Translate this page into:
Imaging findings and interventional management of deep venous thrombosis

*Corresponding author: Hussam Hindi, Department of Radiology, Wayne State University/Detroit Medical Center, Detroit, Michigan, United States. hussamhindi1@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Hindi H, Dongmo G, Goodwin A, Jones S, Loveridge K. Imaging findings and interventional management of deep venous thrombosis. J Clin Imaging Sci 2022;12:26.
Abstract
Deep venous thrombosis (DVT) is a subtype of venous thromboembolism. Lower extremity DVT affects about 1-2% of hospitalized patients. If not managed properly, these thrombi can embolize, causing further complications. Thrombosis risk factors include vascular endothelial injury, venous stasis, and hypercoagulability states. This triad is also known as Virchow’s triad. Although clinical features of lower extremity DVT are nonspecific and many patients are asymptomatic, physicians should maintain a high index of suspicion in patients presenting with leg swelling, pain, warmth, and erythema. Several diagnostic approaches for suspected first DVT have been proposed, and management depends on multiple factors such as location, duration of symptoms, cause of the thrombosis, and recurrence rate.
Keywords
Angioplasty
DVT
Thrombectomy
Thrombolysis
Stent
INTRODUCTION
Deep venous thrombosis (DVT) occurs when a blood clot forms in a deep vein. It can be categorized as provoked and unprovoked. Provoked DVTs are caused by known inciting events, whereas unprovoked DVTs imply that no provoking environmental event is identifiable. Common risk factors include the history of immobilization or prolonged hospitalization, recent surgery or trauma, obesity, previous venous thromboembolism (VTE), malignancy, use of oral contraceptives, pregnancy or postpartum status, age over 65 years, family history of DVT, heart failure, inflammatory bowel disease, and COVID-19 infection.
Although many patients are asymptomatic, DVT usually presents with unilateral extremity swelling, warmth, pain, and erythema. Delay in management may lead to pulmonary embolization or stroke. Another common complication is posted thrombotic syndrome (PTS), which manifests as chronic limb fatigue/heaviness, swelling, pain, and paresthesia, occurring in 50% of patients, accompanied by severe quality of life impairment. Symptoms are aggravated by walking and standing, leading to limited activity and inability to work. Additionally, many patients experience non-healing venous ulcers. The most important factors leading to PTS include the development of valvular reflux and late venous obstruction when the clot is not entirely cleared.
Imaging
Initial clinical suspicion for DVT typically begins with the clinical pretest probability (PTP) and labs, including the D-dimer assay. Several diagnostic approaches for DVT have been identified for confirmation, including ultrasound (US) and alternative imaging modalities such as computed tomography (CT), magnetic resonance imaging (MRI), and venography.
Venous duplex ultrasound (VDUS) uses two main components to assess for DVT, specifically, the brightness mode (B-mode) imaging—using probe compression and Doppler evaluation in addition to color flow Doppler imaging and spectral Doppler waveform analysis.[1]
Lower-extremity duplex ultrasound (LEDUS) can detect characteristics of thrombus chronicity, allowing differentiation of chronic disease from acute DVT. Classification of DVT done using LEDUS utilizes clot echogenicity; adherence to vessel wall; venous distension; compressibility; and recanalization, calcifications, or collaterals. Based on the presence or absence of these findings, thrombus chronicity can be defined as “definitely acute, definitely chronic, or DVT of indeterminate age.”[1]
Characteristics that define “definitely acute” include two or more of the following criteria observed on LEDUS; a hypoechoic thrombus, thrombus loosely attached to a vessel wall or is mobile, a distended vein lumen, and a compressible thrombus [Figure 1]. Abstractly, characteristics of the classification “definitely chronic” must have a combination of two or more of the following features observed on LEDUS; hyperechoic thrombus, a thrombus firmly adherent to the vessel wall, venous wall thickening and scaring with or without calcium deposition, standard size or smaller than average-sized vein lumen, recanalization of the thrombus, or a thrombus that is noncompressible or partially compressible [Figures 2 and 3].
