Translate this page into:
Magnetic Resonance Imaging Determination of Normal Pituitary Gland Dimensions in Zaria, Northwest Nigerian Population
Address for correspondence: Dr. Philip Oluleke Ibinaiye, Department of Radiology, Ahmadu Bello University Teaching Hospital, Zaria, Nigeria. E-mail: pibinaiye@abu.edu.ng
-
Received: ,
Accepted: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Objectives:
To determine the dimensions of normal pituitary gland using T1-weighted magnetic resonance images (MRI) and to determine their relationship with age and sex.
Materials and Methods:
Cranial MRI scans of 100 individuals with clinically normal pituitary function (58 males and 42 females) and in the age range 14–82 years were reviewed in order to obtain volumetric measurements of the pituitary gland. The height, width, and depth of the pituitary were obtained from mid-sagittal and coronal planes, while the volume was calculated from these measured parameters. The data obtained were stratified based on age and sex for analysis. Statistical tests applied included Student's t-test and Pearson correlation. A minimum level of statistical significance was set at P < 0.05.
Results:
The mean pituitary volumes were 334.1 ± 145.8 mm3 and 328.1 ± 129.2 mm3 while the mean pituitary heights were 6.45 ± 1.7 mm and 6.46 ± 1.57 mm in males and females, respectively. Although there was no statistically significant difference between pituitary height and pituitary volume in both sexes, they correlated negatively with increasing age (r = −202, P = 0.04 and r = −410, P = 0.000, respectively). Both parameters were highest in pubertal subjects and declined steadily with age, with a second peak occurring only for pituitary height in the sixth decade. The mean pituitary widths (9.08 ± 2.59 mm and 9.21 ± 1.86 mm) and depths (10.59 ± 1.71 mm and 10.49 ± 1.57 mm) in males and females, respectively, did not show remarkable changes with age and sex in the individuals studied.
Conclusion:
With this study, we have provided reference values in Nigerian population for the dimensions of normal pituitary gland, in order to facilitate assessment and diagnosis in patients with abnormalities of the hypothalamic–pituitary axis.
Keywords
Magnetic resonance imaging
normal
pituitary gland
volume
INTRODUCTION

The pituitary gland was first described anatomically by a Belgian scientist Andreas Vesalius in 1543.[1] It is a small-sized gland with master functions; hence, its size and morphology have been a source of interest for many researchers. Sometimes in imaging, one takes a quick look at the contour of the superior surface of the pituitary gland or the size of the sella turcica as an indication or suggestion of enlargement of the gland. However, this would be misleading as the shape of the superior surface of the normal gland could either be flat, concave, or convex, depending on the hormonal status, age, sex, and even race of the individual.[1] Also, the size of the bony sella is not a sensitive indicator of pituitary gland abnormality since an empty sella can lead to an enlarged fossa. Hence, there is a need for quantitative assessment. Magnetic resonance imaging (MRI) presently supersedes computerized tomography (CT) and plain radiographs in the investigation of the sella, parasellar, and suprasellar regions.[234] MRI allows detailed visualization of the anterior and posterior lobes, pituitary infundibulum, optic chiasma, and other parasellar structures.
The coronal image is considered the best single view for imaging the pituitary gland, while the sagittal image best assesses the relationship of the midline structures.[5]
There is currently paucity of data on the dimensions of normal pituitary gland in the Nigerian population. This study was carried out to fill in this gap and also to compare our findings with those of other authors in previous studies.
MATERIALS AND METHODS
One hundred consecutive subjects (58 males and 42 females) with no evidence of neuroendocrine or neuropsychiatric disease, whose serum prolactin, follicle stimulating hormone (FSH), and luteinizing hormone (LH) were essentially normal, and who had normal pituitary gland on brain MRI scan were included in the study. Pregnant or breastfeeding women, patients with previous brain surgery, and those on exogenous hormone therapy were excluded. This cross-sectional study which spanned from 1 June, 2012 to 31 August, 2013 was conducted at the radiology department of Ahmadu Bello University Teaching Hospital, Zaria, Nigeria. Approval for the study was granted by the ethical committee of our institution. All patients gave informed and written consent prior to the investigation. The patients who met our inclusion criteria were those who were referred to the radiology department for brain MRI. The various indications for brain MRI in these patients are highlighted in Table 1.
