Translate this page into:
Magnetic Resonance Imaging Assessment of Pre- and Post-Surgery Myocardial Changes in Hypertrophic Cardiomyopathy: Correlation with Echocardiography

*Corresponding author: Venkatraman Bhat, 309, Greenwoods Apt. Bommasandra, Bangalore, India. bvenkatraman@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Gadabanahalli K, Bhat V, Kanagasabai K, Rao PV. Magnetic Resonance Imaging Assessment of Pre- and Post-Surgery Myocardial Changes in Hypertrophic Cardiomyopathy: Correlation with Echocardiography. J Clin Imaging Sci 2020;10:4.
Abstract
Hypertrophic cardiomyopathy (HCM) is a common form of cardiomyopathy and a leading cause of sudden death in the young. Magnetic resonance imaging (MRI) is an established pre-operative tool for the evaluating of patients suspected with HCM for morphological assessment and identifying patients at risk of sudden death. Echocardiography and MRI are equally used in the post-treatment assessment of cardiac function and morphology. In this report, we present the comparative role of these two modalities in pre- and post-operative imaging assessment in our patients, treated surgically with the left ventricular myomectomy. Relative merits of MRI and echocardiography are presented and discussed.
Keywords
Hypertrophic cardiomyopathy
Magnetic Resonance Imaging
Echocardiography
Hypertrophic cardiomyopathy
Post-myomectomy
Late gadolinium enhancement
T1 mapping
INTRODUCTION
Hypertrophic cardiomyopathy (HCM) is a genetic disorder involving the cardiac sarcomere characterized by the left ventricular (LV) hypertrophy without obvious etiology.[1] It is the most common inheritable cardiac disorder with autosomal dominant inheritance (50–60%).[2] Morphologically, it is classified as asymmetrical, symmetrical, apical, mass-like (tumefactive), and end-stage form (burned-out phase).[2] The most common variety is asymmetrical type, which frequently involves the anteroseptal myocardium. Echocardiography and magnetic resonance imaging (MRI) provide clinically useful parameters for the evaluation of HCM. Some observations are common in both modalities, others specific to the modality. Commonly assessed parameters are ejection fraction (EF), interventricular septal (IVS) thickness, wall motion, and assessment of chamber lumen. Echocardiography provides quick, reliable assessment of gradients at different parts of the left ventricle. MRI, although capable of assessing gradients, has technical limitations to obtain consistently accurate gradient measurements. Echocardiography is a clinically established technique for the assessment of HCM. However, the technique is limited by operator dependency, poor acoustic window, incomplete visualization of the left ventricular wall, and inaccurate evaluation of the LV mass. MRI is useful in quantitative evaluation of wall thickness and documenting the extent and distribution of disease better than echo, especially in the anterolateral wall of the LV myocardium. MRI also has the ability to accurately evaluate the LV mass, chamber volume, global/regional wall motion abnormalities, and detection of aneurysms. Myocardial fibrosis, detected as foci of delayed myocardial enhancement (DME), also described as late gadolinium enhancement (LGE), is the information unique to the modality.[2] MRI evaluation also has a very effective role in the post-interventional evaluation. Post-interventional changes following alcohol injection are widely reported in patients with HCM.[3].On the other hand, there are few reports of post- myomectomy changes evaluated by MRI.[2]
CASE REPORT
Clinical, MRI, and echo observations of three patients of HCM treated with LV myomectomy are presented. Pre- and post-operative MRI imaging parameters and echo results are presented in Table 1.