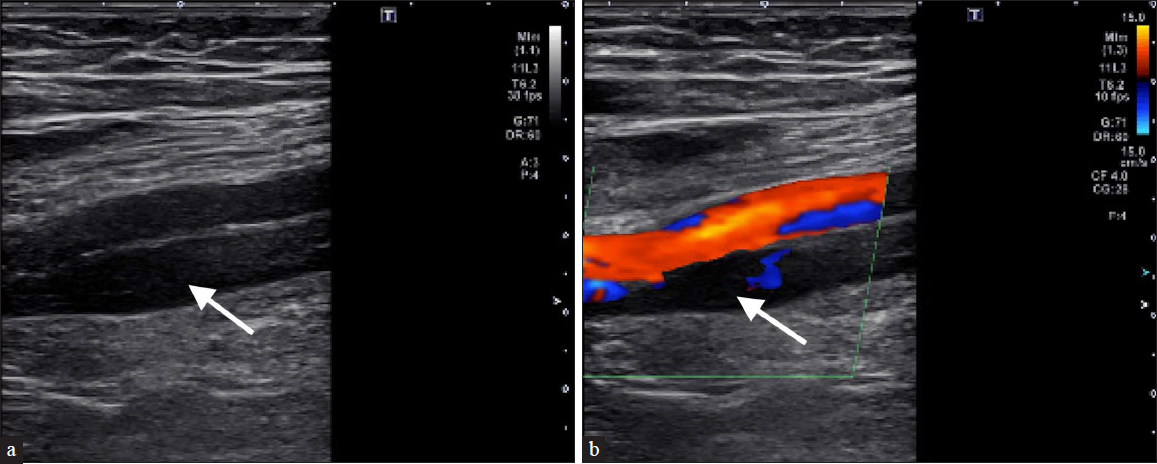
- A 75-year-old male with a history of pulmonary embolism (PE) presents with lower GI bleed. Anticoagulation was subsequently discontinued, and the patient began experiencing right lower extremity pain. (a) Ultrasound (US) venous duplex demonstrates non-echogenic thrombus within the superficial femoral vein (arrow). (b) Demonstrates partial compressibility, and lack of flow on doppler imaging (arrow) consistent with acute DVT. The patient underwent temporary IVC filter placement.
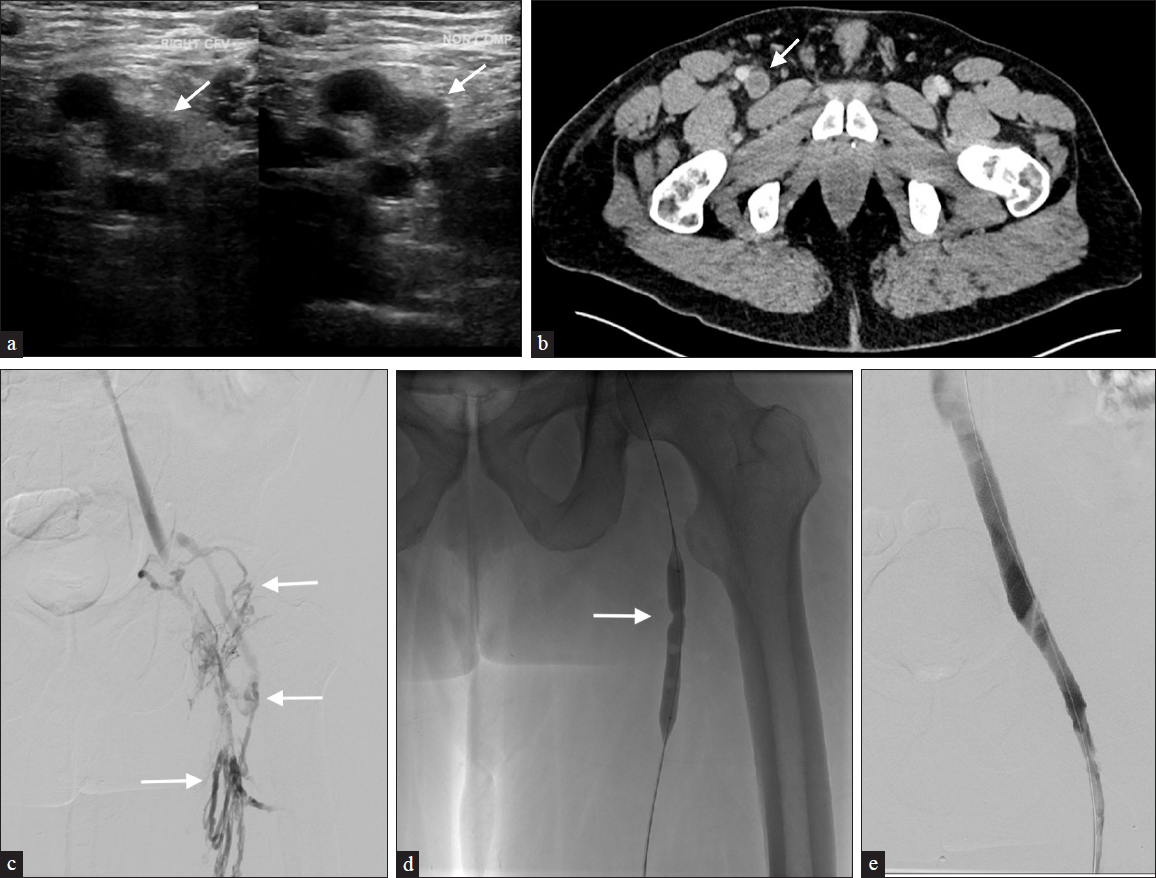
- A 51-year-old male with a history of uncontrolled hypertension presents with right lower extremity swelling and pain. (a) US images show noncompressible thrombus in the right common femoral vein (CFV) (arrow) extending into the superficial femoral vein. (b) Similarly, delayed axial computed tomography (CT) demonstrates a filling defect within the CFV (arrow). (c) Venography reveals chronic thrombus with collateral formation (arrows) extending from the external iliac to the superficial femoral vein. The patient underwent multiple passes with the Inari ClotTriever device. (d) Subsequent balloon angioplasty (16 mm × 40 mm) for residual stenosis (arrow) necessitated a 14 mm × 60 mm CFV and 16 mm × 90 mm external iliac vein Wallstent to maintain patency. (e) Post venography images show improved outflow with non-opacified collaterals.
