Translate this page into:
Multimodality imaging in cardiac sarcoidosis: A case series of diverse phenotypes

*Corresponding author: Minjie Lu, Department of Magnetic Resonance Imaging, Fuwai Hospital, Beijing, China. coolkan@163.com
-
Received: ,
Accepted: ,
How to cite this article: Qiao H, Tian H, Jiang M, Li J, Lan T, Lu M. Multimodality imaging in cardiac sarcoidosis: A case series of diverse phenotypes. J Clin Imaging Sci. 2025;15:5. doi: 10.25259/JCIS_143_2024
Abstract
Cardiac sarcoidosis (CS) represents a rare yet potentially life-threatening condition characterized by non-specific clinical symptoms that maybe easily missed by clinicians. In this case series, the clinical presentations, various imaging modalities’ characteristics, and the management of four patients, each with distinct phenotypes of CS confirmed through endomyocardial biopsy, are discussed. Advanced imaging techniques, including positron emission tomography, revealed the focal septal uptake of 18F fluorodeoxyglucose, which suggests an ongoing inflammation, whereas contrast-enhanced cardiac magnetic resonance demonstrates septal late gadolinium enhancement, which indicates replacement fibrosis. These features of multimodality imaging in CS can assist in patient diagnosis and treatment.
Keywords
Cardiac sarcoidosis
Cardiac imaging techniques
Magnetic resonance imaging
Heart failure
INTRODUCTION
Sarcoidosis refers to a systemic inflammatory disease characterized by non-caseating granulomas in multiple organs. Cardiac sarcoidosis (CS) features non-specific clinical manifestations, which accounts for approximately 5% of patients with sarcoidosis, and low sensitivity of endomyocardial biopsy, which is often overlooked in clinical settings.[1] However, cardiac involvement considerably influences prognosis, which makes the diagnosis of CS particularly challenging.[2-4] With the wide development of cardiac magnetic resonance (CMR) and positron emission tomography (PET) with 18F-fluorodeoxyglucose (FDG), CS has garnered increased attention from clinical physicians.[5,6]
The clinical manifestations of CS are diverse and mainly include arrhythmia, heart failure, and sudden cardiac death.[7] Given that the prognosis of CS depends on phenotype and other factors, an early and precise diagnosis is necessary. Given the great mimicker of CS, some patients with CS are underdiagnosed and misdiagnosed. The use of multimodal imaging results is crucial to improving the sensitivity of CS diagnosis.[8,9] Therefore, we present multimodality imaging findings from four cases with various CS phenotypes confirmed through endomyocardial biopsy at our hospital.
CASE SERIES
Case 1
A 44-year-old male experiencing dyspnea was diagnosed with first-degree atrioventricular block (AVB) and right bundle branch block (Panel A). Transthoracic echocardiogram (TTE) revealed thickening of the ventricular septum and the right ventricle. Elevated levels of C-reactive protein and troponin I were also observed. CMR revealed the uneven thickening of the left and right ventricular walls and multiple significantly enhanced lesions on the late gadolinium enhancement (LGE) sequence (Panels B-D). Chest computed tomography (CT) was negative and without evidence of extracardiac lymphadenopathy (Panel E). 18F-FDG-PET displayed an increased FDG uptake in the ventricular septum and left and right ventricles (Panel F). Myocardial biopsy exhibited intramyocardial focal inflammatory cell infiltration with multinucleated giant cells (Panel K). After the confirmed diagnosis of CS, the patient received steroids and immunosuppressive therapy, which relieved them of their symptoms. A repeat CMR performed 4 months later revealed a reduced size of nodular enhancement (Panel H-J). The characteristics observed in CMR, which are indicative of active inflammatory infiltration, may offer valuable insights into efficacy evaluation during follow-up.
