Translate this page into:
Malignant Mesenchymal Renal Tumor: A Rare Case of Primary Renal Fibrosarcoma
Address for correspondence: Dr. Madanmohan Gupta, Department of Radio Diagnosis, M. P. Shah Medical College, Jamnagar - 361 008, Gujarat, India. E-mail:-madan_gupta05@yahoo.in
-
Received: ,
Accepted: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Malignant mesenchymal neoplasms of kidney constitute a rare group of tumors. Primary fibrosarcoma of kidney is an extremely rare subtype of primary malignant mesenchymal renal neoplasms. An elderly female presented with a gradually increasing abdominal lump and mild abdominal discomfort. On cross-sectional imaging, the lesion showed features suggestive of an atypical renal mass not conforming to either ball or bean type growth pattern. The mass was surgically removed and on histopathological and immunohistological investigations diagnosed to be primary renal fibrosarcoma.
Keywords
Atypical renal mass
malignant mesenchymal renal tumor
primary renal fibrosarcoma
INTRODUCTION

Primary malignant mesenchymal renal masses are a rare group of tumors that account for 1-3% of all malignant renal tumors in adults.[12]
Primary renal fibrosarcoma is an extremely rare malignancy of the kidney, with only few cases reported in literature. Most renal fibrosarcomas arise within the renal capsule, which consists of large amounts of fibrous tissue. Renal fibrosarcoma is commonly seen in patients older than 40 years; with both sexes affected equally.[3]
A 75-year-old female patient presented with a 3-month history of a gradually increasing abdominal lump on the right side and vague abdominal discomfort. No history of fever, backache, hematuria, or renal colic was present.
On palpation, the large abdominal mass localized in the right lumbar region was non-tender with ill-defined margins.
RADIOLOGIC FEATURES
Ultrasound (US) examination of abdomen via subcostal approach using convex (5-7 MHz) probe did not provide much information regarding the mass because of limited field of view in the transverse and coronal planes [Figure 1].
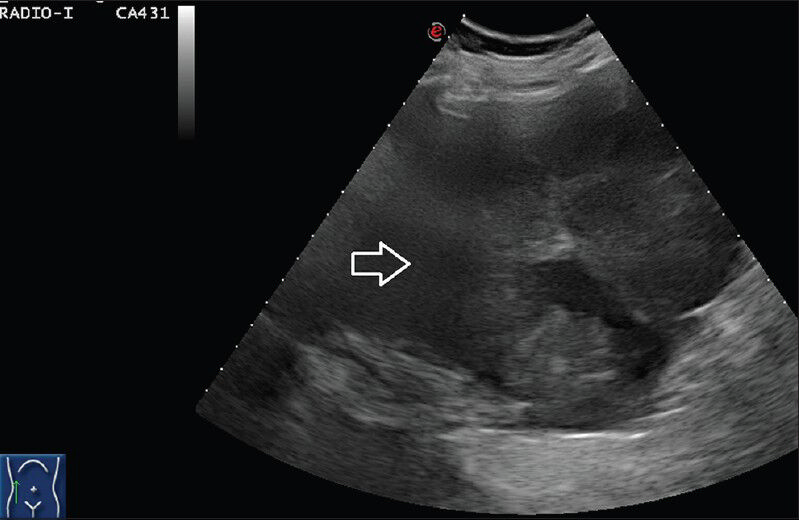
- 75 year-old female with a large abdominal mass diagnosed with primary renal fibrosarcoma. Gray-scale ultrasound scan using subcostal approach in coronal plane with convex (5-7 MHz) probe in right lumbar region shows an ill-defined heterogenous mixed echogenicity mass which cannot be separately made out from liver and kidney.
Patient underwent a non-enhanced computed tomography (NECT) scan on a 16-slice CT scanner (General Electric Brightspeed, Milwaukee, Wisconsin, USA). This was primarily aimed to help decide imaging protocol since the organ of origin was indeterminate.
Since the organ of origin was not clear on US, a plain NECT scan was done first to look for radiologic clues about the organ of origin. NECT images revealed a lobulated soft tissue attenuation lesion in the right renal bed with loss of intervening fat plane with right kidney suggesting renal origin of the mass. A liver mass has a different contrast on contrast-enhanced CT (CECT) than a renal origin mass. The mass in this case showed intact fat plane with liver but not with the kidney on NECT scan, which indicated a renal origin lesion; and thus, helped in deciding a protocol for the renal masses [Figure 2].