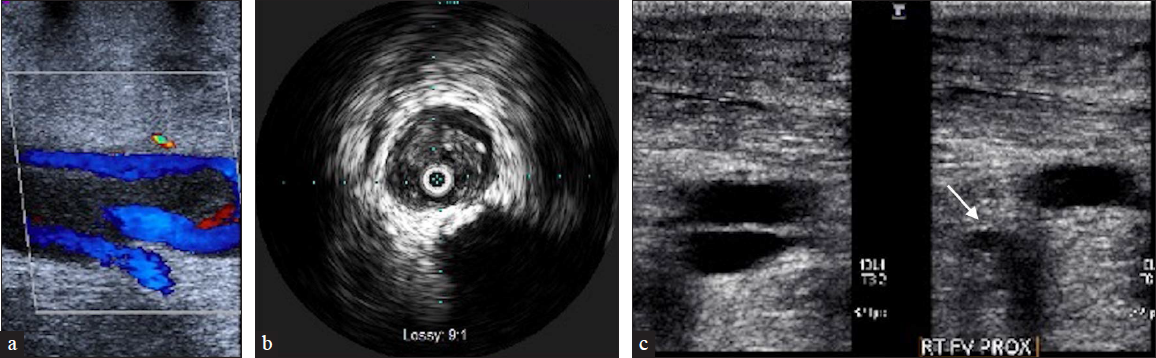
- A 66-year-old male with metastatic prostate carcinoma and a history of lower extremity DVT and right iliac vein stent placement presents with worsening right lower extremity swelling. (a) US demonstrates chronic thrombus with partial recannulation in the superficial femoral vein that extends from the common femoral to the posterior tibial and peroneal veins. (b) Intravenous ultrasound (IVUS) demonstrates echogenic adherent thrombus. (c) After Inari ClotTriever mechanical thrombectomy follow-up US shows compressibility (arrow) in the previously uncompressible femoral vein.
Computed tomography venography (CTV) permits routine evaluation of the US’s deep veins not routinely evaluated, including the iliac veins and inferior vena cava (IVC). The clot is identified as a filling defect within a deep vein [Figure 2]. Acute DVT often expands the vein and may demonstrate venous wall enhancement or perivenous edema.[2]
Other well-studied endovascular imaging modalities, often used in combination, include MRI with non-contrast MR venography (NC-MRV) and MR direct thrombus imaging (MR-DTI); an inversion-recovery water-selective fat gradient-echo acquisition [Figure 4]. Throughout the literature, MR-DTI has been proposed as an alternative that may help establish thrombus age and distinguish between an acute recurrent thrombus and a persisting (evolving) clot in the exact location. The technique is based on the paramagnetic properties of methemoglobin, formed in a fresh thrombus, which decreases the longitudinal relaxation time (T1), resulting in a high signal on T1-weighted images. The MR-DTI highlights this bright thrombus signal by nulling the signals from fat (using a water-selective excitation) and blood (due to its extended T1 property).[3]
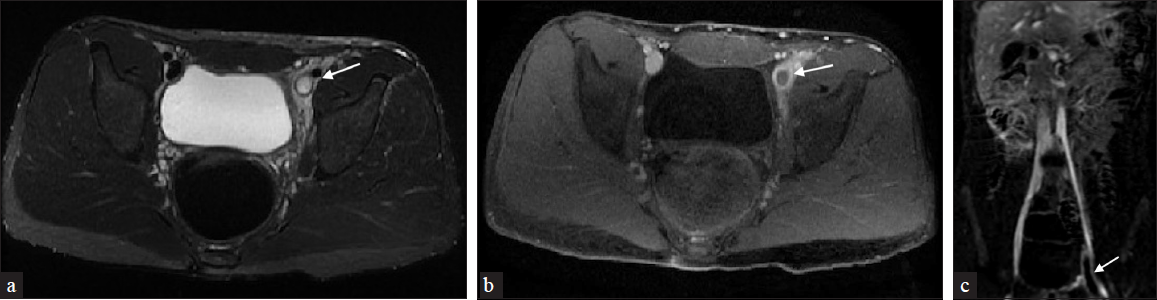
- A 16-year-old male with left groin and lower extremity swelling. (a) Axial T2-weighted fat-saturated magnetic resonance imaging (MRI) demonstrates a hyperintense signal (arrow) extending from the left external iliac to the superficial femoral vein. (b) Axial T1-weighted postcontrast MRI shows filling defect (arrow) in the left common femoral vein. (c) Perivascular edema extending into the superficial femoral vein with venous wall thickening/enhancement on MRI subtraction imaging (arrow).