Case 2
A 52-year-old female was admitted to the hospital after experiencing intermittent heart palpitations that lasted 1 month. Her N-terminal prohormone of brain natriuretic peptide (NT-proBNP) was slightly elevated at 805 pg/mL. Electrocardiogram (ECG) revealed short-array ventricular tachycardia (Panel A). TTE displayed enlargement of the right atrium and left and right ventricles and decreased left ventricular ejection fraction (LVEF) (LVEF 42%) (Panel B). CMR revealed thickening of the left and right ventricle walls, which corresponded to evident patchy transmural LGE (Panels C-H). Endocardial myocardial biopsy confirmed CS (Panels I-J). The patient was eventually implanted with a cardioverter defibrillator. This case may lead to an impression of inflammatory or infiltrative cardiomyopathy.
Case 3
A 37-year-old male presenting with palpitations and shortness of breath for more than half a year was transferred to our hospital. NT-proBNP was elevated (1041 pg/mL). ECG showed first-degree AVB, and abnormal P and Q waves (Panel A). Chest CT was negative (Panel B). TTE revealed a decreased LVEF (37%). CMR unveiled an evidently enlarged left ventricle, thinned ventricular wall, and a patch of LGE areas in the right ventricle, interventricular septum, and ventricular free walls (Panels C-E). 18F-FDG-PET indicated perfusion defects, which is consistent with the lesions observed on CMR imaging (Panel I). Endomyocardial biopsy revealed granulomatous nodules consistent with CS (Panel J). The patient was given steroid therapy, which considerably relieved his symptoms. The case may lead to a misdiagnosis of arrhythmogenic left ventricular cardiomyopathy (ALVC).
Case 4
A 57-year-old male experiencing intermittent chest tightness and shortness of breath for 4 years was admitted to our hospital. NT-proBNP was markedly elevated (5444 pg/mL). ECG showed atrial fibrillation (Panel A). TTE revealed enlargement of the whole heart and decreased function of the left heart (LVEF 20%). CT unveiled multiple enlarged lymph nodes in the mediastinum and both hila (Panel B). CMR displayed an overall enlargement of the heart with scattered mid myocardial LGE areas in the basal interventricular septum (Panels C-E). 18F-FDG-PET showed no significant update in focal FDG update (Panel F). This patient eventually underwent a heart transplant due to end-stage heart failure. The recipient heart was pathologically confirmed to have scattered granulomatous nodules (Panel G). This case may be misinterpreted as dilated cardiomyopathy.
DISCUSSION
In our study, 4 patients with CS had arrhythmia and/or cardiac insufficiency as the main clinical manifestations, and they were easily misdiagnosed as other cardiomyopathies in clinical practice. This finding suggests that multimodal imaging techniques play various important roles in the diagnosis of cardiac nodules.
Modalities, such as echocardiography, CT, CMR, and PET, are highly valuable for the assessing of myocardial involvement in sarcoidosis. Echocardiography is the preferred initial choice, and it can be used to identify abnormal wall motion and reduced ejection fraction, although the findings are not definitive for diagnosis. Chest CT is well recognized for its capability to characterize enlarged lymph nodes in the mediastinum and hilar. However, this procedure is limited in facilitating the early diagnosis of CS.