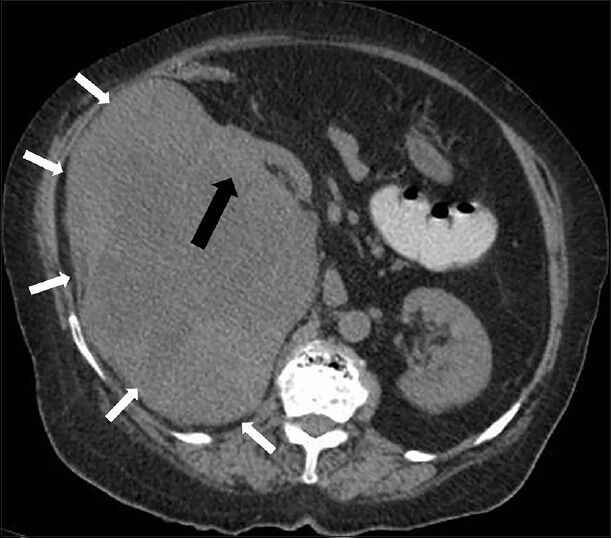
- 75-year-old female with a large abdominal mass diagnosed with primary renal fibrosarcoma. Non-enhanced computed tomography abdomen, Axial section shows a well-defined lobulated heterogeneous soft tissue attenuation lesion in the right renal bed (small white arrows) with loss of intervening fat plane (black arrow).
CECT was done by injecting 120 ml of intravenous contrast iohexol (Omnipaque, General Electric Healthcare) with an 18 gauge needle through the right antecubital vein at a rate of 4 ml/sec. Scanning parameters used were: Tube current of 105 milliampere second (mAs), tube voltage of 130 peak kilovoltage (kVp). Acquisition was done at slice thickness of 5 mm. Image acquisition was done during corticomedullary (35-45 s), nephrographic (70-90 s), and excretory (3 min) phases. The 5 mm thick axial images were reformatted into thinner sections in three orthogonal planes (0.6 mm thick).
CECT images showing the presence of the embedded organ sign confirmed the renal origin of the mass. An embedded organ sign is a cross-sectional imaging sign where an organ appears to be embedded in the tumor. The parenchyma of the organ is stretched toward the tumor and the surface of the organ appears embedded in the tumor at the contact surface [Figure 2]. In addition, interface between mass and kidney is best visualized in nephrographic phase (the maximal difference between peak enhancement of cortex and mild enhancement of mass lesion) [Figure 3]. The mass appeared to be centered on the renal capsule causing significant capsular expansion. No evidence of significant involvement of underlying cortex was noted. The soft tissue mass lesion was predominantly exophytic causing buckling of the underlying renal cortex and pushing the right kidney superomedially. No evidence of filling defect was noted in the inferior vena cava suggesting absence of thrombosis [Figure 3].
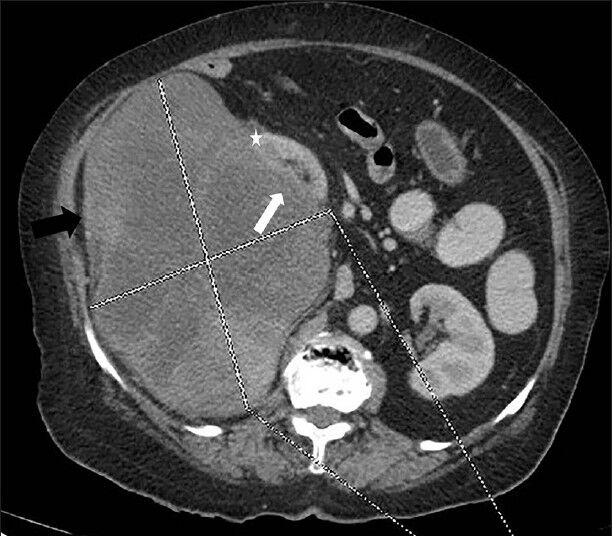
- 75-year-old female with a large abdominal mass diagnosed with primary renal fibrosarcoma. Contrast-enhanced computed tomography abdomen, Axial section in nephrographic phase shows well-defined exophytic heterogeneously enhancing right renal mass (showed by lines) with internal low attenuation areas (black arrow) causing indentation of the underlying renal cortex (white arrow) and displacing kidney superomedially. No evidence of invasion of underlying kidney is noted (Note the distinct mass lesion; cortex interface) (asterisk).