Intravascular ultrasound (IVUS) imaging of veins can provide valuable information regarding defects within the lumen, degree of stenosis, and compressive structures adjacent to the veins. Thrombus from DVT can have a varying spectrum of echogenicity depending on its age. An acute thrombus may not be visible on IVUS due to its high concentration of red blood cells [Figure 6]. In contrast, chronic thrombus can be highly echogenic due to its fibrin composition [Figures 3 and 5]. The VIDIO trial demonstrated increased sensitivity of treatable venous iliofemoral stenosis compared to venography.[2]
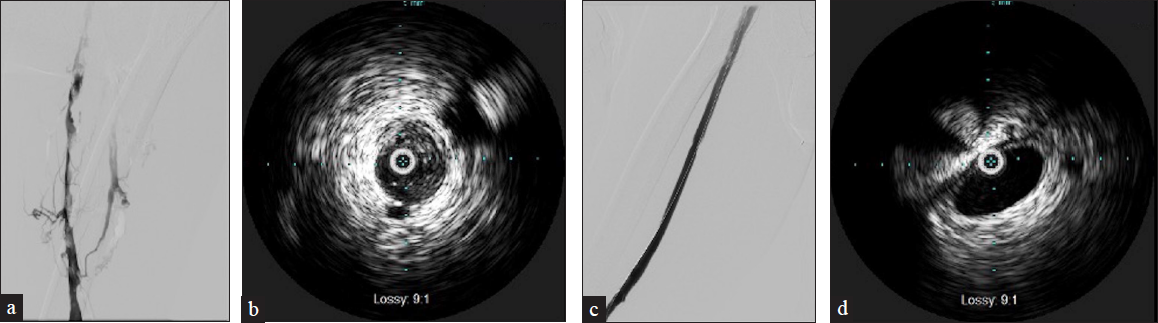
- A 59-year-old male with a history of anticoagulation use for recurrent DVT/PE presents with right lower extremity pain. CT (not shown) demonstrates spontaneous hematoma within the right iliopsoas muscle compressing the right common and external illiac veins. (a) Venography demonstrates multiple filling defects extending from the common illiac to the popliteal vein with collateral formation. (b) IVUS confirms diffuse echogenic thrombus. (c) After Inari ClotTriever mechanical thrombectomy, venography showed improved flow. (d) Repeat IVUS demonstrates a significant reduction of thrombus burden.
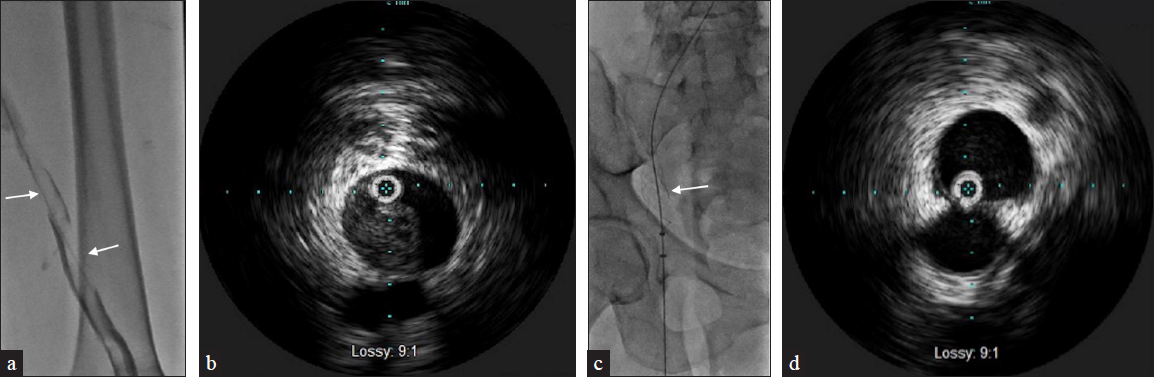
- A 69-year-old female with stage 4 uterine carcinosarcoma presents with left lower extremity swelling. US (not shown) demonstrates thrombus from the left common femoral to popliteal veins. (a) Venography demonstrates multiple filling defects (arrows) beginning within the common iliac vein and extending to the superficial femoral and popliteal veins. (b) IVUS identifies nonechogenic thrombus throughout including the common iliac vein. (c) Patient underwent Inari ClotTriever thrombectomy (arrow) of the lower extremity. (d) Repeat IVUS shows almost complete resolution of acute thrombus.