PET can detect both cardiac and extracardiac inflammation and is valuable for monitoring therapy and predicting patient outcomes. Patients with CS display focal FDG uptake of the myocardium. A “hot spot” of 18F-FDG-PET with a perfusion defect serves as a hallmark in the detection of CS.[10] A recent meta-analysis reported PET sensitivity and specificity at 84% and 83%, respectively.[11] Absent FDG uptake combined with abnormal perfusion indicates end-stage myocardial scarring, as illustrated by the imaging of the patient 3. A mismatch pattern and right ventricle uptake are important predictors of cardiac events.[12,13] The widespread application of these findings is constrained by the variability in methodologies and differences in interpretation approaches.[14]
For patients suspected of having CS, multiparametric CMR imaging can reveal not only cardiac anatomy and function but also myocardial edema and fibrosis. The inflammatory phase of CS is marked by granulomatous infiltration, which may lead to focal myocardial thickness and wall motion abnormalities as observed in cine CMR. Myocardial edema is readily detected in T2-weighted imaging [Figures 1 and 2]. LGE is considered the principal magnetic resonance imaging technique for CS identification, with a reported sensitivity of 75–100% and a specificity of 77–85%.[15] Although CS can affect any part of the myocardium, it typically involves the basal segments of the left ventricle and the right ventricle side of the septum.[16,17] In chronic disease, granulomatous infiltration results in substantial myocardial fibrosis and scarring [Figures 3 and 4]. In addition, risk stratification and prediction of adverse outcomes in patients with CS can be achieved through the quantitative assessment of the degree of LGE intensification.[18] In this case series, we have highlighted the importance of clear identification of the various phenotypes of CS, which include dilated cardiomyopathy-like, hypertrophic cardiomyopathy-like, arrhythmogenic, and other non-specific inflammatory cardiomyopathy. Each phenotype carries distinct diagnostic challenges, treatment strategies, and prognostic implications. Arrhythmogenic phenotypes may require implantable cardioverter defibrillator implantation to prevent sudden cardiac death, and dilated phenotypes primarily focus on heart failure management. CMR and PET can visualize the different stages of CS, which suggests the possibility of personalized treatment plans and different clinical outcomes for patients.
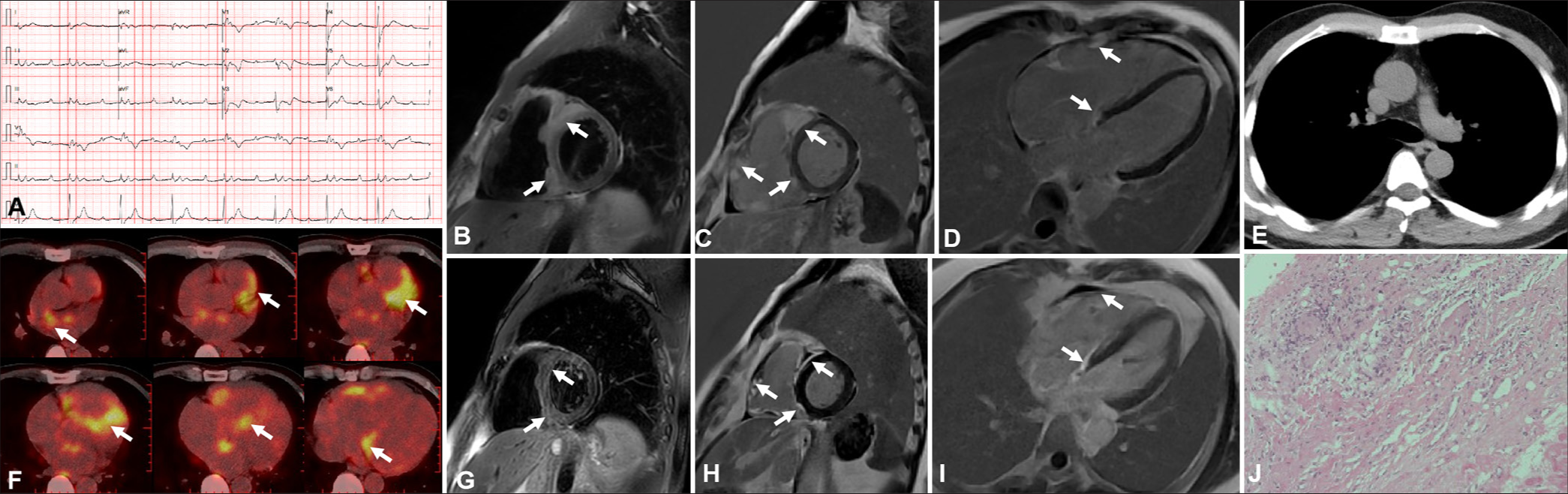
- Imaging results for a 44-year-old patient during the active inflammation phase. (A) First-degree atrioventricular block and incomplete right bundle branch block were detected. (B-D) Cardiac magnetic resonance (CMR) showed nodular thickening of the right ventricular wall and interventricular septum with T2 high signal intensity in the short-axis geometry corresponding to apparent late gadolinium enhancement (white arrow). (E) Computed tomography result was negative. (F) 18F-fluorodeoxyglucose (FDG)-positron emission tomography revealed focal FDG update (white arrow). (G-I) CMR was repeated and revealed that a nodular enhancement was smaller than before (white arrow). (J) Endomyocardial biopsy showed the formation of non-caseating inflammatory granuloma in low-magnification hematoxylin-eosin (HE) staining (×10).
