Translate this page into:
Management of Mucoepidermoid Carcinoma of the Palate Utilizing 18F-FDG PET/CT
Address for correspondence: Dr. S Sudhakar, Department of Oral Medicine and Radiology, St. Joseph Dental College and Hospital, Eluru, Andhra Pradesh, India. E-mail: drsudhakaroralmed@yahoo.co.in
-
Received: ,
Accepted: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Salivary gland carcinomas are a clinically diverse group of neoplasms with histological patterns overlapping other tumors, thus complicating their diagnosis. Mucoepidermoid carcinoma (MEC), first described by Masson and Berger in 1924, is a well-recognized salivary gland neoplasm, accounting for 5–10% of all salivary gland tumors. MEC frequently involves the major salivary glands and is rarely seen involving the jaws. The biological behavior of MEC is usually more aggressive with higher nodal and metastatic status at the time of presentation, which notably reduces the survival rate. Hence, early and accurate diagnosis utilizing advanced imaging modalities can reduce its morbidity. The present case is a rare presentation of MEC involving the palate, where (18) F-fluorodeoxyglucose-positron emission tomography/computed tomography (FDG-PET/CT) was utilized for diagnosis and treatment.
Keywords
Carcinoma
chemotherapy
palate
positron emission tomography scan
radiation
INTRODUCTION

Salivary gland malignancies are rare neoplasms and they comprise only about 0.5% of all malignancies.[1] Many factors affect the prognosis of patients with salivary gland malignancies; among them, the two most significant factors are clinical stage at the time of presentation and histological grading. In order to achieve a good treatment outcome, it is essential to accurately evaluate the extent of disease before deciding the treatment regimen. Thus, various factors such as local invasion of the primary tumors, regional lymph node metastasis, and distant spread of the disease need evaluation. The pre-treatment work-up for salivary gland malignancies depends primarily on the information from contrast-enhanced computed tomography (CT) and magnetic resonance imaging (MRI) scans. Some reports suggest that CT and MRI provide a similar diagnostic accuracy, while other studies suggest that MRI is superior to CT with respect to its ability to distinguish between benign and malignant tumors.[2] However, when bony evaluation is required, CT is considered to be superior to MRI scans. Recently, studies have demonstrated that the positron emission tomography (PET)/CT image data can accurately evaluate the extent of tumor and its stages when compared with CT alone.
CASE REPORT
A 45-year-old man presented to the clinic with a year's history of a painless growth on the right side of the palate. The lesion was initially the size of a peanut and had gradually enlarged to attain the present size of 3 cm. Patient had noticed ulceration over the surface of the growth 15 days prior to seeking our opinion. Personal history showed that he had been a smoker for 25 years and smoked 5–10 cigars/day. The medical, dental, and family histories were non-contributory to the complaint.
Extra-oral examination showed bilateral palpable submandibular lymph nodes. The lymph nodes were single, tender, firm in consistency, and mobile. The other facial structures appeared normal. Intra-oral examination revealed a well-defined ovoid ulcero-proliferative growth approximately 3.5 × 2.5 cm in size. Anteroposteriorly, the growth extended from the posterior-most rugae to the junction of hard and soft palate. Mediolaterally, it extended from the midline of the palate toward the palatal gingiva. Mucosa over the growth showed focal areas of ulceration, and the base of the ulcer contained yellowish white slough. Surrounding mucosa appeared normal and no secondary changes were seen. On palpation, the growth was non-tender, firm in consistency, and fixed to the underlying bone [Figure 1].