Phlegmasia cerulea dolens
Phlegmasia cerulea dolens is a severe form of DVT characterized by complete lower extremity venous occlusion of deep and superficial channels. Associated edema causes a rise in interstitial tissue pressure overcoming arterial closing pressure, causing collapse.[4] Symptoms include pain, motor/sensory deficits, and venous gangrene with significant morbidity and mortality. Endovascular treatments include catheter-directed thrombolysis and pharmacomechanical/mechanical thrombectomy.
Paget-Schroetter syndrome
Paget-Schroetter syndrome is effort thrombosis of the axillosubclavian vein in the setting of repetitive forced abduction of the upper limb [Figure 7]. The subclavian vein traverses the costoclavicular space anterior to the anterior scalene muscle passing adjacent to the junction of the clavicle and first rib. The patient presents with upper extremity pain and swelling, worsened with abduction. The thrombus is treated with anticoagulation and catheter-directed thrombolysis, after which surgical decompression is performed. Residual stenosis can be treated with stent placement only after surgical decompression due to high rates of crushed stent and re-thrombosis. A series following 23 patients demonstrated 100% patency of nine patients that underwent venoplasty alone vs 64% with stent placement.[5]
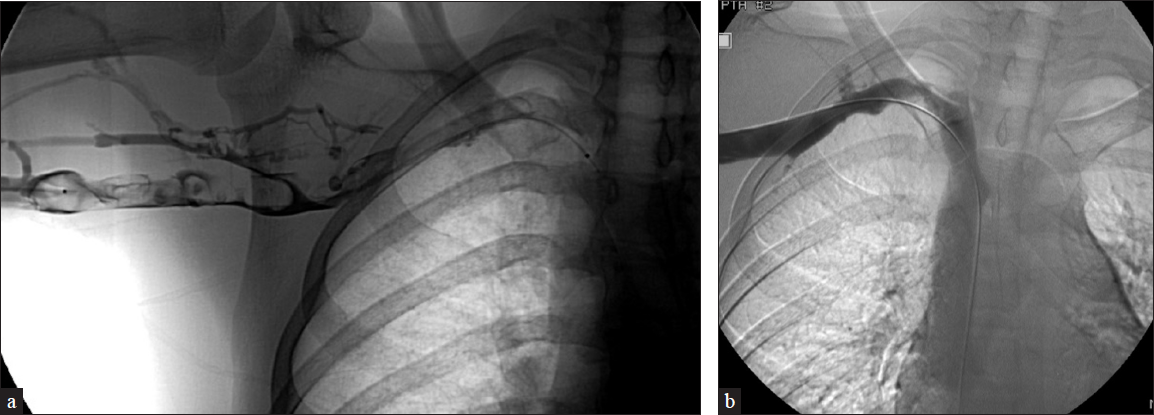
- A 16-year-old male soccer player presents with right upper extremity pain and swelling. US demonstrates right axillary vein DVT. (a) The patient subsequently underwent venogram demonstrating extensive right axillosubclavian thrombosis and collateral formation. The patient underwent staged overnight thrombolysis, subsequent right 1st rib resection, and angioplasty. (b) Repeat imaging shows improved patency with a significant reduction in clot burden.
Management
In most patients, anticoagulation should be started immediately upon diagnosis due to increased embolization risks if treatment is delayed. In the absence of contraindication to anticoagulation, therapy should be started immediately. The authors explore interventional management options for this essay, including catheter-directed lysis, mechanical thrombectomy, pharmaco-mechanical directed lysis, IVC filter placement, and venous stenting.
Catheter-directed thrombolysis
Catheter-directed thrombolysis (CDT) is an image-guided procedure that involves the infusion of a fibrinolytic drug using a multi-side-hole catheter into the venous thrombus, with (EkoSonic Endovascular System) (Boston Scientific Marlborough, MA, USA) or without ultrasound augmentation [Figure 8]. Following this, the underlying vein’s exploration of stenosis is performed by venography or IVUS and treated with angioplasty or stenting, if applicable. Multiple studies found that CDT decreased PTS symptoms compared to anticoagulant therapy alone. Although it is practical, this mode of venous thrombosis treatment can result in complications such as significant bleeds, intracranial bleeds, and the need for monitoring in the ICU for 1-3 days.[6]
- A 71-year-old female with metastatic gallbladder adenocarcinoma incidentally found to have right lower extremity thrombus on CT (not shown). (a) Venography demonstrates multiple filling defects (arrows) within the right common femoral and superficial femoral veins. (b) An Argon Cleaner rotational thrombectomy device (arrow) was used, however, residual thrombus remained. (c) An EKOS (EkoSonic) catheter (arrow) was placed and TPA 1 mg and heparin 500 units per hour were administered over 24 hours with ICU monitoring. (d) Repeat venogram in prone demonstrates significant resolution of thrombus.
