Translate this page into:
Magnetic resonance imaging spectrum of intracranial meningiomas: An institutional review of common and uncommon imaging appearances

*Corresponding author: Singireddy Padmaja, Department of Radiodiagnosis, Apollo Institute of Medical Sciences and Research, Hyderabad, Telangana, India. docpadmajareddys@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Padmaja S, Jakkani A, Vijay KE, Moorthy NLN, Ramesh G. Magnetic resonance imaging spectrum of intracranial meningiomas: An institutional review of common and uncommon imaging appearances. J Clin Imaging Sci. 2024;14:38. doi: 10.25259/JCIS_61_2024
Abstract
Objectives:
Meningiomas are the most common non-glial central nervous system (CNS) tumors, typically presenting as well-defined extra-axial masses with distinct computed tomography (CT) and magnetic resonance imaging (MRI) features. Despite their characteristic appearance, some meningiomas exhibit atypical features that can be diagnostically challenging. This study aims to analyze the imaging findings of meningiomas in a series of 82 cases, covering both common and uncommon locations, and exploring their typical as well as atypical/malignant features.
Material and Methods:
A retrospective review was conducted on 82 patients with pathologically confirmed meningiomas who underwent both CT and diffusion-weighted MRI (1.5 T) at our institution. The imaging data were analyzed to assess various parameters, including age incidence, sex distribution, tumor location, T1- and T2-weighted sequences, contrast enhancement patterns, and the presence of calcifications, cysts, peritumoral edema, hemorrhage, and magnetic resonance spectroscopy findings.
Results:
The analysis revealed a spectrum of imaging characteristics across the 82 meningioma cases. While many tumors exhibited typical imaging features, such as well-circumscribed margins and homogeneous contrast enhancement, others presented with atypical or malignant features, including heterogeneous signal intensity, irregular borders, and evidence of invasion into adjacent structures. The study also identified a range of tumor locations, with both common (e.g., convexity and parasagittal) and uncommon (e.g., intraventricular and skull base) sites represented. Peritumoral edema, calcifications, cystic changes, and hemorrhage were noted in varying frequencies across the cases, providing insights into the diverse radiological presentations of meningiomas.
Conclusion:
Meningiomas are the most common non-glial primary neoplasms of the CNS, and their diagnosis is generally straightforward when located in typical regions with characteristic imaging features. However, it is crucial for radiologists to recognize that meningiomas can present in atypical locations and with misleading or atypical imaging characteristics. A broad understanding of these variations is essential for accurate diagnosis and appropriate management of meningiomas, especially in cases where the imaging findings are not straightforward.
Keywords
Meningioma
Atypical
Typical
Extra-axial
Mimics
INTRODUCTION
Meningiomas are the most common extra-axial broad-based non-glial tumors of the central nervous system (CNS). Meningiomas originate from the arachnoid membrane that envelops the brain, nerves, and spinal cord. They are frequently observed in individuals over 50 years, with a prevalence of 2–3% among the elderly population.[1] While most meningiomas develop spontaneously with an unknown cause, certain recognized risk factors include prior exposure to radiation and genetic disorders, such as neurofibromatosis type 2.[2] Most commonly, meningiomas are asymptomatic, although clinical signs may vary and arise from the compression of brain structures or obstruction of the ventricular system. Meningiomas predominantly occur along the lateral hemisphere convexity (20–34%), parasagittal (18–22%), sphenoid wing, middle cranial fossa (17–25%), and olfactory groove. Less common sites include cerebellopontine (CP) angle (2–4%), intraventricular (2–5%), orbital (<1–2%), clivus (<1%), and ectopic areas such as optic nerve sheath (0.4–1.3%) and choroid plexus (0.5–3%). Histopathologically, while the spinal origin accounts for about 10%, approximately 90% of meningiomas are classified as World Health Organization (WHO) Grade 1 lesions, denoting a benign nature. However, a minority, comprising 5–7%, exhibits atypical features as WHO Grade 2 tumors, while an even smaller fraction, ranging from 1% to 3%, represents the more aggressive WHO Grade 3 anaplastic types. It is important to recognize both the atypical locations and unusual imaging features of these common neoplasms to avoid misdiagnosis.
Imaging findings
The role of plain films in diagnosing meningiomas has largely been replaced by more advanced imaging techniques. While most plain films appear normal, they can occasionally reveal hyperostosis, calcification, or osteolysis associated with meningiomas.
Meningiomas typically present as well-defined, lobulated extra-axial masses with broad-based dural attachment. They may cause inward displacement of cortical gray matter and occasionally display an infiltrative “meningioma en plaque” growth pattern.[3]
Computed tomography (CT) is often the initial imaging method for detecting meningiomas. CT scans are particularly effective at visualizing the tumor and its related features, including calcification, hemorrhage, edema, and changes in the calvarium. On unenhanced CT, meningiomas typically appear as sharply defined, homogeneous, and hyperdense extra-axial masses with a broad dural attachment.[4] In only 1–2% of cases, multiple tumors are present. About 60% of meningiomas appear as hyperdense solid lesions, and up to 20% show calcification. Enhancement with intravenous contrast medium is intense and usually homogeneous. Bone changes associated with meningiomas primarily include osteolysis and hyperostosis, with hyperostosis being the most common, occurring in approximately 20% of cases, particularly in the en plaque form.[3] These changes are best visualized on CT, although they can also be detected on magnetic resonance imaging (MRI). Hyperostosis frequently occurs in tumors located at the skull base and anterior cranial fossa, and its extent is not necessarily related to the tumor size [Figure 1]. Pathologically, hyperostosis may result from[4] direct tumor invasion of the bone or reactive hypervascularity of the periosteum, leading to benign bone formation.[5] Distinguishing between hyperostosis due to en plaque meningiomas and primary intraosseous meningiomas, especially osteoblastic types (seen in 59% of cases), can be challenging, particularly when the latter is accompanied by underlying dural enhancement. However, homogeneous, dense enhancement of the tumor within the skull can help differentiate a primary intraosseous meningioma from an en plaque meningioma.