CECT images showed progressively increasing enhancement of renal parenchyma and mass lesion (especially in peripheral portion) causing blurring of interface between kidney and mass lesion [Figures 4 and 5]. However, the central portion in the mass showed minimal contrast enhancement suggesting necrotic areas. The kidney showed normal contrast excretion with normal appearance of the upper ureter. No evidence of invasion of pelvicalyceal system was noted [Figures 4 and 5].
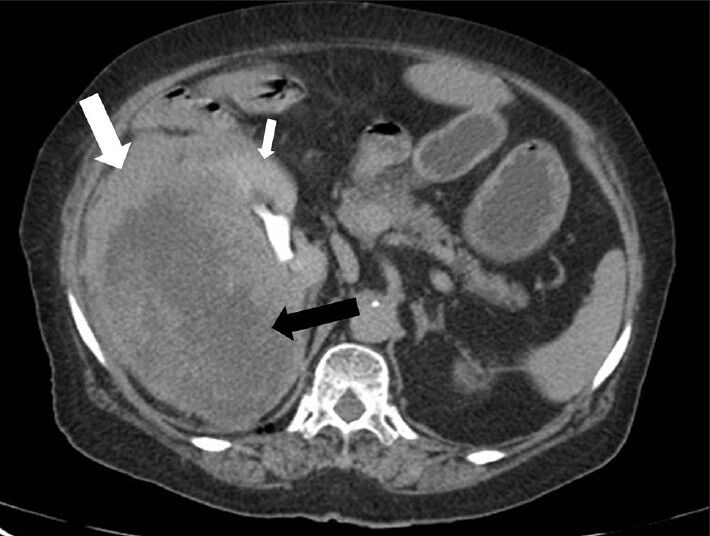
- 75-year-old female with a large abdominal mass diagnosed with primary renal fibrosarcoma. Contrast-enhanced computed tomography abdomen, Axial section in excretory phase reveals progressively increasing enhancement of renal parenchyma (small white arrow) and lesion (especially peripheral part) (white arrow). Central areas of lesion shows minimal contrast enhancement (black arrow).
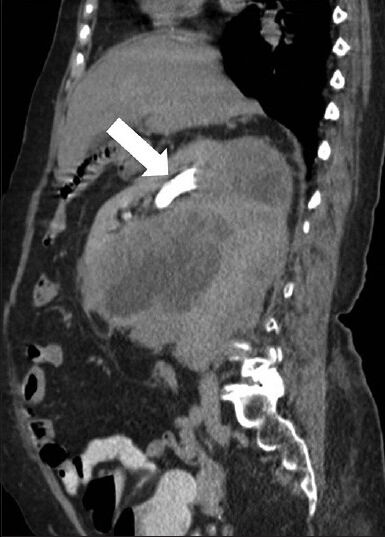
- 75-year-old female with a large abdominal mass diagnosed with primary renal fibrosarcoma. Contrast-enhanced computed tomography abdomen, Sagittal section in excretory phase demonstrates normal contrast excretion (white arrow) from the kidney and no evidence of invasion of pelvicalyceal system. A distinct fat plane is noted between the mass lesion and inferior surface of liver.
In order to better characterize the mass lesion, patient was further evaluated using noncontrast magnetic resonance imaging (MRI). T2-weighted spin echo images showed large, well-defined lobulated extremely heterogeneous signal intense mass with the central part appearing mildy hyperintense and peripheral areas showing significant hypointensity [Figure 6]. On T1-weighted fast spin echo (FSE) images the lesion showed low signal intense areas (central portion) and iso to hypointense areas in the periphery (intensity similar to skeletal muscle). Normal flow related signal void was noted in the inferior vena cava on FSE images, which suggested absence of intraluminal thrombus. Close observation showed a faint interface between mass lesion and the kidney [Figure 7].
Above findings were more suggestive of an atypical renal mass.