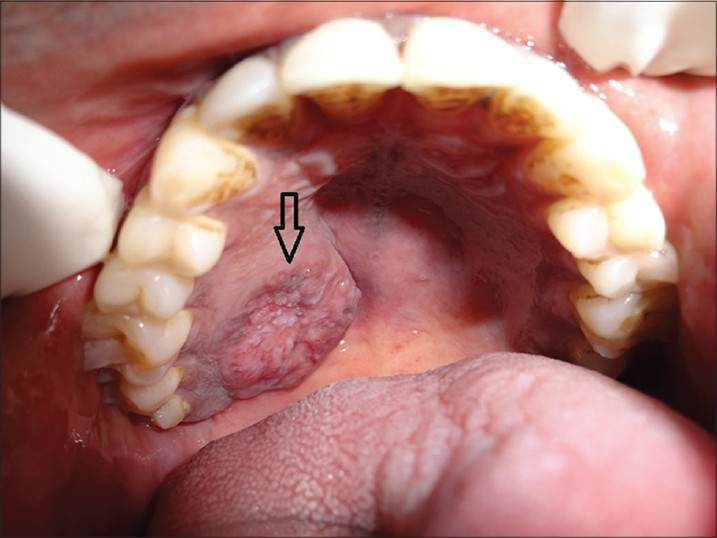
- 45-year-old male patient with lesion on the right side of the palate diagnosed as mucoepidermoid carcinoma – intermediate stage. Intra-oral photograph shows the ulcerated lesion on the right upper palate (arrow).
Maxillary cross-sectional occlusal radiograph showed a faint radiolucency on the right half of the hard palate suggestive of bony erosion. Panoramic radiograph showed a well-defined radiolucent shadow exactly over the right maxillary sinus [Figure 2].
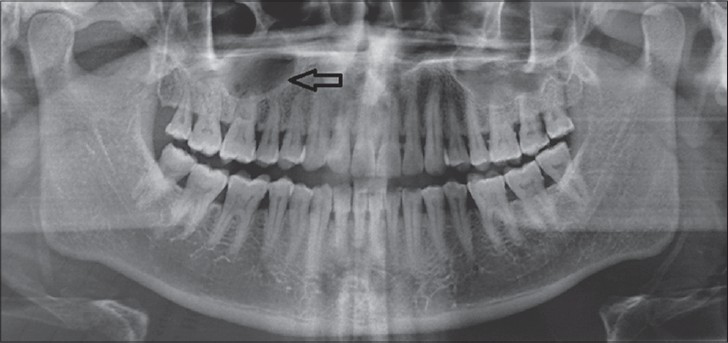
- 45-year-old male patient with lesion on the right side of the palate diagnosed as mucoepidermoid carcinoma – intermediate stage. Orthopantomograph shows radiolucency superimposing the right maxillary sinus (arrow).
Thin spiral CT sections of the neck were studied before and after intravenous IV contrast administration. All the images were studied for soft tissue, bone, as well as air window settings. The findings showed a faintly enhancing lesion, 26 × 11 mm in size, in the soft tissue of the hard palate to the right of the midline, associated with slight thinning of the underlying hard palate and a few small calcific densities within the mass [Figure 3].
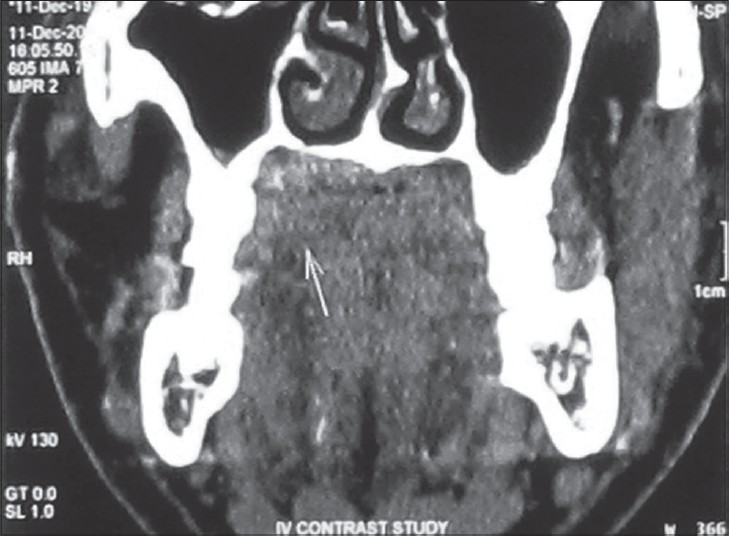
- 45-year-old male patient with lesion over on the right side of the palate diagnosed as mucoepidermoid carcinoma – intermediate stage. Coronal CT image shows the lesion as a faint soft tissue mass (arrow) on the palate.