Percutaneous mechanic thrombectomy
Percutaneous mechanical thrombectomy (PMT) utilizes several mechanisms to remove DVT. Popular devices such as the ClotTriever (Inari Medical Irvine, CA, USA), which uses a scoring element and collection bag, and CLEANER (Argon Medical Frisco, TX, USA), which utilizes a rotational thrombectomy system, are noted to eliminate the need of thrombolytic drugs that carry inherent risks such as bleeding and prolonged ICU stays [Figures 6 and 8].[2] Several large-bore mechanical aspiration devices such as the AngioVac (AngioDynamics Queensbury, NY, USA) and Indigo Aspiration System (Penumbra Alameda, CA, USA) are also utilized.
Pharmacomechanical catheter-directed thrombolysis
Pharmacomechanical catheter-directed thrombolysis (PCDT) combines CDT and PMT that results in thrombus dissolution [Figure 9]. The venous thrombus is primed with the fibrinolytic drugs, which increases the susceptibility to mechanical fragmentation and allows for more effective removal, preventing residual fragment embolizing to the lungs. Due to its effectiveness, this method reduces the thrombolytic drug dose, treatment time, and hospital costs significantly. The patient then undergoes radiolytic thrombectomy utilizing saline jets to create a localized low-pressure zone by the Bernoulli effect to macerate a clot.[7]
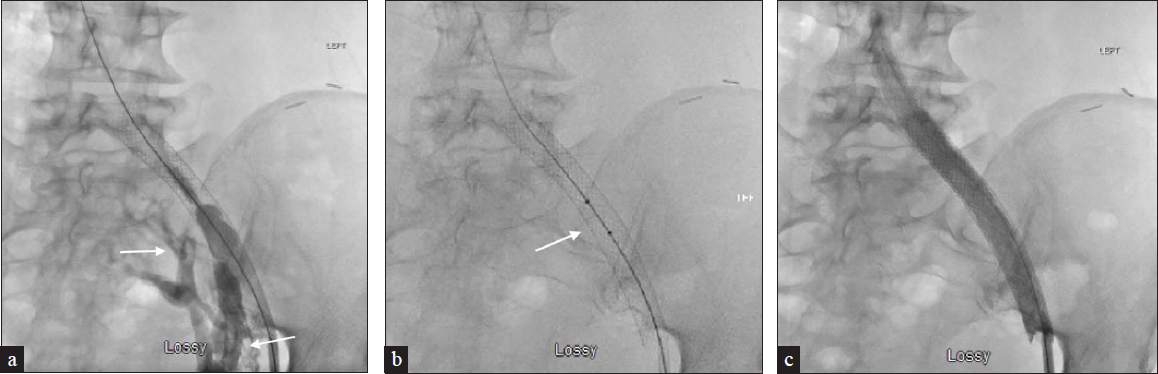
- A 63-year-old female with metastatic breast carcinoma and a history of May Thurner syndrome status post left common iliac stent placement presents with worsening left lower extremity swelling. (a) CT (not shown) demonstrates thrombus extending from the left common iliac to the superficial and profunda femoral veins, which was confirmed on venography with pelvic collaterals visualized (arrows). (b) The patient underwent AngioJet pharmacomechanical thrombectomy (arrow) of the left lower extremity and subsequent 16 mm balloon angioplasty of the stent with proximal extension into the IVC using a 16 mm × 60 mm Wallstent. (c) This shows significantly improved outflow through the stent and decreased pelvic collateralization.