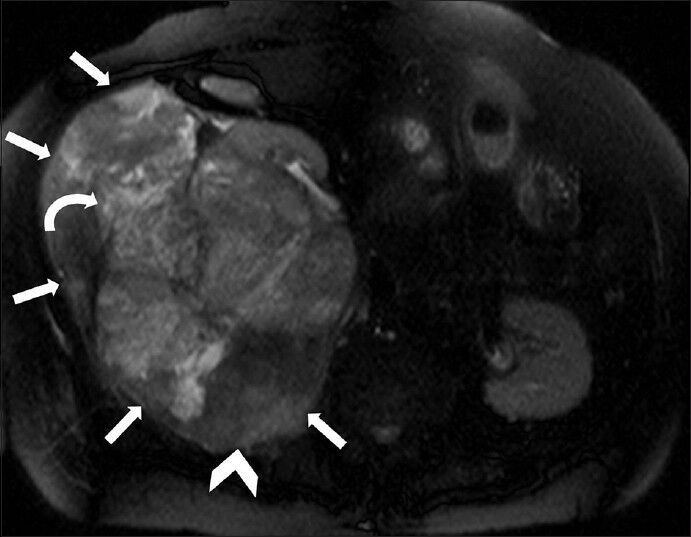
- 75-year-old female with a large abdominal mass diagnosed with primary renal fibrosarcoma. Non-enhanced magnetic resonance imaging of abdomen, T2-weighted axial section shows a large, well-defined, lobulated mass with extremely heterogeneous signal intensity (white small arrows). The central part appears mildly hyperintense (curved arrow) and peripheral areas show significant hypointensity (arrowhead).
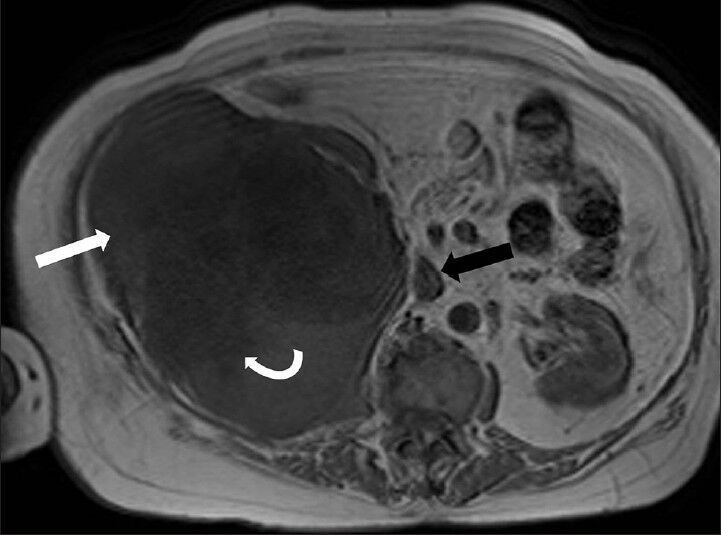
- 75-year-old female with a large abdominal mass diagnosed with primary renal fibrosarcoma. Non-enhanced magnetic resonance imaging of abdomen, T1-weighted axial section reveals that the mass has low signal-intensity areas (curved white arrow) more toward the center and iso to hypointense areas more in the periphery (white arrow) (intensity similar to skeletal muscle). Note the normal flow related signal void in the inferior vena cava on fast spin echo (FSE) images which suggests absence of intraluminal thrombus (black arrow).
PATHOLOGICAL FEATURES
Subsequently, the patient underwent right radical nephrectomy. Grossly resected mass appeared well-circumscribed, fleshy with bosselated external surface. Few scattered areas of hemorrhage and necrosis were seen. On palpation, mass felt firm in consistency [Figure 8].
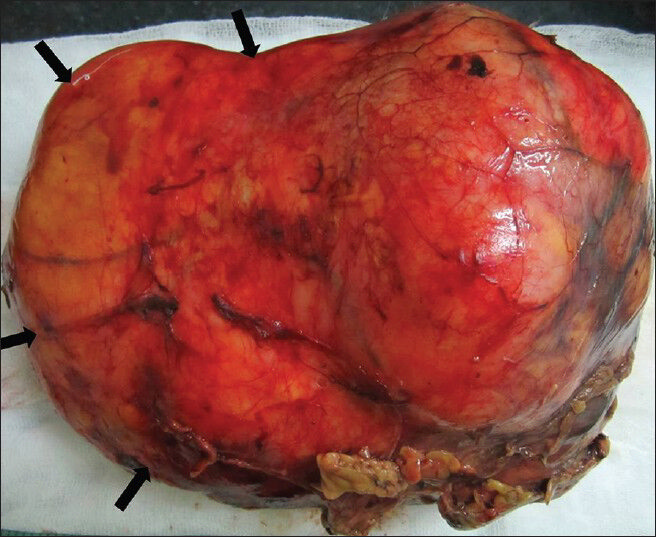
- 75-year-old female with a large abdominal mass diagnosed with primary renal fibrosarcoma. Postoperative specimen of right renal mass. Gross specimen shows bosselated, smooth-surfaced, encapsulated lesion.