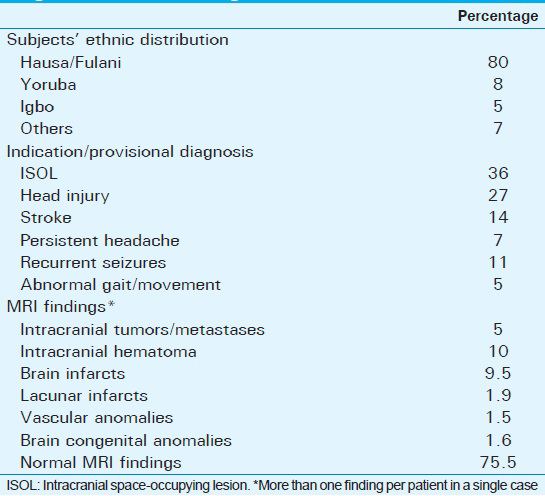
Image acquisition
Axial, sagittal, and coronal images were acquired using a low-field strength (0.2 T) open permanent magnet MR scanner (Magnetom concerto® version; Seimens, Wittelsbacherplatz, Germany) loaded with syngo MR 2004A software (Numaris/4, Siemens software packages, Siemens AG, Germany). T1-weighted images were utilized, as this sequence allows the visualization of the hyperintense posterior pituitary bright spot when present.
T1-weighted sagittal spin echo was acquired with time to repeat (TR) =365–428 ms and time to echo (TE) =9–10 ms, while the post-contrast (the use of gadolinium was part of the clinical indication) coronal images were acquired using TR = 420–530 ms and TE = 13 ms. All images were taken at 3 mm slice thickness and constructed on a 512 × 256 matrix with a field of view of 23 cm.
Measurements
Pituitary gland dimensions were taken as the maximum distance (in mm) between two surfaces (lateral and superoinferior surfaces) using the in-built electronic callipers provided by the software. The pituitary height and depth were measured from the sagittal plane, using a midline image at a section where the cerebral aqueduct was visible [Figure 1], while the pituitary gland width was measured from the coronal plane, at a section where the pituitary stalk was visible [Figure 2]. The pituitary volume (mm3) was obtained by multiplying the height (H) by depth (D) by width (W) by 0.52. The factor 0.52 was derived from sphere volume equation coefficient and cubic volume calculation:[6] (4/3π)(r3)/(2r)3 = 3.1416/6 = 0.52 (0.52 is a constant and correcting factor for the variation in shape that may occur in normal pituitary gland).
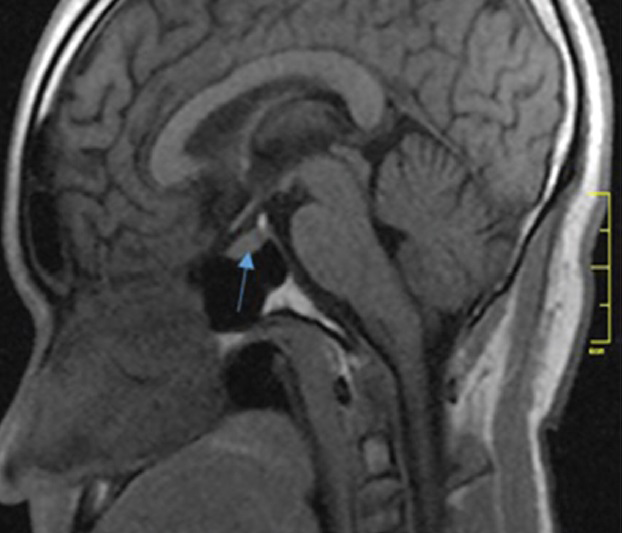
- Mid-sagittal non-contrast T1-weighted MRI in a 26-year-old male shows the pituitary gland (arrow). The neurohypophysis is marked by the posterior bright spot. The pituitary height is measured as the maximum cranio-caudal distance and depth as the antero-posterior distance.