| MRI | Echocardiography | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| LV | EDV (mL) | ESV (mL) | SV (mL) | EF (%) | IVS (mm) | Mass | EF | IVS (mm) | Gra/LVOT | |
| Patient 1 | Pre-operative | 96 | 27 | 69 | 72 | 22 | 154 | 55 | 20 | 90 |
| Post-operative | 81 | 27 | 55 | 67 | 18 | 120 | 55 | 15 | 1520 | |
| Patient 2 | Pre-operative | 101 | 27 | 74 | 73 | 24 | 108 | 55 | 22 | 83 |
| Post-operative | 84 | 42 | 42 | 50 | 17 | 75 | 58 | 10 | 7 | |
| Patient 3 | Pre-operative | 55 | 18 | 37 | 67 | 21 | 144 | 5055 | 19 | 6 |
| Post-operative | 78 | 33 | 45 | 58 | 15.6 | 121 | 50 | 18 | 7 | |
MRI: Magnetic resonance imaging, LV: Left ventricle, EDV: End-diastolic volume, ESV: End-systolic volume, EF: Ejection fraction, SV: Stroke volume, IVS: Interventricular septum, Gr-LVOT: Gradient left ventricular outflow tract, FU Gr-LVOT: Follow-up gradient left ventricular outflow tract
Case 1
A 55-year-old male patient with clinical suspicion of HCM was investigated with echo and MRI. Asymmetrical LV hypertrophy involving the basal (22 mm) and mid-septum was demonstrated [Figure 1]. There was associated systolic anterior motion (SAM) of the mitral valve (MV) with severe LV outflow tract (LVOT) narrowing and flow acceleration. Mild obliteration of the apical LV cavity was seen. There were moderate mitral regurgitation (MR) and mild tricuspid regurgitation (TR). On contrast MRI, mild patchy DME was noted in the basal septum and right ventricular insertion points. He had low-normal indexed LVEDV and LVESV with hyperdynamic LV systolic function. Post-operative echo and MRI at 6 months, after myomectomy of the basal septum, showed minimal residual concentric LV hypertrophy with reduced LVOT narrowing. No regional wall motion abnormality was noted. There were moderate MR and mild TR. On DME, there was a thin rim of mild subendocardial enhancement from basal septum to apex [Figure 1].
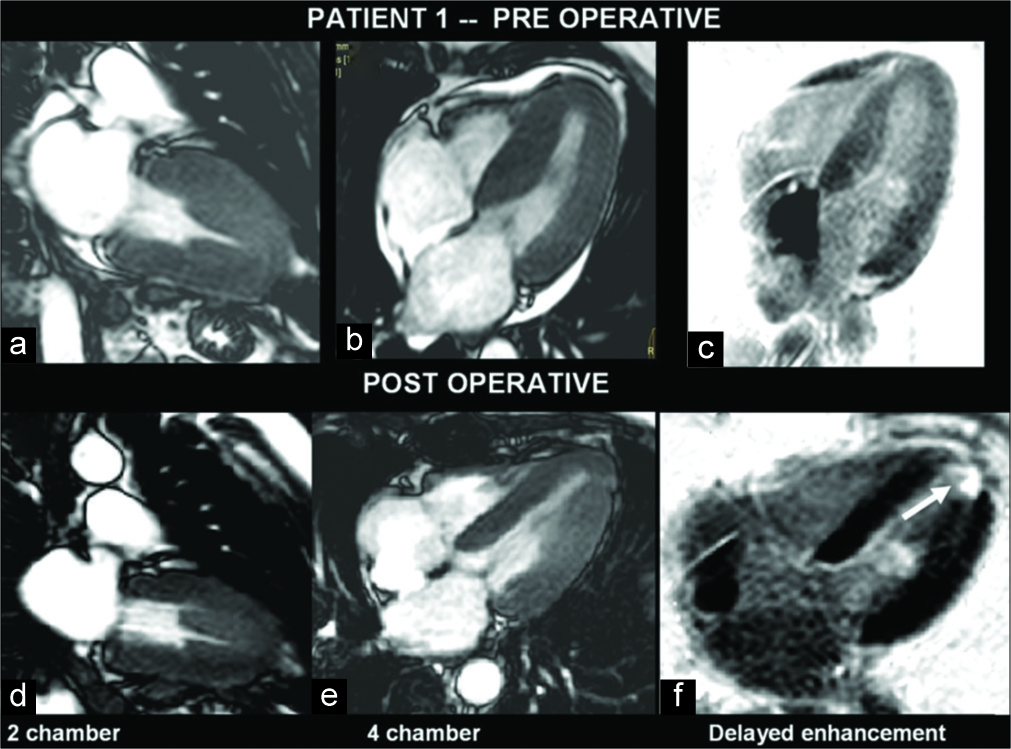
- A 55-year-old man with hypertrophic cardiomyopathy presented with exertional dyspnea. Pre- and post-operative magnetic resonance imaging images show hypertrophied myocardium in the left ventricle (LV) views (a-e). Septal hypertrophy is evident. Gadolinium-enhanced image shows enhancing myocardial scar which is noted at the LV apex (f) (open arrow).
Case 2
A 27-year-old female suspected of HCM was investigated. Asymmetrical hypertrophy of basal, mid-septum, and anterior segments was seen with hypokinesis of the segments and SAM of mitral leaflet with LVOT obstruction. Mild patchy DME was noted. The patient following transaortic extended myomectomy showed impaired LV systolic function with asymmetrical LV hypertrophy (LVH) [Figure 2]. There was persistent hypokinesis of the apex and apical anterior segment. On DME, there was near transmural enhancement [Figure 2].