In 2009, the ATTRACT trial assessed the efficacy of pharmacomechanical catheter-directed thrombolysis with anticoagulation compared to anticoagulation alone. They assessed safety, cost, and ability to prevent PTS between 6 and 24-months follow-ups. An analysis amongst iliofemoral DVT patients demonstrated the VINES-QOL (venous insufficiency epidemiological and economic study-quality of life/symptoms) score change was more significant in the PCDT versus non PCDT group. This was statistically significant at one and six months. Therefore, most of the improvement in quality of life was in the first six months, but not after that.[8] Though PCDT did not prevent post-thrombotic syndrome, it did reduce its severity.[6]
IVC filter
IVC filters are used in patients with contraindication to anticoagulation therapy or failure to avoid iatrogenic pulmonary embolism (PE) [Figure 10]. The PREPIC trial found a significant reduction in PE due to the IVC filter.[9] Another study uncovered a reduction in PE-related death at 30 days in patients with a contraindication to anticoagulant therapy.[10] One apprehension to IVC filter use is the lack of retrieval when concern for PE is no longer present. However, mechanical device-related complications are approximately 2% across various studies. In a study examining DVT patients with and without IVC placement, there was an eightfold reduction in symptomatic PE presentation, indicating the importance of this treatment option.[11]
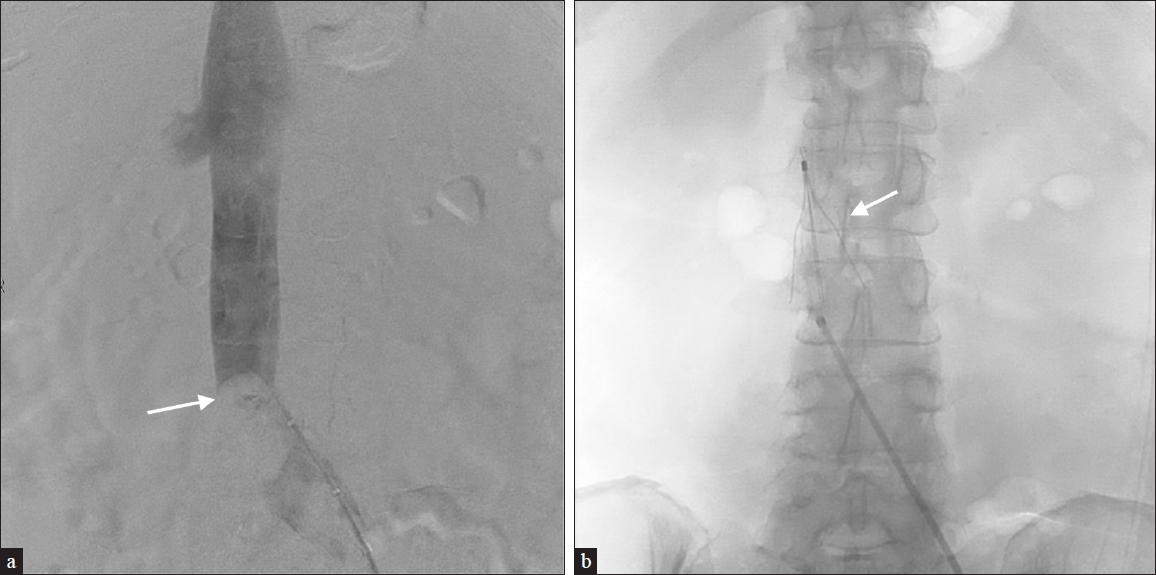
- A 62-year-old female with a history of DVT on anticoagulation presents with disseminated intravascular coagulation and bleeding secondary to sepsis. (a) Venography demonstrates almost complete occlusion of the infrarenal IVC. (b) A Cook Celect filter (arrow) was placed secondary to the patient’s inability to tolerate anticoagulants.
Venous angioplasty/stenting
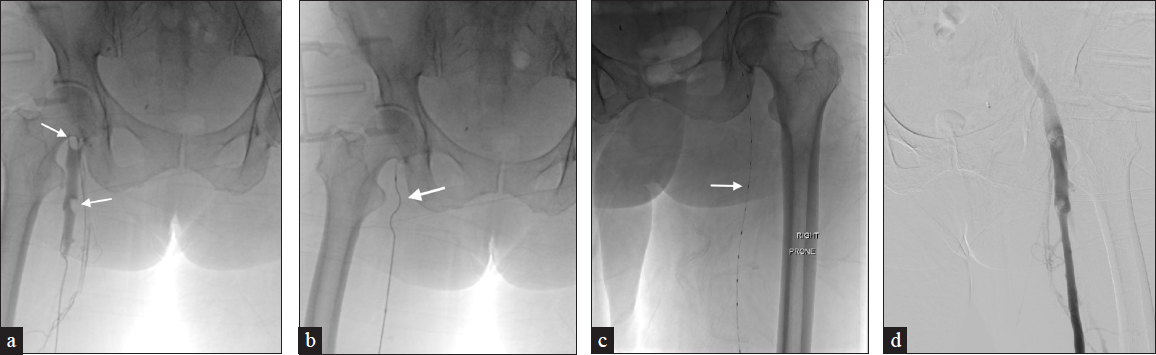
Thrombus maceration with venous angioplasty/stenting is another approach considered for both acute and chronic DVT [Figure 2]. This treatment is classically utilized in May-Thurner syndrome, which refers to chronic compression of the left common iliac vein against a lumbar vertebra secondary to the overlying, pulsating, right common iliac artery causing DVT [Figure 11]. Criteria for angioplasty in acute DVT include iliofemoral DVT and evidence of residual thrombosis or obstruction following mechanical thrombolysis. In contrast, measures in chronic DVT include established PTS with significant symptoms and evidence of iliofemoral stenosis or obstruction confirmed by imaging amenable to stent placement.[12] Research shows that venous stent placement is indicated when the block is >50%, there is apparent formation of superficial collaterals, and evidence of reflux in deep and superficial veins. In both acute and chronic DVT, stent placement requires careful consideration.