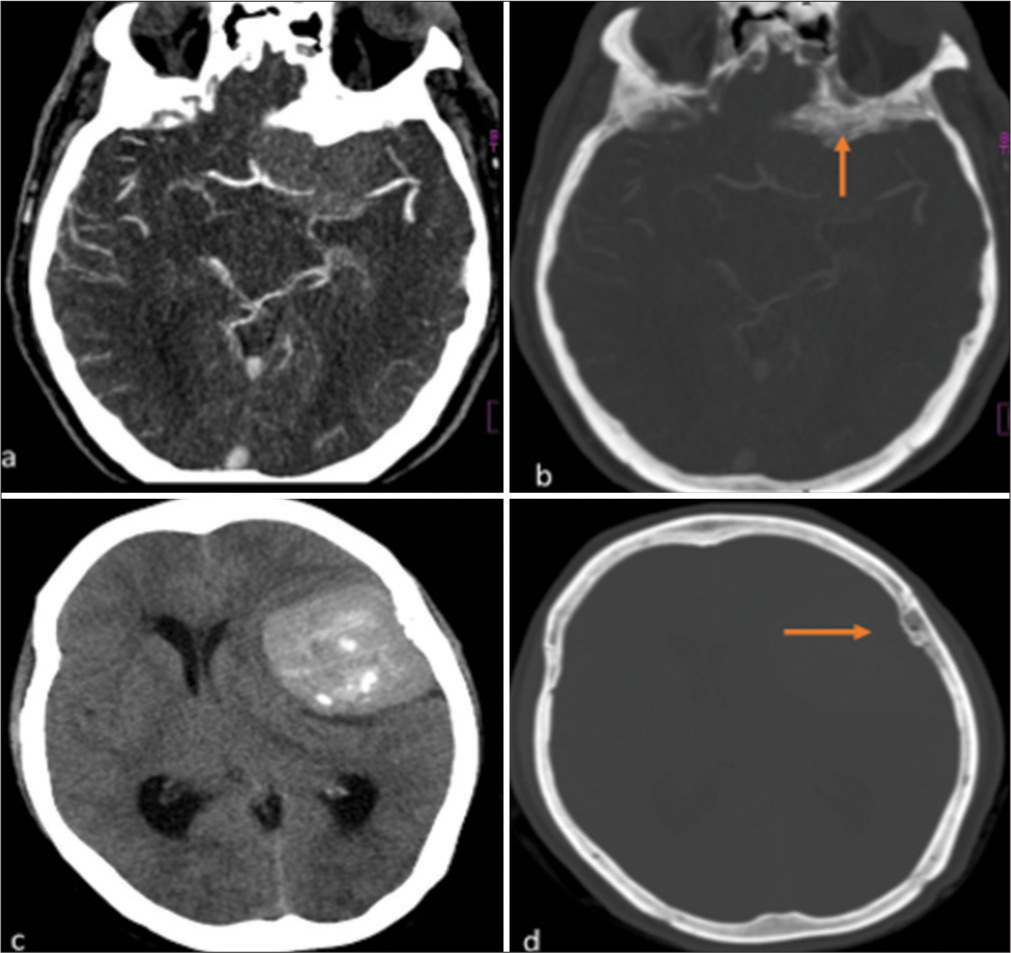
- (a): Axial post-contrast CT brain image of a 46-year-old male, showing a well-defined enhancing hyperdense sphenoid wing meningioma with adjacent hyperostosis of the sphenoid bone. (b) Reformatted CT bone window of the same patient, showing hyperostosis of the sphenoid bone more clearly (orange arrow). (c) Axial non-contrast CT image of a 55-year-old female, demonstrating a hyperdense lateral convexity meningioma. (d) Reformatted CT bone window of the same patient, showing adjacent osseous reaction with subtle hyperostosis of the left parietal bone (orange arrow).
On MRI, they appear iso to slightly hypointense on T1 and isointense to slightly hyperintense on T2/fluid-attenuated inversion recovery, with varying diffusion restriction. Post-contrast, meningiomas usually show avid, homogeneous enhancement, but may have non-enhancing areas such as necrosis or calcification. A dural tail is present in approximately 72% of cases, and a cerebrospinal fluid cleft is characteristic due to its extra-axial location.[6] Encasement of arteries and veins is common, and bone involvement, seen in 20% of cases, can lead to hyperostosis or lytic destruction. Magnetic resonance spectroscopy (MRS) findings in meningiomas are mostly non-specific. Alanine detection inconsistency and lipid presence (0.9/1.3 ppm) are not always reliable indicators of aggressive meningiomas or conclusive evidence of non-benign meningiomas due to variability in results.[7] Meningiomas are highly vascular lesions, typically exhibiting hyperperfusion on perfusion-weighted imaging, with dynamic susceptibility contrast MRI being the most commonly used technique [Figure 2]. Relative cerebral blood volume (rCBV) is generally elevated, with reported values ranging from 6 to 9. Angiomatous subtypes tend to have slightly higher rCBV values, while fibrous subtypes may show slightly lower values.
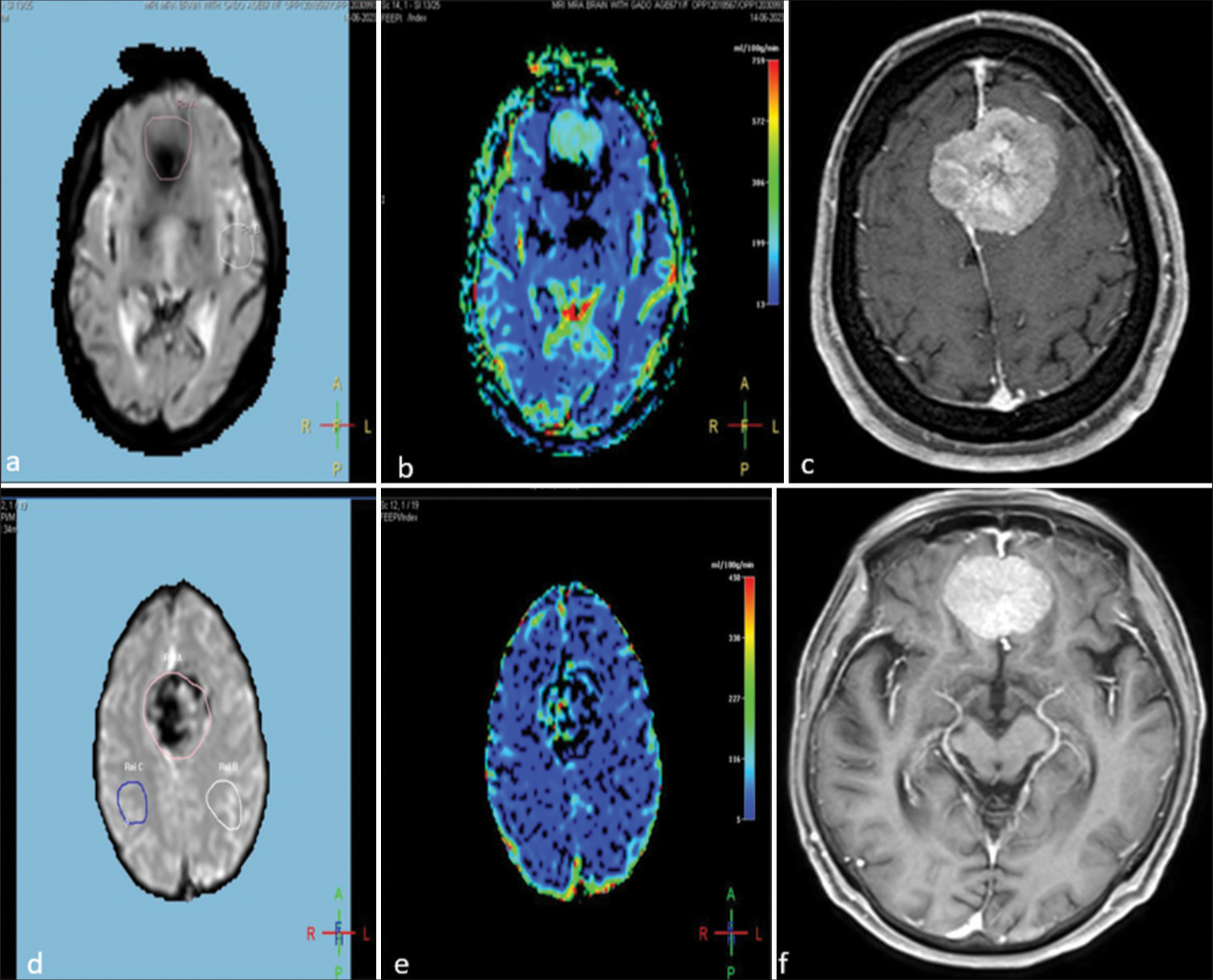
- Imaging of a 43-year-old female and a 51-year-old male with homogeneously enhancing bifrontal meningiomas, demonstrating perfusion characteristics and post-contrast enhancement. (a and b) Perfusion-weighted images of the 43-year-old female. (a) Light pink circle indicating the region of interest (ROI) at the tumor site and a white circle representing the ROI in the normal parenchyma. (b) Raised cerebral blood volume (CBV) at the tumor site, demonstrating increased perfusion. (c) Post-contrast T1-weighted image illustrating homogeneous and intense enhancement of the meningioma. (d and e) Perfusion-weighted images of the 51-year-old male. (d) Light pink representing the ROI at the tumor site and blue and white circles representing the ROI at the normal parenchyma. (e) Increased CBV at the tumor site, demonstrating increased perfusion. (f) Post-contrast T1-weighted image of the 51-year-old male, showing homogeneous and intense enhancement of the meningioma.