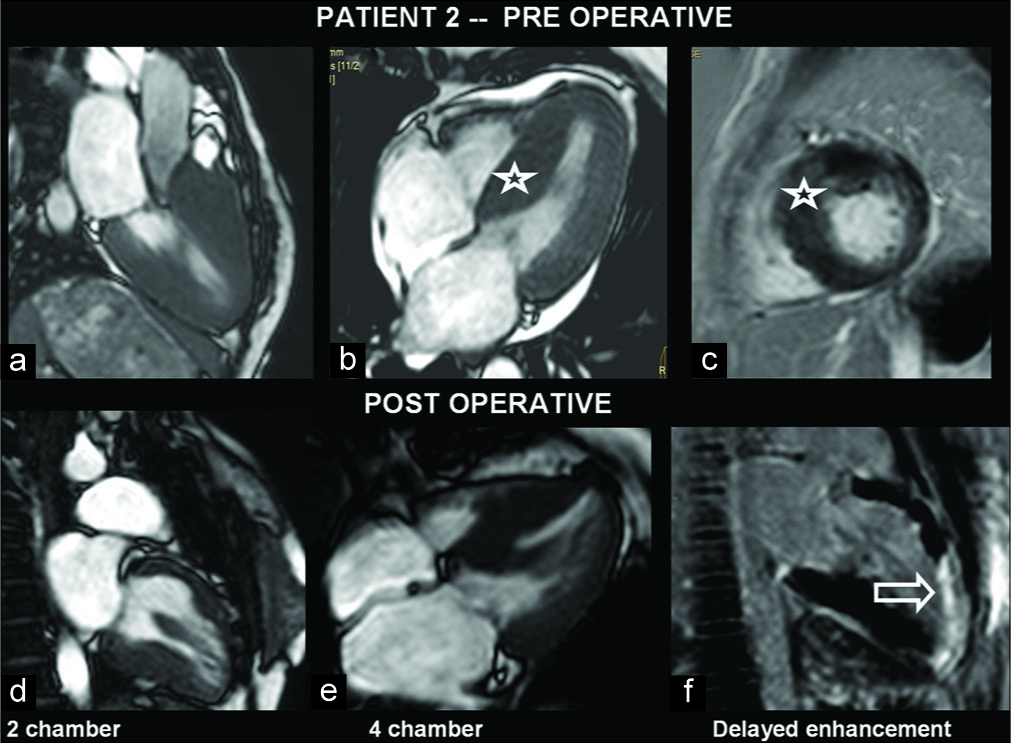
- A 27-year-old female with hypertrophic cardiomyopathy presented with dyspnea. Pre- and post-operative images show hypertrophied myocardium, especially in the septum in the left ventricle (LV) views (a-e). Gadolinium-enhanced image shows enhancing myocardial scar which is noted at the LV anterior wall and apex (f) (open arrow).
Case 3
A 57-year-old male was a known case of triple-vessel disease with worsening of angina. Echocardiography showed LVOT turbulence, concentric LVH, and hyperechoic septum. MRI confirmed LV asymmetrical septal hypertrophy at mid-cavity, anteroseptal segment, and obliteration of the LV apical cavity during systole [Figure 3]. There was LVOT narrowing with SAM. Hypokinesis of the basal and mid-septum was noted.