On histopathological analysis [Figure 9], cells arranged in whorls showed eosinophilic cytoplasm and vesicular elongated nuclei with interspersed areas of edema and myxoid change.
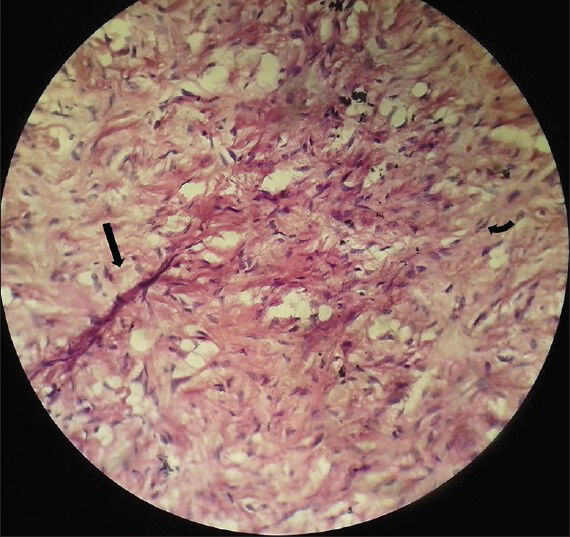
- 75-year-old female with a large abdominal mass diagnosed with primary renal fibrosarcoma. Photomicrograph of Hematoxylin and Eosin stained (×40) specimen shows elongated spindle cells (black arrow) arranged in whorls with nuclear atypia (curved arrow).
On prior imaging and histological analysis, main differential diagnosis consisted of atypical renal cell carcinoma, fibrosarcoma, and leiomyosarcoma.
Immunohistochemical analysis revealed diffuse positivity for vimentin [Figure 10] and Ki-67 (nuclear protein for cellular proliferation named after Kiel, a city in Germany) [Figure 11]. Diffuse positivity for vimentin indicated differentiation of mesenchymal nature. Further, immunohistochemical analysis showed negativity for cytokeratin [Figure 12] and desmin ruling out sarcomatoid renal cell carcinomas and leiomyosarcomas, respectively. Vimentin is a generalized soft tissue marker found in large variety of soft tissue tumors. Ki-67 is a marker for degree of cellular proliferation and positivity indicates a high grade malignant lesion. Mass showed focal positivity for S-100 (one of a family of calcium binding proteins), [Figure 13] which is a nonspecific immunological marker, often present in clear cell sarcomas and benign peripheral nerve sheath tumors.
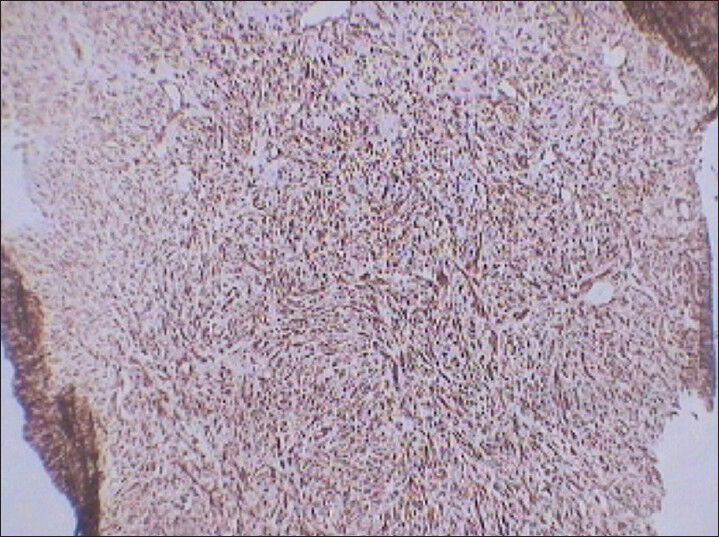
- 75 year-old female with a large abdominal mass diagnosed with primary renal fibrosarcoma. Photomicrograph (×10) of immunohistochemical staining for Vimentin (generalized soft tissue marker) shows diffuse reactivity.
