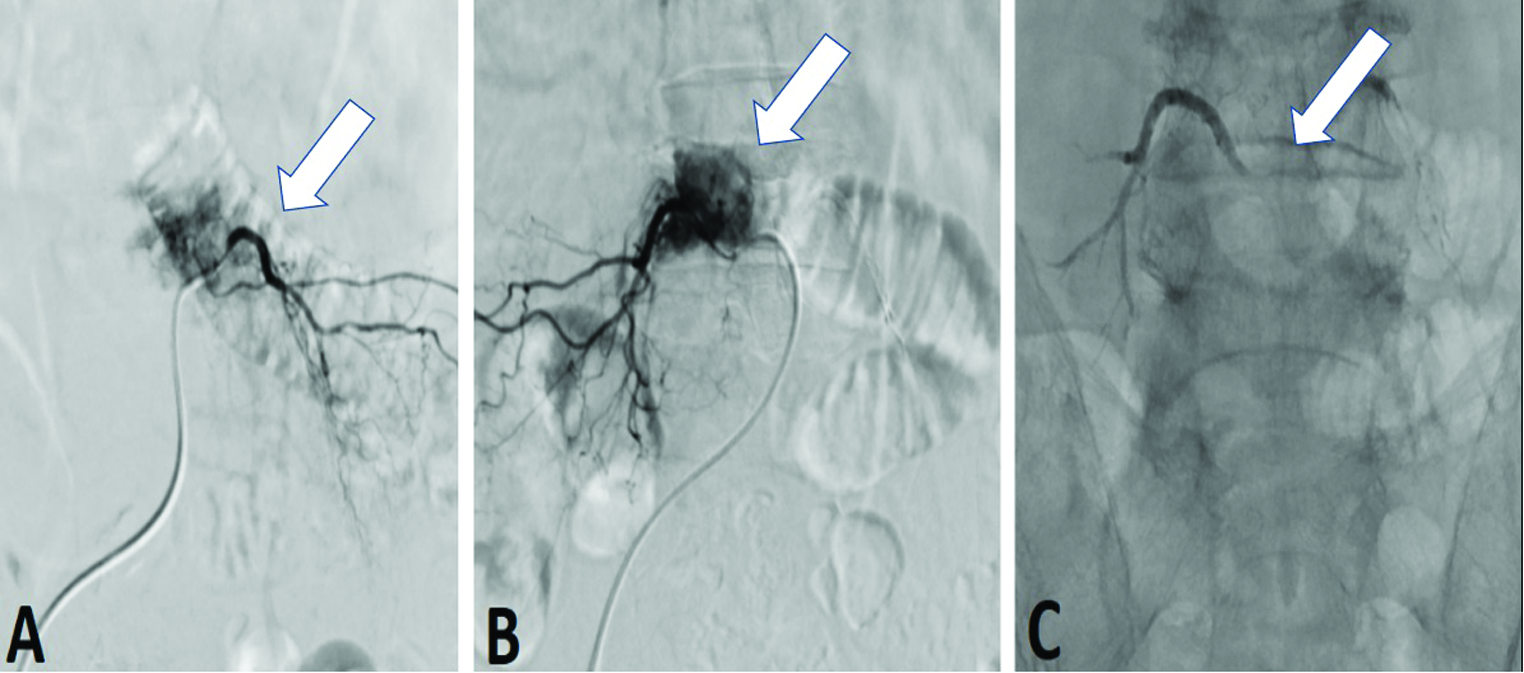
- A 68-year-old man with moderate back pain that radiated to the legs underwent digital subtraction angiography. A markedly hypervascular tumor at the L4 vertebral was observed, receiving blood from the bilateral L4 segmental arteries (A and B) (arrow). (C) Post-embolization angiogram demonstrated no residual tumor (arrow).
DISCUSSION
Spinal tumor PE is often used to prevent potential complications and improve resectability, especially in cases of hypervascular tumors. Metastatic tumors represent 74.1% of spinal tumors.[5] Hemangioblastoma, aggressive vertebral hemangiomas, and aneurysmal bone cysts are the most commonly reported highly vascular primary spinal tumors,[6] whereas the most commonly identified hypervascular metastatic spinal tumors are renal cell carcinoma and thyroid carcinoma.[7]
Multiple embolic agents are available, including coils, particles, liquid embolic agents, chemical embolic agents, or combinations of these options, and their use will depend on the tumor vascularity and anatomy.[8] The tumor angioarchitecture, surrounding anatomy, and surgical intent must be considered before performing embolization.[8] Coil embolization is a simple and safe technique, especially when radiculomedullary anastomoses are detected, which are associated with a high risk of spinal cord ischemia if other agents are used.[8] Coils are effective options when a super-selective catheterization approach cannot be identified or for embolizing surrounding arteries to facilitate successful surgery. However, coil embolization is associated with a high risk of tumor revascularization.[8,9]
Particle embolization is another option for tumor devascularization. Although a wide range of particle diameters are available, 150-250-micron-diameter particles are most commonly used to achieve occlusion of the capillary bed.[5] Smaller particle sizes may also be used to achieve complete devascularization. However, particle delivery can be difficult to control. In cases with intratumoral shunting, larger particles (>250 mm) are often used to reduce the risk of spinal cord ischemia, but larger particles may also prevent complete devascularization.[9]
N-butyl-2 cyanoacrylate is a commonly used liquid embolic agent that can rapidly and completely fill blood vessels with precise control.[5] However, this agent is associated with a risk of reflux or adherence to microcatheters, affecting the parent artery or other non-target sites. The choice of embolization method depends on many factors, and the use of combination methods is sometimes necessary upon consultation with the spine surgeon.