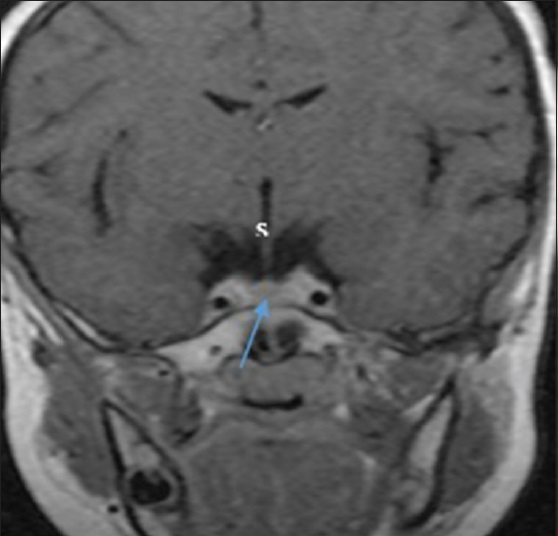
- Coronal post-contrast T1-weighted MRI shows the pituitary gland (arrow), infundibulum, or stalk (s) and the laterally related intracavernous internal carotid artery. The width was measured as the maximum distance to the left and right sides of the pituitary gland.
Blood sample was taken from each participant who had normal pituitary gland on MRI for further hormonal (prolactin, FSH, and LH) analyses, and only those with normal values were recruited into the study.
Statistical analysis
The statistical analysis was done using SPSS software version 17 (SPSS, Inc., Chicago, IL, USA). The patients were stratified on the bases of age and sex. Data were expressed as means and standard deviation and illustrated using tables and graphs. The relationship between pituitary gland dimensions with age was examined statistically using the Pearson's correlation, while gender differences were evaluated using the Student's t-test. P < 0.05 was considered to indicate statistically significant difference.
RESULTS
The subjects’ ethnic distribution, indication/provisional diagnosis, and MRI findings are presented in Table 1.
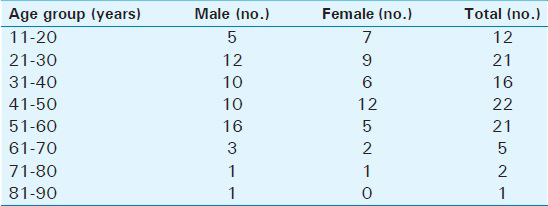
Out of the 100 subjects studied (58 males, 42 females), the youngest was 14 years old while the oldest was 82 years old (mean age ± SD = 40.63 ± 15.32 years). The frequency distribution by age and sex is presented in Table 2.
The mean pituitary height was 6.45 ± 1.7 mm and 6.46 ± 1.57 mm in males and females, respectively [Table 3]. Maximum values were seen in the 11–20 years age group (7.62 ± 2.0 mm3 and 7.81 ± 1.60 mm3 for males and females, respectively) and the value was higher in females than in males.

The range for pituitary volume for the total population was 143.90–370.80 mm3 [Table 4]. However, it was larger in males (mean volume 334.1 ± 145.8 mm3) than in females (mean volume 328.1 ± 129.2 mm3), although not significant statistically, as seen in Table 3.