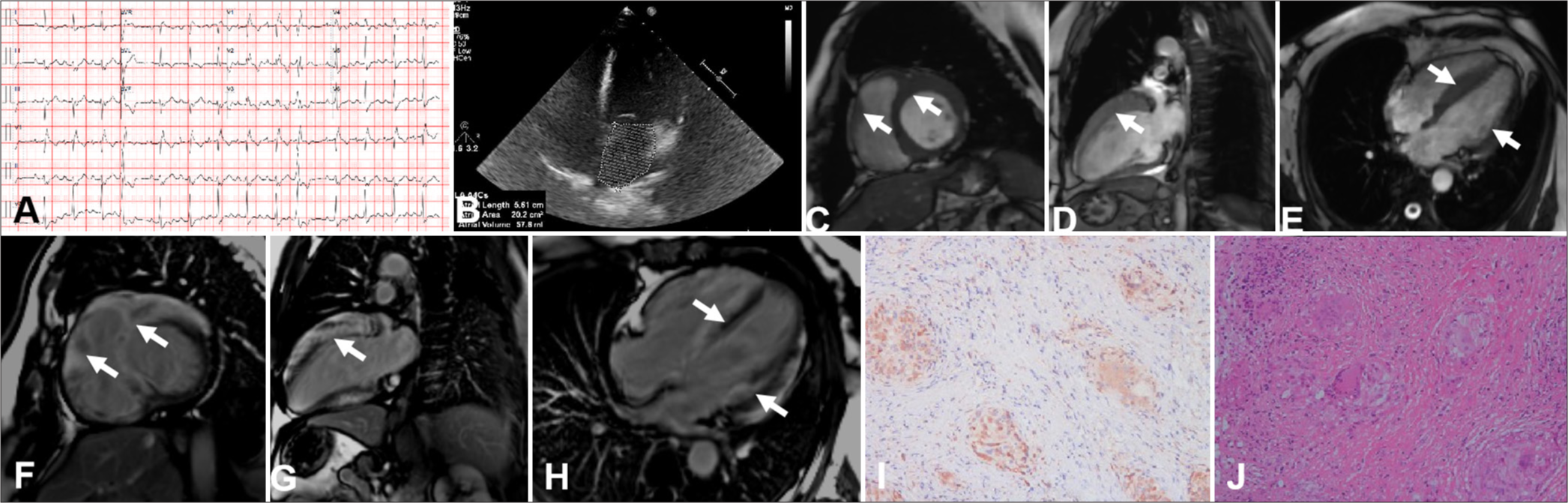
- Imaging results for a 52-year-old woman with a hypertrophic cardiomyopathy-like phenotype of cardiac sarcoidosis. (A) Electrocardiogram showed short-array ventricular tachycardia. (B) Transthoracic echocardiogram revealed enlargement of the right atrium and left and right ventricles. (C-H) Cardiac magnetic resonance unveiled the ventricular septum, right and anterior left ventricles, corresponding to an apparent patchy transmural late gadolinium enhancement (white arrow). (I) Non-caseating epithelioid granulomas were shown on a photomicrograph ((Hematoxylin-eosin HE), x10). (J) CD68 immunostaining highlighted macrophages and giant cells (x10).