With the patient fasting for 6 h, 10 mci of (18) F-fluorodeoxyglucose (FDG) was injected intravenously and three-dimensional positron emission tomography (3D PET) scan was performed. Physiological concentration was observed in the heart, gut, brain, kidneys, and bladder. Increased concentration of FDG was seen in the following regions [standardized uptake values (SUV) as per body weight]: focal lesion on the right hard palate- SUV maximum (max) 16.5, right level II lymph node- SUVmax 4.8, and left posterior cervical lymph node- SUVmax 4.4. The PET impression was that of a focal erosion of the hard palate, associated with 1.9 × 1.2 × 1.6 cm nodular enhancing soft tissue, possibly of neoplastic etiology, and few mildly prominent right upper jugular and left posterior cervical lymph nodes raising suspicion of metastatic or reactive nature of the lesion [Figure 4].

- 45-year-old male patient with lesion on the right side of the palate diagnosed as mucoepidermoid carcinoma – intermediate stage. PET image shows a prominent nodular enhancing lesion over the palate (outline arrow) and mildly enhancing lymph nodes (solid arrows). The SUVmax over the palate was 16.5 and on the right level II node was 4.8.
Considering the history, clinical and imaging findings, incisional biopsy of the lesion and fine needle aspiration cytology of the lymph nodes were performed [Figure 5]. Histopathology of the specimen showed presence of stratified squamous parakeratinized epithelium. The underlying connective tissue showed sheets of proliferating mucus cells and epidermoid cells, with areas of cystic degeneration. Intermediate cells were also seen occasionally. Concentric lamellated calcifications were evident suggestive of mucoepidermoid carcinoma (MEC) – intermediate grade [Figure 6]. Cytology of the aspirate collected from the lymph nodes showed hyperplastic follicles with round to plump chronic inflammatory cells. The findings were suggestive of reactive lymphadenopathy.
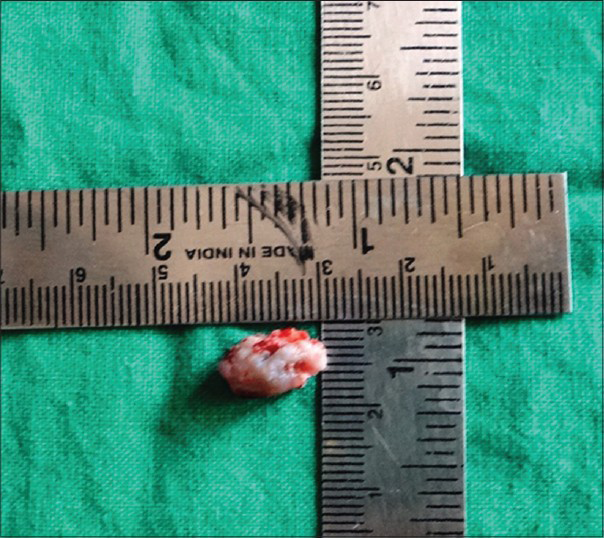
- 45-year-old male patient with lesion on the right side of the palate diagnosed as mucoepidermoid carcinoma – intermediate stage. Incisional biopsy specimen suggested mucoepidermoid carcinoma – intermediate stage.

- 45-year-old male patient with lesion on the right side of the palate diagnosed as mucoepidermoid carcinoma – intermediate stage. Photomicrograph of the hematoxylin and eosin stained biopsy tissue (10×) shows presence of mucus (black arrow) and epidermoid cells (white arrow) with connective tissue septum (red arrow) suggestive of mucoepidermoid carcinoma – intermediate stage.
Based on PET/CT and histological analysis, a combination therapy of surgical excision, radiotherapy, and chemotherapy regimen, followed by prosthetic rehabilitation of the tumor site was planned. Partial maxillectomy (right side) was performed to excise the tumor mass along with a margin of 1 cm normal tissue around it. Later the surgical defect was rehabilitated with a palatal obturator. Two weeks post surgery, the patient was treated with 4 weeks of chemotherapy with cisplatin followed by 6600 cGy of radiation therapy for 6 weeks using image-guided radiotherapy (IGRT). The patient tolerated the treatment protocol well and showed good signs of healing. The patient reported for the first 6-month follow-up and was found to be tumor free [Figure 7].