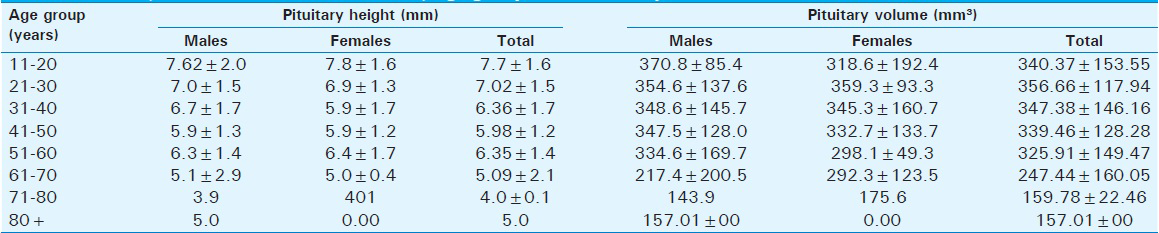
Table 3 also shows that the mean pituitary dimensions (height, width, and depth) were similar in both males and females (P = 0.98, 0.79, and 0.77, respectively).
Both the pituitary volume and height correlated negatively and significantly with age (P < 0.05). The pituitary volume measured showed the largest value (370.8 ± 85.4 mm3) in the age group 11–20 years and it was found in males [Table 4]. In females, however, the largest volume (359.3 ± 93.3 mm3) was found in the age group 21–30 years. The lowest values were recorded in the seventh and eighth decades in both sexes [Figures 3 and 4].
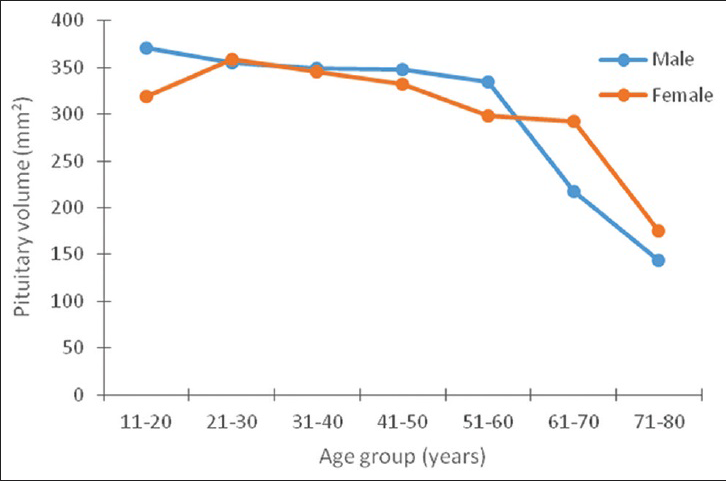
- Relationship between the height of the pituitary gland and age in both sexes.
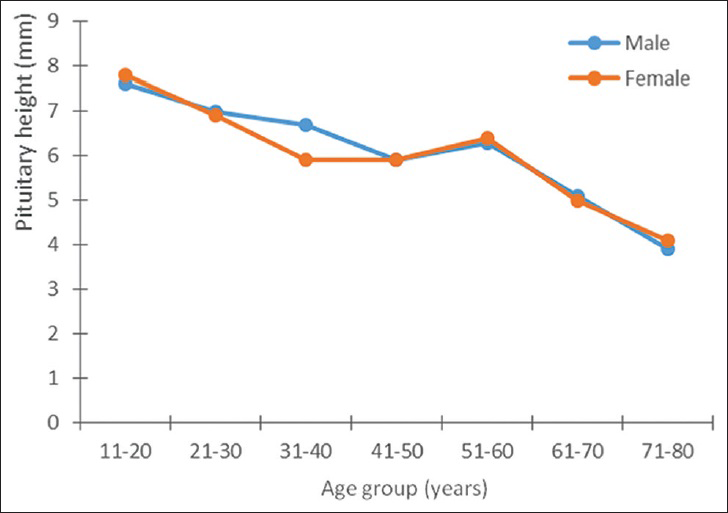
- Relationship between pituitary volume and age in both sexes.
The pituitary width and depth did not show any statistically significant correlation with age.
Out of all pituitary dimensions (height, width, and depth), only height showed correlation with pituitary volume.