Meningiomas are highly vascular tumors, often showing a significant tumor blush and delayed washout on catheter angiography. They are primarily supplied by meningeal branches of the external carotid artery, with additional supply from the internal carotid or vertebral arteries, and sometimes pial vessels. These tumors can encase or displace major vessels and may occlude large veins and cerebral venous sinuses. Identifying these vascular features preoperatively is crucial to reduce the risk of intraoperative hemorrhage or postoperative infarction due to vascular damage.
MATERIAL AND METHODS
A retrospective review was conducted on 82 patients with pathologically confirmed meningiomas who underwent both CT and diffusion-weighted MRI (1.5T) at our institution. The imaging data were analyzed to assess various parameters, including age incidence, sex distribution, tumor location, T1- and T2-weighted sequences, contrast enhancement patterns, and the presence of calcifications, cysts, peritumoral edema, hemorrhage, and MRS findings.
RESULTS
This study analyzed 82 surgically treated intracranial meningioma cases using MRI. It focused on age incidence, gender distribution, tumor location, T1 and T2 characteristics, contrast enhancement, calcification, cysts, peritumoral edema, hemorrhage, and MRS findings.
Meningiomas occurred in individuals aged 21–84, peaking at 40–60 for females and above 60 for males [Graph 1]. The tumors are more common in female patients 58 out of 82 and the rest are male. Left-sided cases numbered 48, right-sided 34. Parasagittal tumors (30%) and lateral hemisphere convexity were predominant locations, aligning with existing literature [Pie Chart 1, Figures 3 and 4].
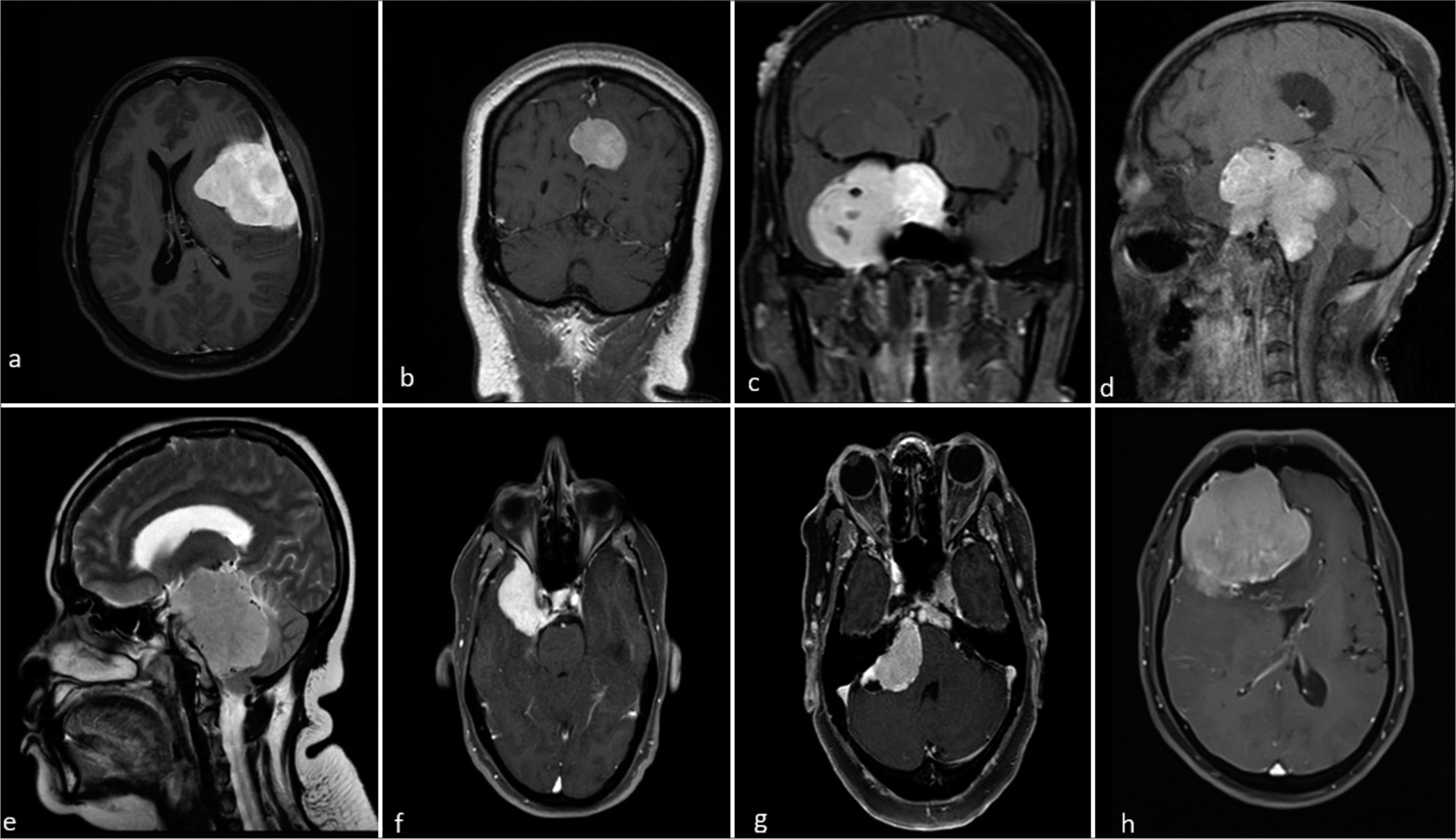
- Magnetic resonance imaging post-contrast images of different patients with meningioma at typical and atypical locations. (a) Lateral convexity meningioma, (b) parasagittal meningioma, (c) sphenoid wing meningioma, (d) sella turcica meningioma, (e) posterior cranial fossa meningioma, (f) middle cranial fossa meningioma, (g) cerebello-pontine angle meningioma, and (h) anterior cranial fossa meningioma.