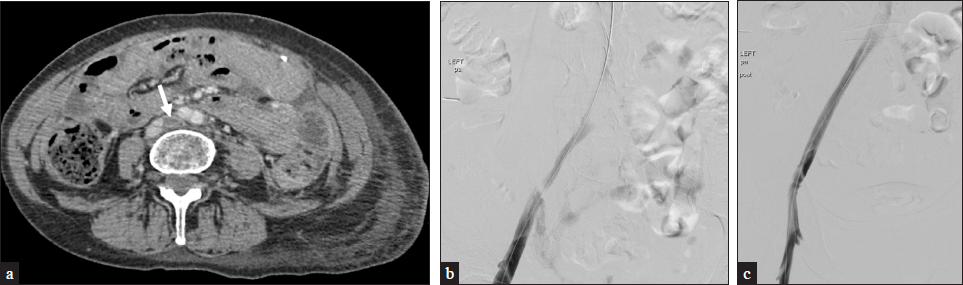
- A 62-year-old female presents with left lower extremity pain and swelling. (a) CT demonstrates a compressed left common iliac vein by the right common iliac artery with thrombus extending into the femoral veins, consistent with May-Thurner syndrome. (b) Venogram demonstrates similar findings. (c) The patient underwent ClotTriever mechanical thrombectomy and subsequent Wallstent placement with the resolution of symptoms.
CONCLUSION
DVT is a multifaceted condition that affects about 300,000 to 600,000 people in the US each year. It should be suspected in patients presenting with extremity swelling, pain, warmth, and erythema. With extensive research and advancement in technology, significant progress has been made using various imaging modalities, including ultrasound, CT, venography, and MR. Traditionally, management has been mostly done with anticoagulation. However, multiple large-scale clinical trials have demonstrated the efficacy, safety, and benefits of interventional radiology techniques in cases of severe DVT.
Declaration of patient consent
Patient consent is not required as the patient’s identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Duplex ultrasound for evaluation of deep venous blood flow in fractured lower extremities. Pol J Radiol. 2018;83:e47-53.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Interventional management of venous thromboembolism: State of the art. AJR Am J Roentgenol. 2017;208:891-906.
- [CrossRef] [PubMed] [Google Scholar]
- Combined MR direct thrombus imaging and non-contrast magnetic resonance venography reveal the evolution of deep vein thrombosis: A feasibility study. Eur Radiol. 2017;27:2326-32.
- [CrossRef] [PubMed] [Google Scholar]
- Trends in the management of phlegmasia cerulea dolens. Vasc Endovascular Surg. 2011;45:5-14.
- [CrossRef] [PubMed] [Google Scholar]
- Long-term results in patients treated with thrombolysis, thoracic inlet decompression, and subclavian vein stenting for Paget-Schroetter syndrome. J Vasc Surg. 2001;33:S100-S5.
- [CrossRef] [PubMed] [Google Scholar]
- Pharmacomechanical catheter-directed thrombolysis for deep-vein thrombosis. N Engl J Med. 2017;377:2240-52.
- [CrossRef] [PubMed] [Google Scholar]
- Deep venous thrombosis: The opportunity at hand. AJR Am J Roentgenol. 2009;193:922-7.
- [CrossRef] [PubMed] [Google Scholar]
- Quality of life after pharmacomechanical catheter-directed thrombolysis for proximal deep venous thrombosis. J Vasc Surg Venous Lymphat Disord. 2020;8:8-23.e18.
- [CrossRef] [PubMed] [Google Scholar]
- A clinical trial of vena caval filters in the prevention of pulmonary embolism in patients with proximal deep-vein thrombosis. Prévention du risque d’embolie pulmonaire par interruption cave study group. N Engl J Med. 1998;338:409-16.
- [CrossRef] [PubMed] [Google Scholar]
- Thirty-day outcomes in patients with acute pulmonary embolism who discontinued anticoagulant therapy before 90 days. Am Heart J. 2018;206:1-10.
- [CrossRef] [PubMed] [Google Scholar]
- Role of IVC filters in endovenous therapy for deep venous thrombosis: The FILTER-PEVI (filter implantation to lower thromboembolic risk in percutaneous endovenous intervention) trial. Cardiovasc Intervent Radiol. 2012;35:1408-13.
- [CrossRef] [PubMed] [Google Scholar]
- Role of venous stenting for venous thromboembolism. Hematology Am Soc Hematol Educ Program. 2020;1:606-11.
- [CrossRef] [PubMed] [Google Scholar]