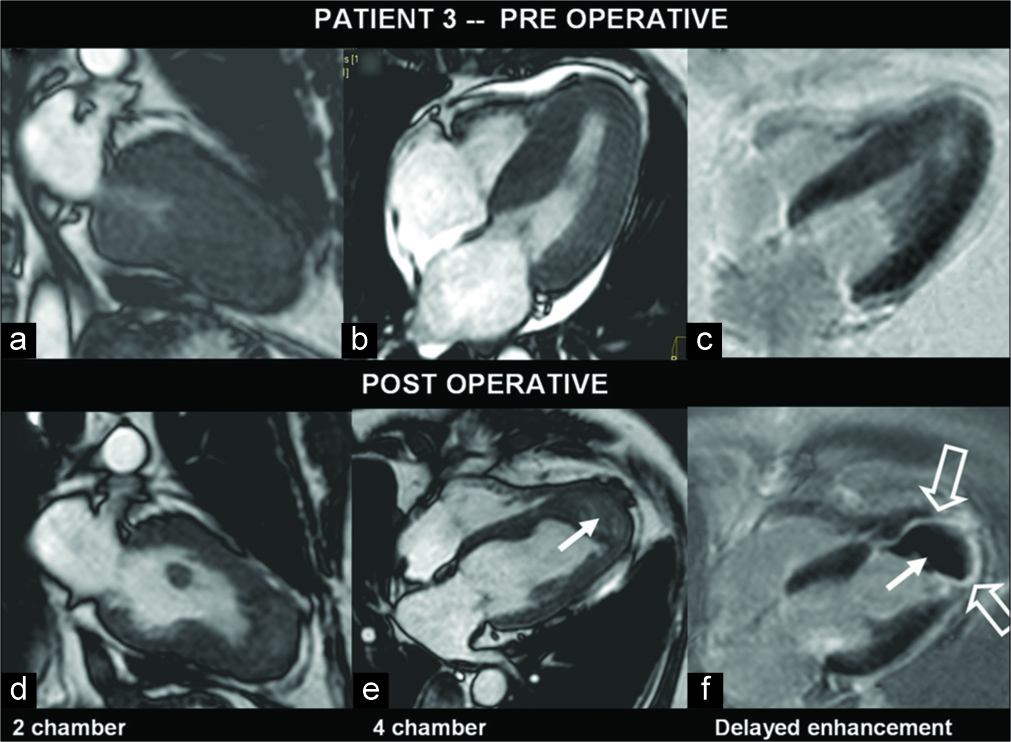
- A 57-year-old man with hypertrophic cardiomyopathy, known to have a triple-vessel disease, presented with increasing angina. Pre- and post-operative images of myocardium in various left ventricle (LV) views (a-e) show predominant septal hypertrophy. In the post-operative gadolinium-enhanced image, there is enhancing myocardial scar (open arrows) and clot at LV apex (f) (arrows).
There were mild MR and TR. There were patchy areas of subendocardial enhancement at LV apex. FU after transaortic septal myomectomy revealed no significant gradient across LVOT or wall motion abnormality. There was asymmetrical LVH with severe hypokinesia of the apical, basal, and mid- segments of the septum. There was a lamellated, adherent LV thrombus at apex [Figure 3]. On gadolinium-enhanced images, there was near transmural enhancement of the LV apex with subendocardial septal enhancement.
DISCUSSION
Established MRI abnormalities in patients with HCM are asymmetrical thickening of septum and narrowing of LVOT (20–30%).[4] The extent of narrowing varies at rest, may be latent or labile. A gradient of 30 mmHg or more is now generally considered significant for prognostic importance.[2] LVOT narrowing and outflow gradients are best assessed with echocardiography. On MRI contrast-enhanced examination, patchy DME of mid-septum occurs in up to 80% of patients.[5] Abnormalities of the MV movement, such as SAM, occur at the portion of the anterior mitral leaflet distal to the coaptation point as the valve being displaced into the LVOT by Venturi (drag) forces.[2] Other findings are diastolic dysfunction and LA enlargement. Apical form of HCM is a rare presentation, wherein absolute apical wall thickness of >15 mm is noted or a ratio of apical LV and basal LV wall thicknesses of ≥1.3–1.5 is seen.[3] HCM with mid-ventricular obstruction presents as mass-like thickening of the LV. “Burned-out phase” is noted in up to 10% of patients, often presents with LV dilatation, loss of myocardial thickness, and replacement fibrosis. Detailed evaluation of myocardial interstitial changes and altered flow kinetics is well shown with presently available MR techniques.[6] The risk of sudden death in HCM patients increases with the following MR criteria:
LV maximal wall thickness of 30 mm or more.
LVOT gradient of 30 mmHg or more at rest or 50 mmHg or more with provocation.
LV dilatation with depressed EF.
The presence of fibrosis, perfusion defect, and reduced functional reserve flow.[7,8] Septal myomectomy is considered the gold standard among septal reduction techniques currently in use.[4,9] Techniques for septal myomectomy are LV and transaortic routes. The transaortic approach is indicated in patients with LVOT obstruction.
In our first patient, both echo and MRI showed interval resolution of LVOT narrowing and flow acceleration. No regional wall motion abnormality was detected in both modalities. MRI showed better EF (67% – MRI and 55% – echo) accurate measurement of residual septal thickness (18 mm – MR and 15 mm – echo). The DME on MRI showed the extent of post-operative scar tissue. In the second patient, MRI detected residual septal thickening in mid- septum which was not detected by echo. Both examinations showed interval resolution of LVOT narrowing and flow acceleration. EF was comparable. MRI additionally showed hypokinesis of the basal and mid anteroseptal segments and the apex with near transmural DME. In the third patient, there was LV thrombus at the apex, post-operative scar with akinesia of the apical segment, and hypokinesia of basal and mid-septum only shown on MRI. Assessment of EF, interval resolution of LVOT narrowing, and residual septal thickness was comparable in both modalities.