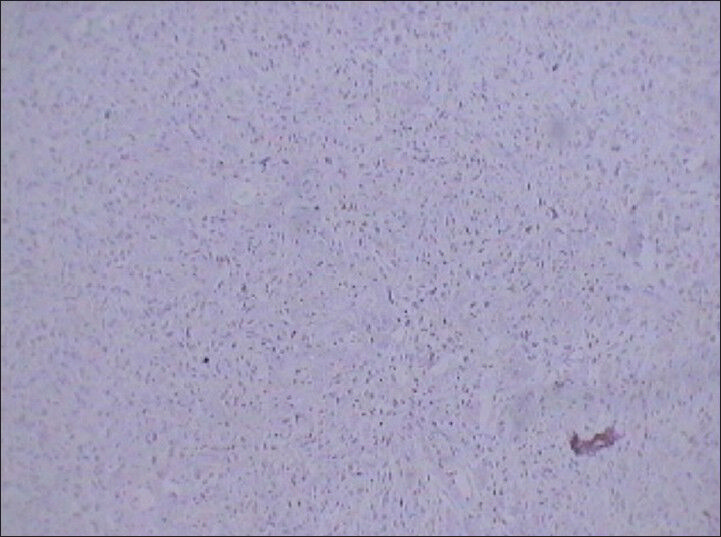
- 75 year-old female with a large abdominal mass diagnosed with primary renal fibrosarcoma. Photomicrograph (×10) of immunohistochemical staining for Ki-67 (cellular proliferation marker) shows diffuse reactivity. Positive reaction to this marker indicates malignant lesions.
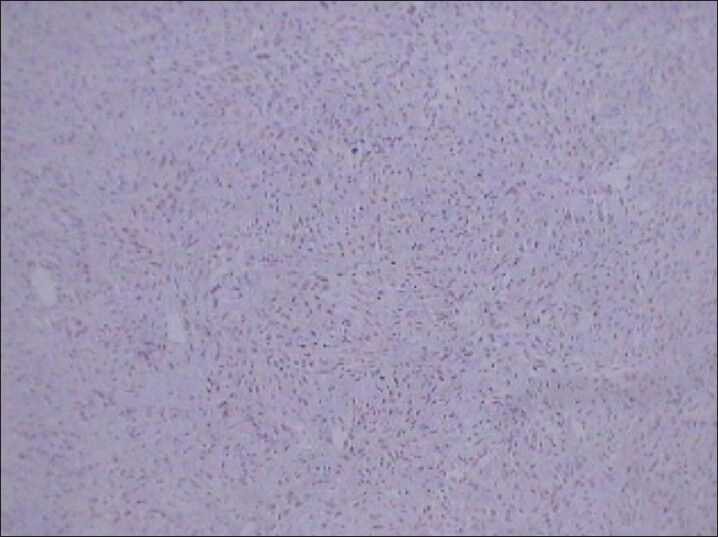
- 75 year-old female with a large abdominal mass diagnosed with primary renal fibrosarcoma. Photomicrograph (×10) Immunohistochemical staining for CK (Cytokeratin) not showing reactivity for tumor cells.
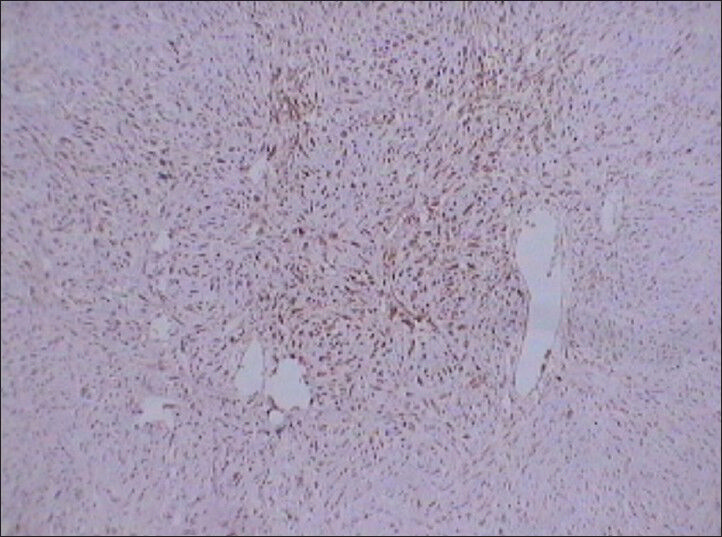
- 75 year-old female with a large abdominal mass diagnosed with primary renal fibrosarcoma. Photomicrograph (×10) of immunohistochemical staining for S-100 shows positive reaction to the marker indicating tumor cells.