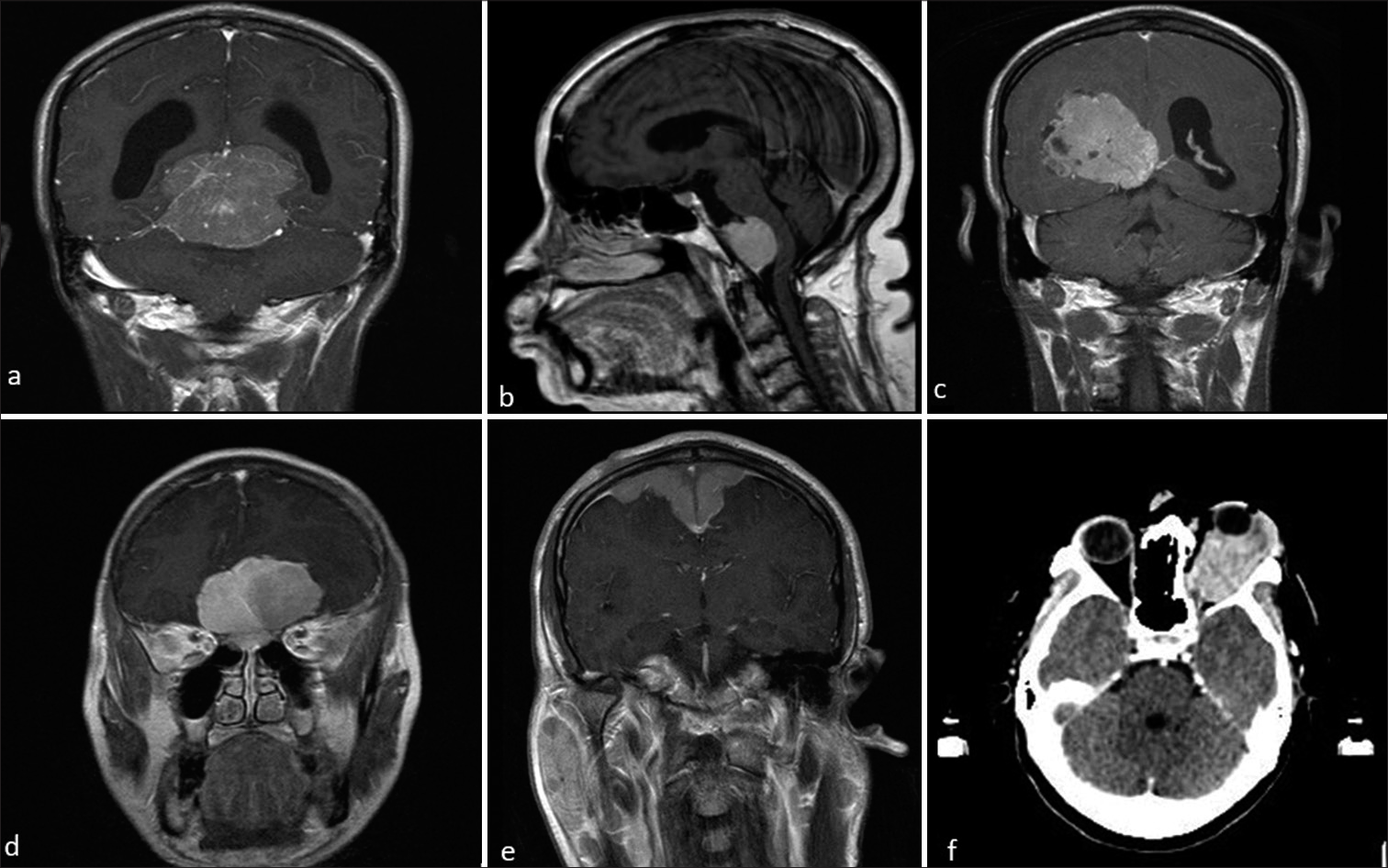
- Magnetic resonance imaging post-contrast images of different patients with meningioma at atypical locations. (a) Tentorial meningioma, (b) retroclival meningioma, (c) intraventricular meningioma, (d) bifrontal meningioma, (e) en-plaque type meningioma, and (f) axial computed tomography brain showing optic nerve meningioma.
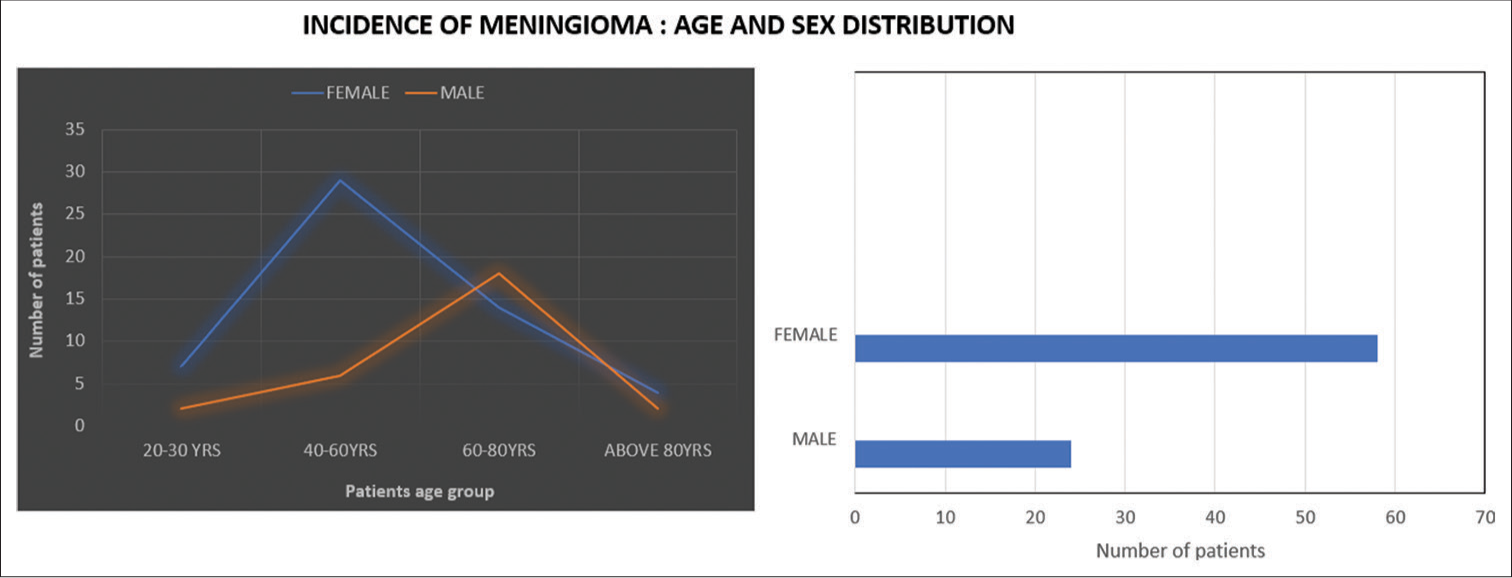
- Age distribution of meningioma cases by sex. (Blue line represents female patients and orange line represents male patients.)