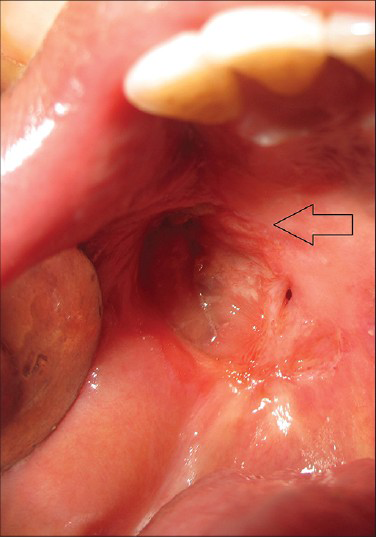
- 45-year-old male patient with lesion on the right side of the palate diagnosed as mucoepidermoid carcinoma – intermediate stage. Post treatment intra-oral photograph shows complete healing of the tumor site (arrow).
DISCUSSION
MEC is a malignant epithelial tumor composed of epidermoid, mucus, intermediate, columnar, and clear cells. On the basis of morphologic and cytologic features, these tumors are divided into low, intermediate, and high types. All these types have tendency for recurrence and metastases.[3]
The etiology of MEC is not well known; however, certain cases show translocation of gene t (11;19)(q21;p13.1) as a possible abnormality.[4] Chemical carcinogens or oncogenic viruses are not known to be associated with MEC; however, prior ionizing radiation has been documented as a contributing factor.[5]
MEC occurs more commonly in the fifth or sixth decade of life and has a slight male predominance. Among the major salivary glands, MEC is seen predominantly associated with the parotid and the most frequent site of its association among the minor salivary glands is the palate. The clinical presentation of MEC may vary according to the histological grade. Low-grade and intermediate-grade MECs generally present as slow-growing masses; high-grade tumors enlarge rapidly.[3] The case presented here is of intermediate grade. Regardless of the location, majority of the cases present as an asymptomatic swelling and only a few are symptomatic. Our patient did not have any pain and the growth gradually increased in size over a period of 1 year. Patients with MEC of the minor salivary glands, as seen with the present case, have diverse clinical presentations. The clinical presentation varies depending on the location of the tumor and it includes dysphagia, dysphonia, pain, paresthesia, ulceration, or hemorrhage. Our patient had only a superficial ulceration. Enlargement of the cervical nodes, related to metastasis, is more common in patients with submandibular gland tumors and recurrent high-grade parotid lesions. Nodal enlargement is seldom found in patients with minor salivary gland neoplasms. Our patient presented with enlargement of upper right jugular and left posterior cervical lymph nodes.
CT, MRI, and ultrasonography (USG) are the standard imaging modalities that are used routinely for the evaluation of salivary tumors. Usually, contrast-enhanced CT and MRI are used for determining the tumor size and nodal involvement, and to distinguish between benign and malignant lesions. However, these findings are not very specific. Moreover, after surgical exploration, anatomical distortion makes it difficult to distinguish residual or recurrent tumor from postoperative changes.[6] On the other hand, FDG-PET uptake is based on tumor metabolism. It is essentially independent of tumor location and size. FDG uptake in a tumor is proportional to the metabolic rate of viable tumor cells which have increased demand for glucose. Literature suggests that PET imaging is consistently more accurate compared to CT and MRI for the detection of recurrence and monitoring of therapy response. The advantage of FDG-PET is that it can image the whole body at one time and can detect unsuspected lesions otherwise not seen with other modalities. It provides more accurate evaluation of the primary site, possible recurrence, lymph nodes, and distant metastases, which frequently results in changing patient management.[7] The diagnostic accuracy of FDG-PET for detecting lymph node metastases is superior with a sensitivity and specificity of up to 90% and 94%, respectively, compared with CT values of 82% and 85%, respectively, and MRI values of 88% and 79%, respectively.[6] Considering this, in the present case, FDG-PET/CT was done, and the cytological analysis of the aspirate from the suspected lymph node suggested reactive lymphadenitis which was treated and a possible neck dissection was avoided, thus improving the patient's quality of life.