In the first patient, the tumor involved the body, posterior elements, and surrounding soft tissues of the T2 vertebra. After consulting the spine surgeon, complete removal was determined to be nearly impossible. Due to the observed hypervascular nature of the tumor, we desired maximal devascularization to reduce potential blood loss, resulting in the selection of particle embolization. In the second patient, complete embolization was unnecessary because the goal of the surgery was spinal fixation. Therefore, we opted to perform glue-based embolization to control the flow of the embolic agent and avoid reflux to the radiculomedullary artery.
To date, no guidelines have been established for the optimal timing of PE and surgery. The timing described for PE in prior studies has ranged from 24 hours to 14 days before surgery.[5] Blood loss appears to be less likely when surgery is performed within three days after embolization.[6] One study indicated that embolism is most effective when embolization is performed within 24 hours of surgery because tumor swelling can compress the spinal cord if the interval to surgery is too long.[5] By contrast, another study found no correlation between the effectiveness of embolization and time to surgery.[10] However, we recommend that surgery should be performed as soon as possible following embolization to reduce the risk of rapid revascularization or increased spinal cord compression secondary to tumor swelling or hemorrhage.
PE may improve the visualization of the operative field, decrease complications associated with intraoperative blood loss, and reduce surgical operating time.[4] Several studies have indicated that patients who undergo PE experience surgical and clinical benefits compared to patients who did not undergo PE.[2,11-13] Complete embolization can be achieved in 50-86% of patients.[4] Incomplete embolization can occur due to the presence of nearby radiculomedullary arteries, the inability to selectively catheterize feeding arteries, and intratumoral arteriovenous shunting.[4,8] Intraoperative bleeding remains a risk in cases of partial embolization, and complete devascularization is preferable.[8] Studies have demonstrated that intraoperative blood loss in the embolization group is significantly reduced compared to the non-embolization group.[2,11,13,14]
The complication rate associated with the embolization procedure is typically low, at approximately 3%.[7,9,11] Reported complications include groin hematoma, cardiac event secondary to anesthesia, common femoral artery thrombosis, bleeding, and neurologic injuries, such as acute stroke, nerve root injury, and spinal cord ischemia.[2,7,12,14] Spinal cord infarction and the deterioration of neurological status are major complications associated with embolization.[2] Spinal cord infarction can occur secondary to the occlusion of the anterior or posterior spinal arteries, whereas acute stroke can occur due to the reflux of embolic material into the parent artery, followed by transport to the brain.[5] Neurologic injuries can also be caused by postembolization tumoral swelling, which may compress the spinal cord.[5]
CONCLUSION
PE performed in tumors located in the spine is an effective procedure for reducing surgical complications and blood loss. Neurologic injury is a major and serious complication of this procedure. Choosing appropriate embolic agents is important and consultation with the surgeon regarding the type of operation is required to ensure the best outcome.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Intraoperative blood loss in oncological spine surgery. Neurosurg Focus. 2021;50:E14.
- [CrossRef] [PubMed] [Google Scholar]
- Only tumors angiographically identified as hypervascular exhibit lower intraoperative blood loss upon selective preoperative embolization of spinal metastases: Systematic review and meta-analysis. Front Oncol. 2021;10:597476.
- [CrossRef] [PubMed] [Google Scholar]
- Transfemoral selective embolisation in the treatment of some cranial and vertebro-spinal vascular malformations and tumours. Preliminary results. J Neurosurg Sci. 1974;18:233-8.
- [PubMed] [Google Scholar]
- Embolization of spinal tumors: Vascular anatomy, indications, and technique. Tech Vasc Interv Radiol. 2011;14(3):129-40.
- [CrossRef] [PubMed] [Google Scholar]
- Neurologic complications of preoperative embolization of spinal metastasis: A systemic review of the literature identifying distinct mechanisms of Injury. World Neurosurg. 2020;143:374-88.
- [CrossRef] [PubMed] [Google Scholar]
- Preoperative embolization of hypervascular spinal tumors: Current practice and center experience. Neurol Res. 2014;36:502-9.
- [CrossRef] [PubMed] [Google Scholar]
- Preoperative embolization of hypervascular thoracic, lumbar, and sacral spinal column tumors: Technique and outcomes from a single center. Interv Neuroradiol. 2013;19:377-85.
- [CrossRef] [PubMed] [Google Scholar]
- The evolution of pre-operative spine tumour embolization. Br J Radiol. 2019;92:20180899.
- [CrossRef] [PubMed] [Google Scholar]
- Preoperative embolization of spinal tumors: A systematic review and meta-analysis. World Neurosurg. 2016;87:362-71.
- [CrossRef] [PubMed] [Google Scholar]
- Risk factors for major complications in surgery for hypervascular spinal tumors: An analysis of 120 cases with adjuvant preoperative embolization. Eur Spine J. 2015;24:2201-8.
- [CrossRef] [PubMed] [Google Scholar]
- Preoperative embolization in patients with metastatic spinal cord compression: Mandatory or optional? World J Surg Oncol. 2017;15:45.
- [CrossRef] [PubMed] [Google Scholar]
- Retrospective analysis of preoperative embolization of spinal tumors. Am J Neuroradiol. 2010;31:656-60.
- [CrossRef] [PubMed] [Google Scholar]
- Preoperative embolization in surgical treatment of spinal metastases: single-blind, randomized controlled clinical trial of efficacy in decreasing intraoperative blood loss. J Vasc Interv Radiol. 2015;26:402-12.e1.
- [CrossRef] [PubMed] [Google Scholar]
- Systematic review and meta-analysis of effectiveness of preoperative embolization in surgery for metastatic spine disease. J NeuroInterventional Surg. 2018;10:596-601.
- [CrossRef] [PubMed] [Google Scholar]