Further evaluation was required using a more specific panel of markers since fibrosarcoma has no specific marker of their own based on which they can be directly identified. HMB-45 (melanocytic marker) [Figure 14], cytokeratin (CK; sarcomas of epithelial origin), CD-31 (vascular origin), and BCl-2 (lymphomatous lineage) [Figure 15], and smooth muscle actin (SMA; smooth muscle origin) [Figure 16] markers were all found to be negative.
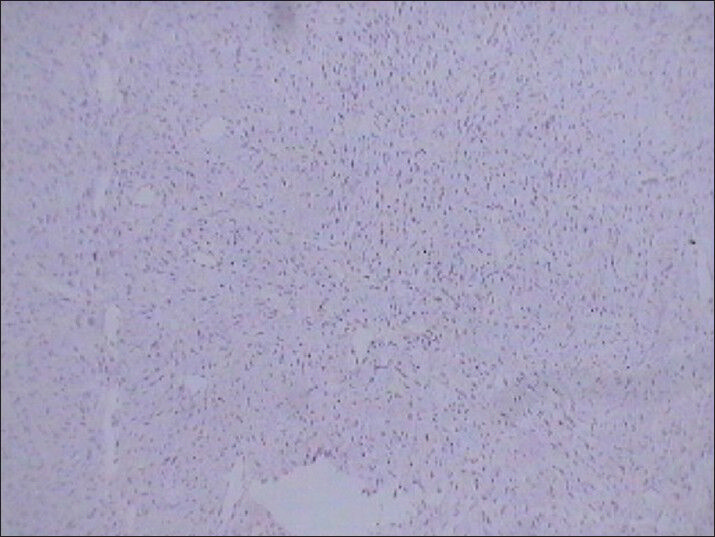
- 75 year-old female with a large abdominal mass diagnosed with primary renal fibrosarcoma. Photomicrograph (original magnification, ×10;) of immunohistochemical staining for HMB-45 (melanocytic marker) shows no reactivity.
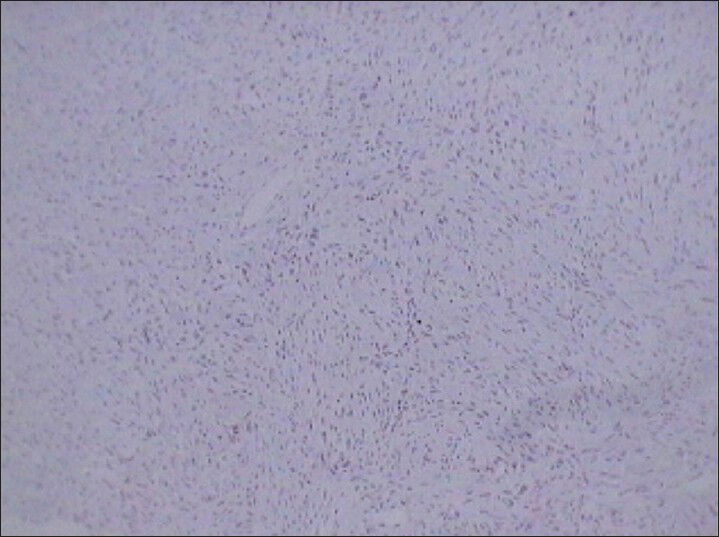
- 75 year-old female with a large abdominal mass diagnosed with primary renal fibrosarcoma. Photomicrograph (×10) of immunohistochemical staining for Bcl-2 (lymphomatous lineage) shows no reactivity.
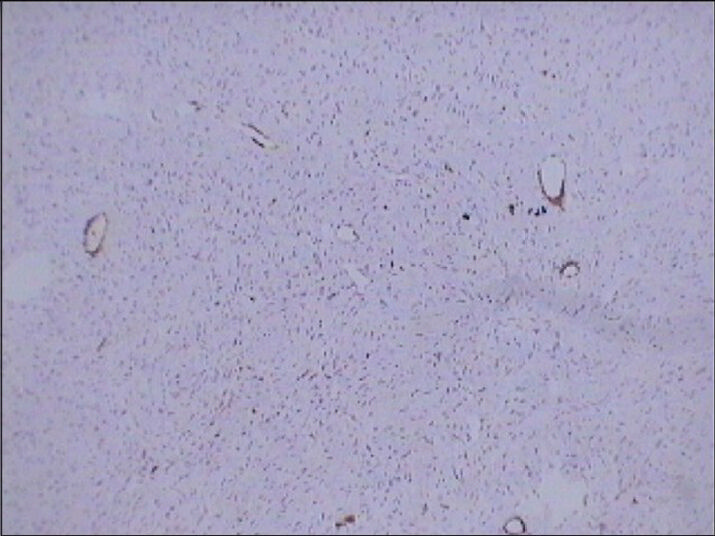
- 75 year-old female with a large abdominal mass diagnosed with primary renal fibrosarcoma. Photomicrograph (×10); of immunohistochemical staining for SMA-10 (smooth muscle origin) shows no reactivity.