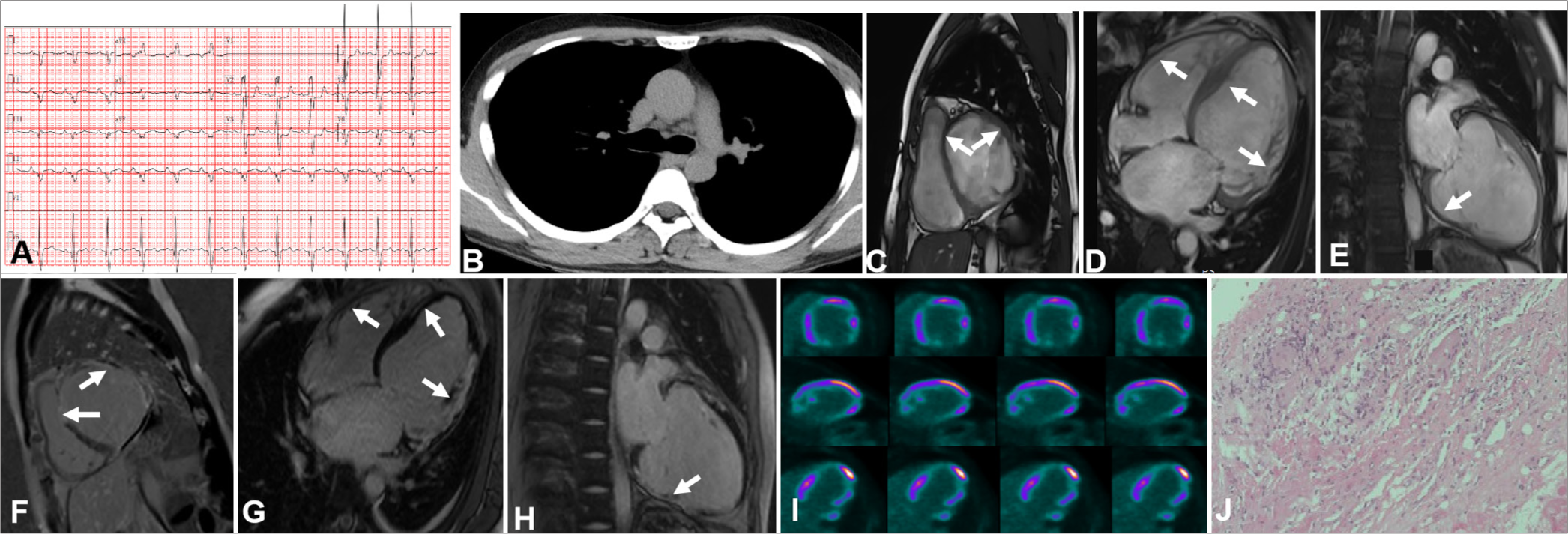
- Imaging findings for a 37-year-old patient in the late phase of cardiac sarcoidosis (CS). (A) First-degree atrioventricular block and abnormal P and Q waves were observed. (B) Computed tomography results were negative. (C-H) Cardiac magnetic resonance (MR) revealed general thinning of the ventricular wall and late gadolinium enhancement in the interventricular septum, right ventricle, and ventricular free walls (white arrow). (I) 18F-fluorodeoxyglucose-positron emission tomography unveiled fixed perfusion defects corresponding to the affected areas seen in MR imaging. (J) Pathological images displayed the advanced phase of CS, which was characterized primarily by myocardial fibrosis with granulomas and chronic inflammation ((Hematoxylin-eosin HE), x10).