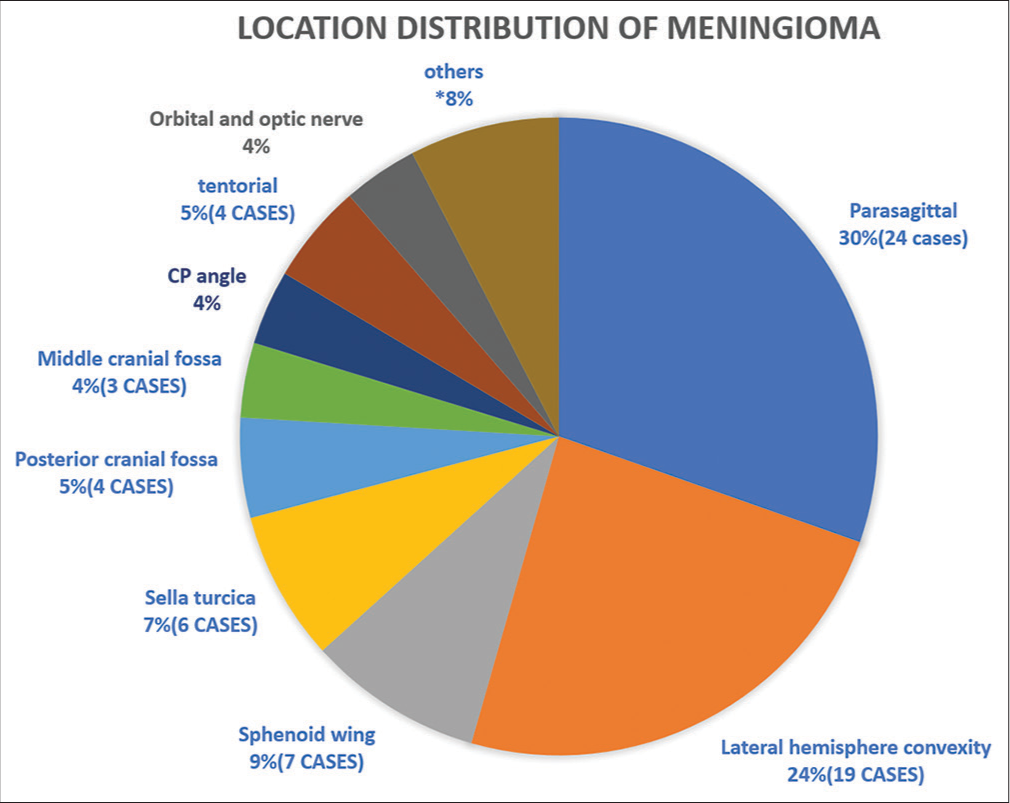
- Summary of locational distribution of meningioma.
Most tumors showed typical magnetic resonance signal intensities and homogeneous contrast enhancement. Atypical features (22 cases) included heterogeneous signal, calcification, diverse contrast patterns, cysts, and necrosis. Dural tail signs and peritumoral edema occurred in over one-third of cases. Hyperostosis (24 cases) surpassed osteolysis. MRS indicated increased choline and creatine peaks, and alanine presence in 12 cases. Six cases had intratumoral/peritumoral hemorrhage; one had spontaneous intracerebral hemorrhage, confirming an anaplastic subtype. Encasement of carotid arteries and dural sinuses occurred in six cases. Four patients had recurrent meningiomas, and one case each of plaque meningioma and falx/tentorium origin was identified [Table 1, Figures 5 and 6]. Age, sex, location, and salient features of different cases given below.
| Findings | Number of cases |
|---|---|
| Peri-tumoral edema | 38 |
| Mass effects | 31 |
| Dural tail sign | 27 |
| Necrosis | 21 |
| Bone hyperostosis/erosion | 21 |
| Cystic changes | 10 |
| Abnormal MR spectroscopy | 12 |
| Intra/peritumoral hemorrhage | 6 |
| Vascular encasement | 6 |
| Calcification | 4 |
MR: Magnetic resonance
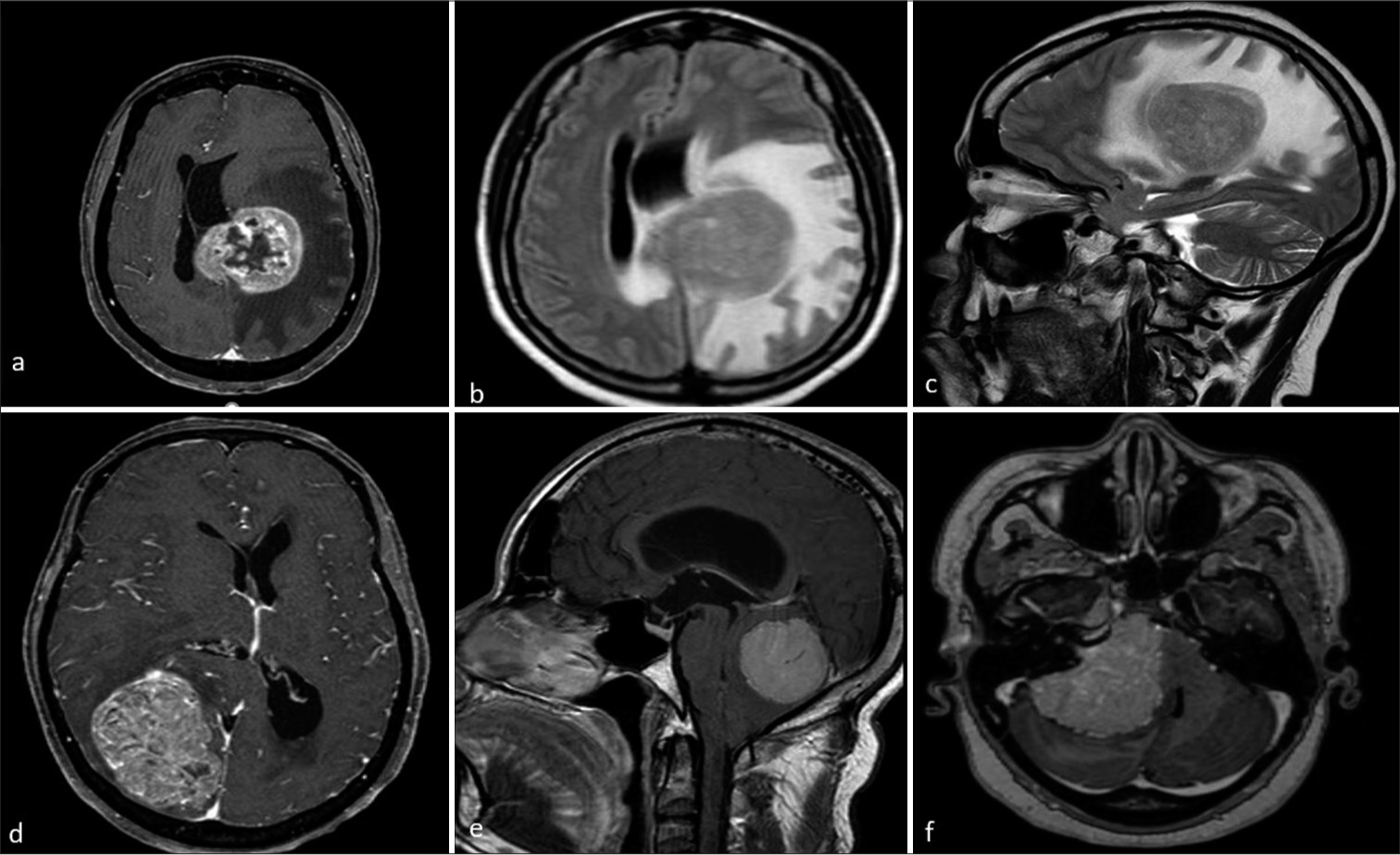
- Magnetic resonance (MR) imaging of different patients with meningioma showing atypical features. (a-c) MR axial post-contrast image showing left parasagittal meningioma with central non-enhancing necrotic areas axial and sagittal T2W images show parasagittal meningioma with moderate to gross perilesional edema. (d) Axial post-contrast image shows meningioma causing significant mass effect by compressing adjacent cortical areas and causing midline shift. (e and f) Sagittal and axial post-contrast images show posterior fossa and cerebello-pontine angle meningioma causing compression of brainstem and fourth ventricle with tonsillar herniation and hydrocephalus.