Imunohistochemistry helps to rule out other possible tumors in a kidney with a specific line of differentiation. Absence of SMA rules out tumor of smooth muscle origin. Cytokeratin negativity makes epithelial sarcomas unlikely. Similarly, vascular and melanocytic origins were ruled out by negative staining for CD-31 and HMB-45. Absence of BCl-2 rules out lymphomatous lineage. Thus, fibrosarcoma is a diagnosis of exclusion based on combined histology and immunochemistry.
DISCUSSION
The classical imaging algorithm used for renal masses divides them into ball and bean variety.
Ball-type masses (e.g., renal cell carcinoma) grow by additive expansion deforming normal renal outline causing distortion and displacement of pyelocalyceal system. A distinct interface is seen between the mass and surrounding renal parenchyma on post-contrast images. In contradistinction, bean-type lesions (e.g., transitional cell carcinoma) infiltrate into renal parenchyma causing renal enlargement without altering reniform shape. The margins of the mass are ill-defined causing distortion of renal sinus anatomy.[4]
Renal origin benign mesenchymal masses are extremely common for example angiomyolipoma. On contrary, their malignant counterparts are rare, like leiomyosarcoma and fibrosarcoma. No cross-sectional imaging findings can distinguish between the various subtypes.
Histopathology of a typical renal fibrosarcoma typically shows spindle-shaped cells arranged in parallel rows intersecting each other at acute angles giving a herring bone pattern (broken zigzag pattern) and intermixed areas of hemorrhage and necrosis. Cells show scant to moderate eosinophilic cytoplasm. Nuclei are oval-shaped with irregular nuclear membrane and irregularly distributed chromatin.[5]
Immunohistochemistry is a biochemical technique used to identify specific molecules in different kinds of tissue. The tissue is treated with antibodies that bind the specific molecule. These are then made visible under a microscope by using a color reaction, a radioisotope, or a fluorescent dye. Mesenchymal masses like fibrosarcoma, leiomyosarcoma, and sarcomatoid renal cell carcinoma can appear similar on histopathology. Immunohistochemistry (IHC) is definitive method to rule out latter two, since fibrosarcoma is usually a diagnosis of exclusion. Fibrosarcomas are positive for vimentin (nonspecific), negative for CK and desmin; whereas, sarcomatoid renal cell carcinoma and leiomyosarcoma are diffusely positive for CK and desmin, respectively.[5]
CONCLUSION
Imaging of renal masses helps in identifying atypical renal masses. Malignant mesenchymal masses show atypical features on imaging and ultimate characterization depends on histopathology and immunohistochemistry (IHC).
Fibrosarcoma is a diagnosis of exclusion on IHC which is a must for diagnosis of rhabdomyosarcomas (desmin and myogenin), gastrointestinal stromal tumors (CD117), vascular tumors (factor VIII-related antigen, CD31), epithelioid sarcoma, sarcomatoid carcinoma (cytokeratin), and clear cell sarcoma (S-100 protein, HMB45, and melan A).
Available FREE in open access from: http://www.clinicalimagingscience.org/text.asp?2013/3/1/52/122322
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- Primary sarcomas of the kidney: A clinicopathologic and DNA flow cytometric study of 17 cases. Cancer. 1990;65:1611-8.
- [Google Scholar]
- Infiltrative renal lesions: Radiologic-pathologic correlation. Radio Graphics. 2000;20:215-43.
- [Google Scholar]
- Leiomyosarcoma of the kidney: CT and MR appearance. J Comput Assist Tomogr. 1993;17:656-8.
- [Google Scholar]
- Renal masses: Assessment of corticomedullary-phase and nephrographic phase CT scans. Radiology. 1995;196:445-51.
- [Google Scholar]
- Primary renal fibrosarcoma: A rare case report and review of literature. Indian J Pathol Microbiol. 2008;51:409-10.
- [Google Scholar]