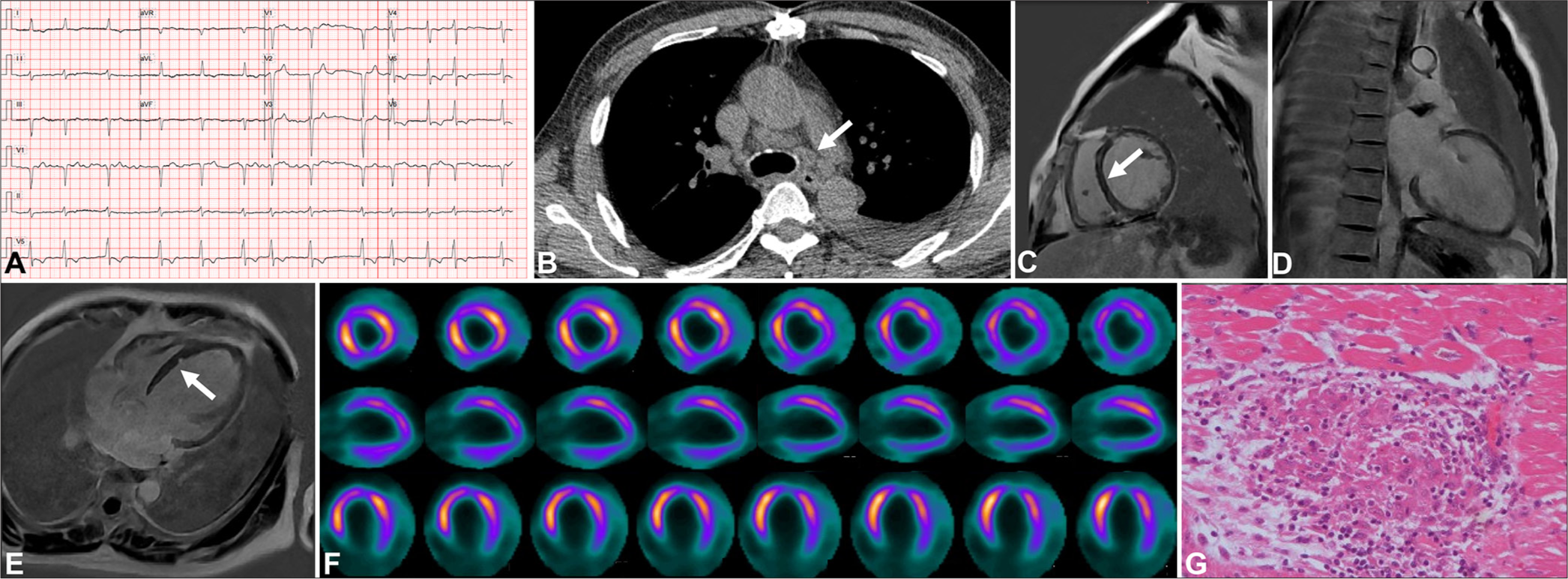
- Imaging findings for a 57-year-old patient with a dilated cardiomyopathy-like phenotype of cardiac sarcoidosis. (A) Electrocardiogram showed atrial fibrillation. (B) Computed tomography revealed extracardiac lymphadenopathy (white arrow). (C-E) Cardiac magnetic resonance displayed scattered mid myocardial late gadolinium enhancement areas in the basal interventricular septum (white arrow in sub-figures C and E). (F) 18F-fluorodeoxyglucose (FDG)-positron emission tomography revealed no significant focal FDG update. (G) Photomicrographs illustrated lesions in a heart explant specimen composed of non-caseating epithelioid granulomas ((Hematoxylin-eosin HE), x20).
Accurate diagnosis of CS presents a challenge due to the overlap of clinical and imaging findings with other infiltrative and inflammatory cardiomyopathies, such as giant cell myocarditis and ALVC. Giant cell myocarditis and CS display similar presentations and are hardly distinguishable; the former progresses rapidly, and the pathology shows the absence of non-caseating granuloma to support the diagnosis.[19] ALVC is an rare inherited cardiomyopathy characterized by fibrofatty replacement of left ventricle myocytes, and LGE is observed in fibrofatty infiltrated areas.[20]
CONCLUSION
An effective CS diagnostic approach necessitates the recognition of multimodality imaging features and addressing differential diagnostic challenges. This strategy aids in improving the early diagnosis, optimizing treatment plans, and enhancing the clinical outcomes of CS patients.
Ethical approval
The Institutional Review Board has waived the ethical approval for this study.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
This study was supported by the National Natural Science Foundation of China (No. 82302187 for H.Y.Q); Top Talent Support Program for young and middle-aged people of Wuxi Health Committee (BJ2023044 for H.Y.Q).
References
- Diagnosis of isolated cardiac sarcoidosis based on new guidelines. ESC Heart Fail. 2020;7:2662-71.