Treatment of MEC is mainly surgery, especially in patients with an early stage of disease. Surgical exploration of the neck is performed, only when there is clinical and histopathological evidence of lymphatic spread. In the present case, submandibular lymph node metastasis was ruled out by cytopathological analysis. Radiation therapy alone is used if poor cosmetic results are anticipated with surgical resection or in patients with advanced unresectable disease. However, reports suggest that radiotherapy as a sole curative modality in advanced stages of salivary gland carcinomas is only about 20%. Conversely, combination of surgery and radiation therapy showed a potential benefit to control locoregional recurrences in patients with positive surgical margins.[8] Chemotherapy is used in cases of locally advanced, metastatic, or recurrent disease. Several studies have evaluated the role of chemotherapy, either as induction, adjuvant, or neoadjuvant, in the treatment of salivary gland carcinomas.[9] Numerous agents have been used in combination or alone and their response rates vary between 10% and 46%. Vinorelbine and Cisplatin are described as the most effective single agents.[10] Combinations of cyclophosphamide, doxorubicin, and cisplatin, and doxorubicin and 5-fluorouracil (5-FU) are also in use, but the increased side effects have to be recognized. Docetaxel has shown impressive anti-tumor activity in patients with squamous cell carcinoma. With its promising response rates, this drug seems to be a logical alternative in salivary gland tumors. In the present case, the treatment modality included a combination of surgical with adjunct chemotherapeutic agents and radiation therapy.
The prognosis of patients with MEC depends on the clinical stage, grade of the tumor, location, and adequacy of treatment. Patients are more likely to experience a recurrence if the margins of resection are positive, regardless of grade. Other studies found that the survival rate was significantly associated with the presence of nodal disease and distant metastases implying a poor prognosis.[3]
CONCLUSION
Intermediate-grade MEC with a clinically enlarging lymph node is a rare presentation. Literature suggests that the diagnostic accuracy of FDG-PET in detecting lymph node metastases is superior, when compared with CT and MRI. Based on this, in the present case, FDG-PET/CT was done. The cytological analysis of the aspirate from the suspected lymph node suggested reactive lymphadenitis which was treated and a possible neck dissection was avoided, thus improving the patient's quality of life.
Available FREE in open access from: http://www.clinicalimagingscience.org/text.asp?2014/4/2/5/145898
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- Salivary gland tumors: Diagnostic value of gadolinium-enhanced dynamic MR imaging with histopathologic correlation. Radiology. 2003;226:345-54.
- [Google Scholar]
- Atlas of Tumor Pathology: Tumor of the salivary glands. Washington, DC: Armed Forces Institute of Pathology; 1996. p. :155-75. 353-5
- [Google Scholar]
- Mucoepidermoid carcinoma of minor salivary gland with t (11;19)(q21;p13.1) as the only karyotypic abnormality. Cancer Genet Cytogenet. 1996;87:29-33.
- [Google Scholar]
- Salivary gland tumors among atomic bomb survivors, 1950-1987. Cancer. 1997;79:1465-75.
- [Google Scholar]
- Positron emission tomography in the evaluation of stage III and IV head and neck cancer. Head Neck. 2001;23:1056-60.
- [Google Scholar]
- The impact of FDG-PET in the management of patients with salivary gland malignancy. Ann Nucl Med. 2005;19:691-4.
- [Google Scholar]
- Role of radiotherapy for mucoepidermoid carcinoma of salivary gland. Oral Oncol. 1999;35:105-11.
- [Google Scholar]
- Cisplatin, doxorubicin, and 5-fluorouracil chemotherapy for salivary gland malignancies: A pilot study of the Northern California Oncology Group. J Clin Oncol. 1987;5:951-5.
- [Google Scholar]
- Cisplatin-based chemotherapy for neoplasms arising from salivary glands and contiguous structures in the head and neck. Cancer. 1988;62:2313-9.
- [Google Scholar]