DISCUSSION
Despite its small size, subtle alterations in the pituitary gland could have substantial effects on other neuroendocrine organs. The Growth Hormone Society summary statement of 2000 indicated the need for more data on pituitary dimensions.[7] We also found that there is dearth of literature on African subjects. In the present study, the mean pituitary height for African population was 6.45 mm and the volume was 331.59 mm3. This is comparable to earlier reports.[89] Our peak values for pituitary height (7.62 ± 2.0 mm and 7.81 ± 1.60 mm in males and females, respectively) were, however, higher than the findings of Faisal et al.,[8] (6.3 ± 1.4 mm and 5.9 ± 1 mm, respectively), Denk et al.,[9] (5.7 ± 0.2 mm and 5.6 ± 0.2 mm, respectively), Elster et al.,[10] and Tsunoda et al.,[11] (5.33 ± 1.2 mm and 4.93 ± 1.0 mm, respectively). These variations can be explained as due to the differences in the populations studied.
The pituitary dimensions vary in different age groups and this has been well documented.[891011] It reflects changes in the intricate hormonal milieu of the gland at different phases of life. Some authors found that these changes are significantly different between sexes, with larger glands found in females.[811] Though our findings support that female have larger glands, the results were not statistically significant. In the studies of Denk et al.,[9] and Kato et al.,[12] results revealed that boys less than 10 years of age had statistically larger glands than females.
After birth, serum levels of growth hormone and prolactin decrease, which leads to a decrease in gland height in the immediate postnatal period. After the second month of life, the pituitary gland gradually increases in height to reach its maximum at puberty. This can be explained by the rapid hormonal changes at puberty, especially in the levels of gonadotropins (LH and FSH). Similar to previous studies,[8910] we found that the pituitary height peaked at the second decade of life in both sexes, with higher values found in females. Some authors differ on this finding and have reported peak values in the third decade.[1112] Apart from pituitary height, the shape of the upper surface and the signal intensity were also reported to show more significant changes in females.[1213]
We have observed that both males and females attained the peak pituitary height in a single decade of life (2nd decade). This is, however, contrary to the finding by Faisal et al.,[8] who reported that females achieved the peak heights in their second decade, while in males it was achieved in the third decade. They explained that peak height is a determinant factor for the development of puberty and this is achieved in females 5 years earlier than in their male counterparts. They believe that this fact could be responsible for early achievement of maximal height in females.
In addition, we recorded a second peak in pituitary height in individuals at the sixth decade, which was also greater for females. This is in agreement with the reports of Tsunoda et al.,[11] and Denk et al.[9] This second peak is thought to reflect the increased activity triggered as a negative feedback mechanism by the waning hormonal levels in the target organs.
There were no subjects with a pituitary height greater than 9 mm in either sex in this study.
When we further analyzed the differences in mean height with respect to the various age groups in both sexes, the sex differences were not statistically significant, unlike seen in other reports.[141516]
Our findings on pituitary volume, however, differed slightly with respect to age and sex. The peak age was in the third decade, while the peak value was higher in males. No second peak in pituitary volume was recorded in individuals in the sixth decade. However, both pituitary height and volume declined steadily thereafter, and the lowest levels were recorded after the seventh decade.
In addition, we also observed that among the four parameters that we studied in relation to the pituitary gland, pituitary height changed most remarkably with respect to age and sex. This is in partial agreement with the opinion that mid-sagittal height of the pituitary gland reflects the variation in pituitary morphology more accurately.[131718]
Lurie et al.,[18] opined that there are no age-related effects on gland width or depth, which is in agreement with our findings. We also believe that the unremarkable variation in pituitary volume in our study could be partly explained by the fact that volume measurements calculated from linear parameters are indirect estimations. Lurie SN et al., believe that 3D volumetry should be the new standard for measuring pituitary sizes.[19]
Limitations
Our study is limited by selection bias due to the fact that the high cost of the examination did not permit us to study normal volunteers. Hence, patients with other conditions, but without clinical or imaging evidence of neuroendocrine or neuropsychiatric pathology were selected.