In all patients, MRI provided additional, more accurate information compared to echo with better understanding of post-operative state and some valuable information for immediate patient care. MRI was superior in measurement of residual thickness and detection of regional wall motion abnormalities. T1 mapping techniques appear to have a potential role in identifying and quantifying diffuse myocardial disease.[10] LGE is sensitive in detecting focal myocardial fibrosis, which is an end-stage feature of the disease. However, LGE is less sensitive to diffuse inflammation and myocardial remodeling, hence underestimates the overall disease burden. Patients with HCM show high T1 indices in overt disease as well in subclinical disease state. Thus, T1 mapping may find an important role which is identifying subclinical disease in genetically susceptible population and monitoring the course of the disease. Evolving MR techniques and ease of affordability certainly will drive this advantage further by shorter examination time and more accurate assessment of structural and functional parameters. Clinical impact of observed “MRI advantage” on the overall patient management needs to be evaluated further in larger data sets. Echocardiography certainly has the great advantage in ease of use and clinician comfort. Availability, cost factor of MRI examination, and gadolinium contrast-related issues are important factors which will play a significant role in selection of the examination.
CONCLUSION
Cardiac MRI is an extremely valuable non-invasive tool to assess pre- and post-operative state of HCM. At present, echo assessment remains a comprehensive, practical tool for post-operative assessment. However, our study demonstrates superiority of MRI inputs in the assessment of patients following septal myomectomy. Clinically, relevant observations not evident on echocardiography were noted in our patients. Emerging T1 mapping techniques appear to have a role in assessing focal and diffuse myocardial changes in overt as well as subclinical disease. We see a definite role for a post-operative MRI in certain patients such as the one with incomplete clinical relief, in those with suboptimal echocardiography information, and in patients with post-operative complications. Considering availability, cost factor, and contrast-related issues with MRI, we defer recommending routine MRI post-myomectomy in all patients. In view of greater availability and rapid developments in MRI technology, it is worth exploring the role of MRI in these groups of patients for more accurate results.
Acknowledgments
The authors would like to thank Dr. Devi Prasad Shetty, Chairman of the Institution, for supporting the work and providing valuable guidance.
Declaration of patient consent
The patient’s consent not required as the patient’s identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- A comprehensive evaluation of myocardial fibrosis in hypertrophic cardiomyopathy with cardiac magnetic resonance imaging: Linking genotype with fibrotic phenotype. Eur Heart J Cardiovasc Imaging. 2014;15:1108-16.
- [CrossRef] [PubMed] [Google Scholar]
- MRI of hypertrophic cardiomyopathy: Part I, MRI appearances. AJR Am J Roentgenol. 2007;189:1335-43.
- [CrossRef] [PubMed] [Google Scholar]
- The value of magnetic resonance imaging of the left ventricular outflow tract in patients with hypertrophic obstructive cardiomyopathy after septal artery embolization. Circulation. 2000;101:1764-6.
- [CrossRef] [PubMed] [Google Scholar]
- Detection of apical hypertrophic cardiomyopathy by cardiovascular magnetic resonance in patients with non-diagnostic echocardiography. Heart. 2004;90:645-9.
- [CrossRef] [PubMed] [Google Scholar]
- 4D flow MRI and T1-Mapping: Assessment of altered cardiac hemodynamics and extracellular volume fraction in hypertrophic cardiomyopathy. J Magn Reson Imaging. 2016;43:107-14.
- [CrossRef] [PubMed] [Google Scholar]
- Noninvasive cardiac imaging in patients with hypertrophic cardiomyopathy. J Am Coll Cardiol. 2006;48:2410-22.
- [CrossRef] [PubMed] [Google Scholar]
- Toward clinical risk assessment in hypertrophic cardiomyopathy with gadolinium cardiovascular magnetic resonance. J Am Coll Cardiol. 2003;41:1561-7.
- [CrossRef] [Google Scholar]
- Long-term clinical and echocardiographic follow-up after surgical correction of hypertrophic obstructive cardiomyopathy with extended myectomy and reconstruction of the subvalvular mitral apparatus. Circulation. 1995;92:II122-7.
- [CrossRef] [PubMed] [Google Scholar]
- T1 Mapping in Characterizing Myocardial Disease: A Comprehensive Review. Circ Res. 2016;119:277-99.
- [CrossRef] [PubMed] [Google Scholar]