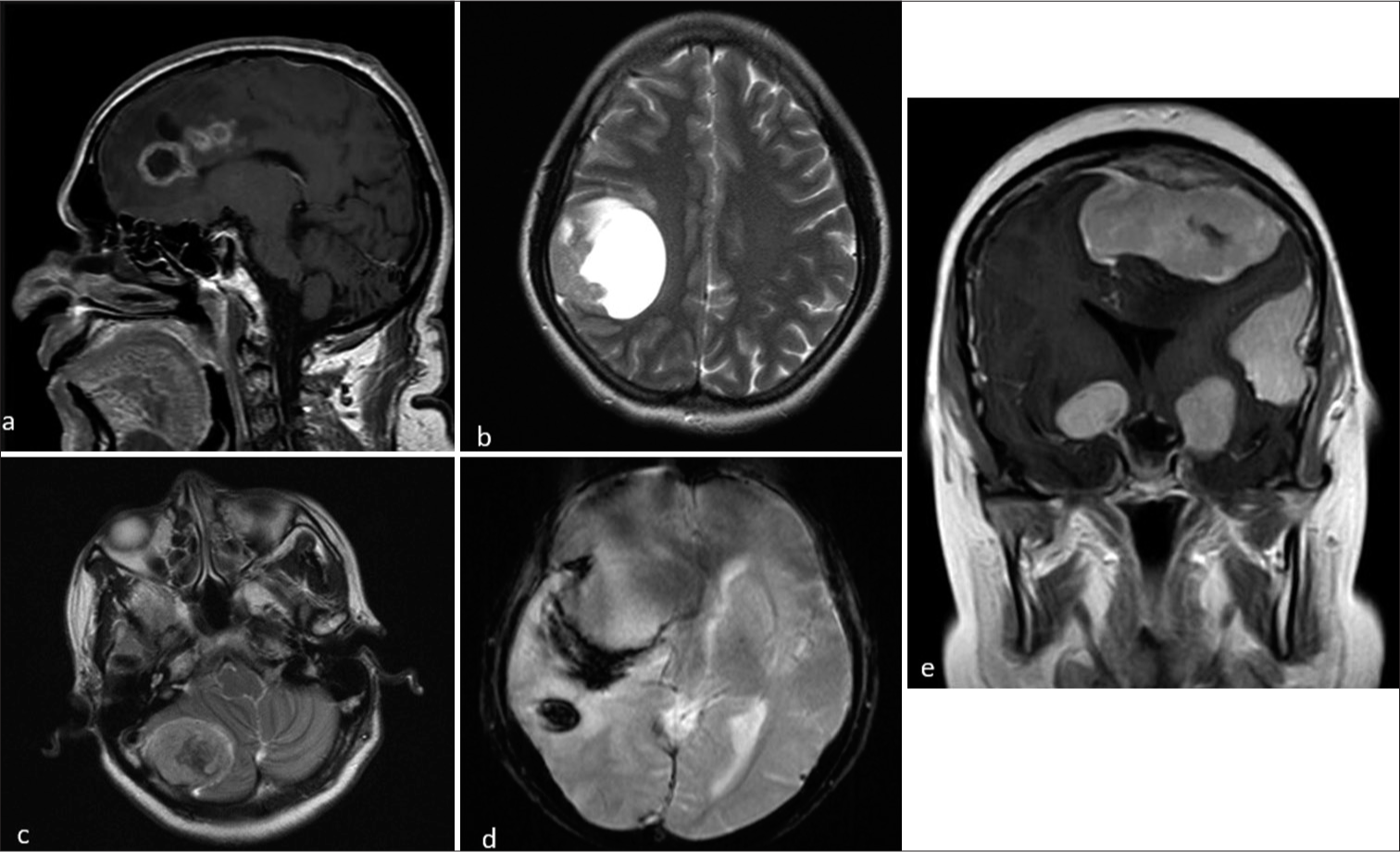
- Magnetic resonance imaging of different patients with meningiona showing atypical features. (a and b) Sagittal post-contrast and axial T2-weighted (T2W) images showing meningioma with cystic changes. (c and d) Axial T2W and susceptibility-weighted imaging sequences showing meningioma with intra tumoral hemorrhage and intracerebral hemorrhage. (e) Coronal contrast images shows multiple meningioma involving supra and infratentorial regions.
Atypical imaging features
Distinguishing atypical meningiomas from the WHO I meningiomas before treatment is crucial for surgical planning and better outcomes. Atypical meningiomas show robust but heterogeneous contrast enhancement. Key imaging features include restricted diffusion, notably lower apparent diffusion coefficient (ADC) compared to typical meningiomas. Perfusion MRI may reveal elevated rCBV, especially in peritumoral edema. MRS often indicates elevated alanine levels, a distinctive marker for atypical meningiomas. Recognizing these features is essential for accurate pre-treatment classification, guiding surgeries, and improving overall treatment success.[8] Prevalence of atypical imaging features in this study given below [Table 2]. Meningiomas from atypical locations are seen to have atypical features compared to typical locations [Table 3].