- [CrossRef] [PubMed] [Google Scholar]
- Cardiovascular outcomes in sarcoidosis. J Am Coll Cardiol. 2020;76:778-80.
- [CrossRef] [PubMed] [Google Scholar]
- Challenges of sarcoidosis and its management. N Engl J Med. 2021;385:1018-32.
- [CrossRef] [PubMed] [Google Scholar]
- Cardiac sarcoidosis outcome differences: A comparison of patients with de novo cardiac versus known extracardiac sarcoidosis at presentation. Respir Med. 2022;198:106864.
- [CrossRef] [PubMed] [Google Scholar]
- HRS expert consensus statement on the diagnosis and management of arrhythmias associated with cardiac sarcoidosis. Heart Rhythm. 2014;11:1305-23.
- [CrossRef] [PubMed] [Google Scholar]
- JCS 2016 Guideline on diagnosis and treatment of cardiac sarcoidosis-digest version. Circ J. 2019;83:2329-88.
- [CrossRef] [PubMed] [Google Scholar]
- Cardiac sarcoidosis: Phenotypes, diagnosis, treatment, and prognosis. Eur Heart J. 2023;44:1495-510.
- [CrossRef] [PubMed] [Google Scholar]
- The harm of delayed diagnosis of arrhythmogenic cardiac sarcoidosis: A case series. Europace. 2020;22:1376-83.
- [CrossRef] [PubMed] [Google Scholar]
- Long-term adverse cardiac outcomes in patients with sarcoidosis. J Am Coll Cardiol. 2020;76:767-77.
- [CrossRef] [PubMed] [Google Scholar]
- A joint procedural position statement on imaging in cardiac sarcoidosis: From the Cardiovascular and Inflammation and Infection Committees of the European Association of Nuclear Medicine, the European Association of Cardiovascular Imaging, and the American Society of Nuclear Cardiology. Eur Heart J Cardiovasc Imaging. 2017;18:1073-89.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnostic performance of F-18 FDG PET for detection of cardiac sarcoidosis; A systematic review and meta-analysis. J Nucl Cardiol. 2020;27:2103-15.
- [CrossRef] [PubMed] [Google Scholar]
- Localization of myocardial FDG uptake for prognostic risk stratification in corticosteroid-naïve cardiac sarcoidosis. J Nucl Cardiol. 2022;29:2132-44.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnostic accuracy and prognostic value of simultaneous hybrid 18F-fluorodeoxyglucose positron emission tomography/magnetic resonance imaging in cardiac sarcoidosis. Eur Heart J Cardiovasc Imaging. 2018;19:757-67.
- [CrossRef] [PubMed] [Google Scholar]
- PET Imaging in cardiac sarcoidosis: A narrative review with focus on novel PET tracers. Pharmaceuticals (Basel). 2021;14:1286.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnostic accuracy of cardiac MRI versus FDG PET for cardiac sarcoidosis: A systematic review and meta-analysis. Radiology. 2022;304:566-79.
- [CrossRef] [PubMed] [Google Scholar]
- Comprehensive cardiovascular magnetic resonance assessment in patients with sarcoidosis and preserved left ventricular ejection fraction. Circ Cardiovasc Imaging. 2016;9:e005022.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of techniques for the quantification of myocardial scar of differing etiology using cardiac magnetic resonance. JACC Cardiovasc Imaging. 2011;4:150-6.
- [CrossRef] [PubMed] [Google Scholar]
- Prognostic value of cardiovascular magnetic resonance in patients with biopsy-proven systemic sarcoidosis. Eur Radiol. 2020;30:3702-10.
- [CrossRef] [PubMed] [Google Scholar]
- Idiopathic giant cell myocarditis or cardiac sarcoidosis? A retrospective audit of a nationwide case series. ESC Heart Fail. 2020;7:1362-70.
- [CrossRef] [PubMed] [Google Scholar]
- Arrhythmogenic left ventricular cardiomyopathy: From diagnosis to risk management. J Clin Med. 2024;13:1835.
- [CrossRef] [PubMed] [Google Scholar]