The non-availability of 3D software in our center might have affected the accuracy of our measurements.
CONCLUSION
With this study, we have provided reference values for the normal pituitary gland dimensions in a Nigerian population, which will facilitate assessment and diagnosis in patients with abnormalities in pituitary function. Our study points to racial variation in pituitary dimensions and showed a higher pituitary height and no sex difference when compared to previous reports on Caucasian populations.
Financial support and sponsorship
Nil.
Conflict of interest
There are no conflict of interest.
Available FREE in open access from: http://www.clinicalimagingscience.org/text.asp?2015/5/1/29/157853
REFERENCES
- Weight and dimensions of the pituitary in Northwestern Indians. Pituitary. 2006;9:19-26.
- [Google Scholar]
- A parallel study of the roentgen anatomy of the sella turcica and histopathology of the pituitary gland in 205 autopsy specimens. Neuroradiology. 1981;21:55-65.
- [Google Scholar]
- Role of magnetic resonance imaging in the diagnosis and prognosis of growth hormone deficiency. Clin Endocrinol (Oxf). 1996;45:21-6.
- [Google Scholar]
- Comparative morphological study of the pituitary gland by computed tomography and magnetic resonance imaging. S Afr Med J. 1988;74:406-7.
- [Google Scholar]
- 3D volumetry comparison using 3T magnetic resonance imaging between normal and adenoma-containing pituitary glands. Neurol India. 2011;59:696-9.
- [Google Scholar]
- Growth Hormone Research Society. Consensus guidelines for the diagnosis and treatment of growth hormone (GH) deficiency in childhood and adolescence: Summary statement of the GH Research Society. GH Research Society. J Clin Endocrinol Metab. 2000;85:3990-3.
- [Google Scholar]
- Pituitary height on magnetic resonance imaging observation of age and sex related changes. J Pak Med Assoc. 2008;58:261-5.
- [Google Scholar]
- Height of normal pituitary gland on MRI: Differences between age groups and sexes. Okajimas Folia Anat Jpn. 1999;76:81-7.
- [Google Scholar]
- Pituitary gland: MR imaging of physiologic hypertrophy in adolescence. Radiology. 1990;174:681-5.
- [Google Scholar]
- MR height of the pituitary gland as a function of age and sex: Especially physiological hypertrophy in adolescence and in climacterium. AJNR Am J Neuroradiol. 1997;18:551-4.
- [Google Scholar]
- Morphological changes on MR imaging of the normal pituitary gland related to age and sex: Main emphasis on pubescent females. J Clin Neurosci. 2002;9:53-6.
- [Google Scholar]
- Influence of age and sex on signal intensities of the posterior lobe of the pituitary gland on T1-weighted images from 3 T MRI. Jpn J Radiol. 2013;31:186-91.
- [Google Scholar]
- MR assessment of pituitary gland morphology in healthy volunteers: Age- and gender-related differences. AJNR Am J Neurodiol. 1992;13:1295-9.
- [Google Scholar]
- Height of normal pituitary gland on MR imaging: Age and sex differentiation. J Comput Assist Tomogr. 1990;14:36-9.
- [Google Scholar]
- Height of normal pituitary gland as a function of age evaluated by magnetic resonance imaging in children. Pediatr Radiol. 1991;21:247-9.
- [Google Scholar]
- MR imaging diagnosis of central precocious puberty: Importance of changes in the shape and size of the pituitary gland. AJR Am J Roentgenol. 1994;162:1167-73.
- [Google Scholar]
- Development and aging of brain midline structures: Assessment with MR imaging. Radiology. 1989;172:171-7.
- [Google Scholar]
- In vivo assessment of pituitary gland volume with magnetic resonance imaging: The effect of age. J Clin Endocrinol Metab. 1990;71:505-8.
- [Google Scholar]