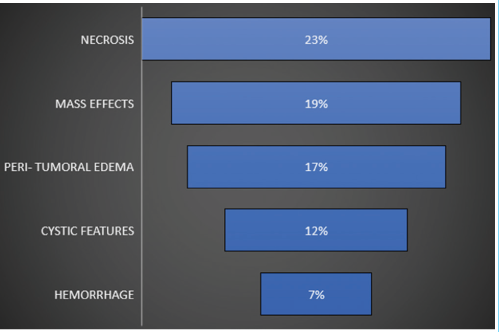 |
| Location | Necrosis | Hemmorhage | Cystic changes | Peri-tumoral edema[severe] |
|---|---|---|---|---|
| Parasagittal[24] | 6 | 2 | 4 | 5 |
| Lateral hemisphere convexity[19] | 3 | - | 2 | 2 |
| Sphenoid wing[7] | 2 | - | 1 | 1 |
| Sella turcica[6] | 3 | 2 | 1 | 1 |
| Posterior cranial fossa[4] | 2 | - | 1 | 1 |
| Middle cranial fossa[3] | 1 | 1 | - | - |
| CP angle[3] | 2 | - | - | 1 |
| Tentorial[3] | 1 | - | 1 | 1 |
| Others | 1 | 1 | - | 1 |
X-Axis (Age Groups): The current grouping of ages into broader intervals (e.g., 20-40 years, 40-60 years) was chosen to maintain clarity and readability given the sample size in each group. While we considered more granular intervals, the existing groupings reflect meaningful stages in patient age distribution and allow for a more streamlined comparison between age groups. We believe this format effectively communicates the trends in the data. Y-Axis (Number of Patients): The y-axis intervals were selected based on the maximum value of patients (35) in the dataset. To avoid unnecessary complexity, we used consistent increments of 5, which we believe balance accuracy and simplicity. We opted not to extend the y-axis beyond 35, as this would create empty space without additional informative value.
DISCUSSION
More than 80% of meningiomas fall into the meningothelial, fibrous, and transitional subtypes, classified as WHO Grade 1, exhibiting typical imaging features described earlier. However, a smaller subset labeled as WHO Grade 2 and Grade 3 meningiomas displays distinct and atypical imaging findings.[7]
Meningothelial meningiomas, the most common subtype, typically present with hyperintensity on T2-weighted sequences, homogenous contrast enhancement featuring a dural tail sign, and associated bone hyperostosis. Fibrous meningiomas, characterized by collagen presence, show relative T2 hypointensity. Transitional meningiomas exhibit mixed iso- and hyperintensity on T2-weighted sequences. Psammomatous meningiomas, besides typical features of the WHO Grade 1, are distinguished by calcifications. Angiomatous meningiomas showcase multiple flow voids due to hypervascularity, accompanied by significant peritumoral edema. Reticular type contrast enhancement with peritumoral edema characterizes microcystic meningiomas.
Secretory meningiomas, common at the skull base, display high-signal intensity on T2-weighted sequences with high ADC values, unlike typical meningiomas, along with notable peritumoral edema. Lymphoplasmacyte-rich meningiomas in young patients present with irregular tumor margins invading adjacent brain tissue, displaying heterogenous contrast enhancement and significant peritumoral edema. Clear cell meningiomas, mainly seen in young patients at CP angles, often recur. Their MRI features include heterogenous contrast enhancement, cysts, peritumoral edema, and lytic bone destruction.
Atypical and anaplastic meningiomas, more common in males, demonstrate irregular tumor margins, an indistinct tumor-brain interface, heterogenous signal intensities, restriction on diffusion-weighted sequences, underlying bone destruction, peritumoral edema, necrosis, and calcification.[7,9] Metaplastic meningiomas, containing cartilaginous, osseous, or lipomatous tissue, present varied imaging findings based on tumor composition. Although approximately 60% of meningiomas are extra-axial, about 60% are associated with vasogenic cerebral edema. Microcystic meningiomas, in particular, exhibit a high incidence of peritumoral edema. Cystic changes, either extratumoral or intratumoral, are rare in meningiomas with 10 cases in this study. Intratumoral hemorrhages are occasionally observed, with six cases in this study, one associated with spontaneous intracerebral hemorrhage.[8] While avid and homogenous contrast enhancement is typical, heterogenous or ring enhancement may occur, especially in cases with central cyst formation, hemorrhage, or necrosis [Table 4].
| Pathological subtype | Distinguishing imaging features |
|---|---|
| Meningothelial | Classical meningioma pattern |
| Fibrous | T2W hypointensity |
| Transitional | T2W heterogeneous appearance |
| Psammomatous | Dense calcification |
| Angiomatous | Large, prominent intratumoural and peritumoral feeding vessels |
| Microcystic | T1W hypointensity |
| Secretory | Skull base location, T2W hyperintensity |
| Lymphoplasmacyte-rich | En-plaque pattern |
| Clear cell | Cerebellopontine angle location, osteolysis |
| Chordoid | High mean ADC values |
| Metaplastic | Mesenchymal components on background of meningioma |
| Atypical and WHO grade III | Ill-defined margins, diffusion restriction |
WHO: World Health Organization, ADC: Apparent diffusion coefficient, T2W: T2-weighted, T1W: T1-weighted
Numerous studies have sought to correlate MRI patterns with the various histopathological subtypes of meningiomas, providing valuable insights into accurate diagnosis and classification.[7,10]
Hoover et al. (2011)[7]: Distinguished soft from firm/fibrous meningiomas based on MRI. Soft meningiomas showed hypointensity on T1 and hyperintensity on T2, while firm/fibrous tumors appeared isointense on T1 and hypointense on T2. This aids surgeons during operations
Hale et al. (2018)[10]: Retrospective study of 128 patients identified predictors of high-grade meningioma through MRI. Predictors included tumor necrosis, increased peritumoral edema, location along the falx or convexity, presence of draining veins, and tumor volume.
Differential diagnosis and mimics for extra-axial tumors
Meningiomas necessitate differentiation from similar extra-axial dural-based conditions, and MRI plays a pivotal role in this diagnostic challenge [Table 5]: [9,11]
| Type | Distinguishing features |
|---|---|
| Solitary fibrous tumour | T2WI: mixed signal due to hypointense regions of collagen and these hypointense regions enhance. Separate regions of different T2 signal can cause Yin-Yang sign |
| Dural Metastasis | T2W –variable, typical rCBV values of less than 2 |
| Schwannoma | Do not have a propensity to involve the internal auditory canal (which is a fairly constant feature of schwannomas) |
| Lymphoma | may be lobulated with indistinct ‘fluffy borders’ and restriction on DWI . |
| Sarcoidosis | Presence of pulmonary sarcoidosis can aid in distinguishing it from meningiomas |
| Glioblastoma | High lipid and high lipid-choline ratio on MRS,restriction on DWI. |
| Venous vascular malformation of the dura or venous sinuses | Delayed slow centripetal “filling in” of the mass on dynamic contrast-enhanced MR is suggestive of hemangioma. |
| Melanocytic tumour | Usually contain areas of T1 hyperintensity due to melanin and haemorrhage. |
T2WI: T2-weighted image, rCBV: Relative cerebral blood volume, DWI: Diffusion-weighted imaging, MRS: Magnetic resonance spectroscopy
Several neoplastic and non-neoplastic entities can mimic meningiomas both clinically and radiologically. Solitary fibrous tumor, rare WHO grade II neoplasms, differ from meningiomas by lacking calcification and hyperostosis but may cause skull erosion and display heterogeneous enhancement with prominent internal flow voids [Figures 1 and 2]. These tumors often have a narrow or broad dural attachment. Dural metastases, which may present with an enhancing dural tail, typically appear hyperintense on T2-weighted MRI sequences. Common sources include breast carcinoma, adenocarcinomas, squamous cell carcinoma of the lung, and renal cell carcinoma, with multiple lesions often suggesting metastases over meningiomas.
Secondary CNS lymphoma can also mimic meningiomas, presenting as dural-based lesions that are isointense to hypointense on T2-weighted sequences with intense post-contrast enhancement. These lesions may involve the leptomeninges and present as multiple. Vestibular schwannoma is also a close mimicker of CP angle meningiomas; however, vestibular schwannomas have a propensity to involve the internal auditory canal (which is a fairly constant feature of schwannomas) [Figure 7]. Sarcoidosis, affecting the CNS in about 5% of cases, can involve various CNS structures including the dura, leptomeninges, perivascular subarachnoid spaces, cranial nerves, and brain parenchyma. Sarcoid lesions may mimic meningiomas, appearing hypointense on T1-weighted sequences and hyperintense on T2-weighted sequences, with homogeneous post-contrast enhancement. The presence of pulmonary sarcoidosis can aid in distinguishing it from meningiomas. Other less important mimics are venous vascular malformation of the dura or venous sinuses, glioblastoma, and melanocytic tumor. The dural tail sign, commonly associated with meningiomas, should be interpreted carefully, as it also appears in other dural conditions.
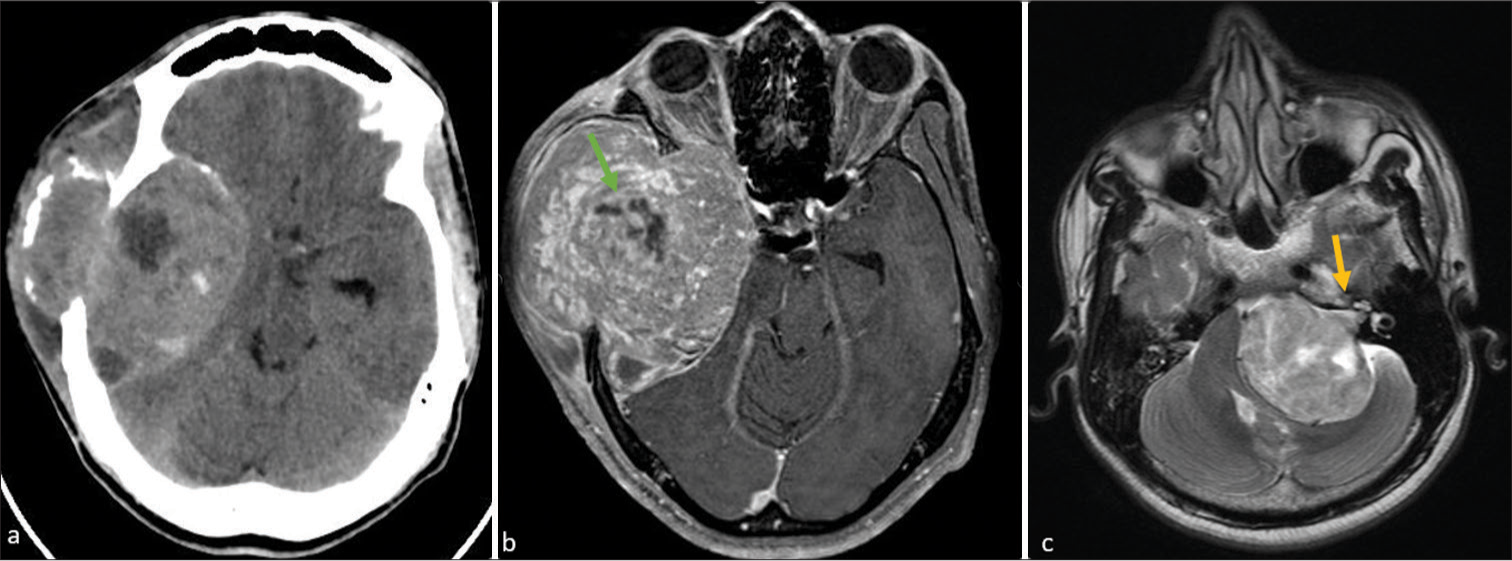
- (a) Axial computed tomography shows a large ill-defined lobulated heterogeneous mixed-density extra-axial mass noted in the right parietal-temporal region with adjacent bone destruction. (b) On post-contrast T1-weighted magnetic resonance imaging show the lesion heterogeneous enhancement with prominent internal flow voids (straight green arrow). (c) Axial T2-weighted image showing a large heterogenous extra-axial mass in the left cerebellopontine angle causing compression and displacement of the left cerebellar hemisphere, pons, as well as fourth ventricle. Note that mass arises within the right internal auditory meatus causing its widening (straight yellow arrow).
CONCLUSION
Meningiomas are the commonest extra-axial tumors of the CNS and can have a varied appearance on imaging. There are a number of typical and atypical MRI features of meningiomas that are described and it is important for the reporting radiologist to have a broad understanding of their variable potential appearances. MRI is the most important preoperative investigation in the diagnosis and grading of meningioma and it also helps to differentiate it from other conditions that mimic meningioma.
Acknowledgment
The authors would like to express their deepest gratitude to Dr. N. L. N. Moorthy for his editorial support.
Ethical approval
The research/study was approved by the Institutional Review Board at Apollo institute of medical sciences and research, number AIMSR/IRB/RC/2024/07/036, dated July 24, 2024.
Declaration of patient consent
Patient’s consent is not required as there are no patients in this study.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Atypical imaging appearances of intracranial meningiomas. Clin Radiol. 2007;62:10-7.
- [CrossRef] [Google Scholar]
- Typical, atypical, and misleading features in meningioma. Radiographics. 1991;11:1087-106.
- [CrossRef] [Google Scholar]
- Hyperostosis associated with meningioma of the cranial base: Secondary changes or tumor invasion. Neurosurgery. 1999;44:742-6.
- [CrossRef] [Google Scholar]
- Neuroradiological findings of atypical meningiomas. CMIG Extra Cases. 2004;28:33-V9.
- [CrossRef] [Google Scholar]
- Use of preoperative magnetic resonance imaging T1 and T2 sequences to determine intraoperative meningioma consistency. Surg Neurol Int. 2011;2:142.
- [CrossRef] [Google Scholar]
- Variants of meningiomas: A review of imaging findings and clinical features. Jpn J Radiol. 2016;7:459-69.
- [CrossRef] [Google Scholar]
- Magnetic resonance imaging of meningiomas: A pictorial review. Insights Imaging. 2014;5:113-22.
- [CrossRef] [Google Scholar]
- Differentiating meningioma grade by imaging features on magnetic resonance imaging. J Clin Neurosci. 2018;48:71-5.
- [CrossRef] [Google Scholar]
- Distinguishing grade I meningioma from higher grade meningiomas without biopsy. Oncotarget. 2015;6:38421-8.
- [CrossRef] [Google Scholar]






