Translate this page into:
Imaging Features of AlloDerm® Used in Postmastectomy Breast Reconstructions
Address for correspondence: Dr. Christine Lee, Department of Radiology, Mayo Clinic, 200 Frist Street SW, Rochester MN 55905, USA. E-mail: lee.christine@mayo.edu
-
Received: ,
Accepted: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
The purpose of this pictorial essay is to demonstrate the imaging features (ultrasound, mammogram, and magnetic resonance imaging (MRI)) of AlloDerm®(LifeCell Corp.; Branchburg, NJ), an acellular dermal matrix sometimes used in both primary and reconstructive breast surgeries. AlloDerm® is derived from cadaveric dermis and provides an immunologically inert scaffold in tissue reconstruction. Since there is little literature on the imaging of this substance, radiologists may be unfamiliar with its appearance in breast imaging. For this manuscript, ex vivo and in vivo images of AlloDerm® in postmastectomy patients were evaluated using different imaging modalities. The appearance of AlloDerm® can vary based on length of time postsurgery and incorporation into the host. AlloDerm® appears as an isodense to glandular tissue on a mammogram and isoechoic to glandular tissue on ultrasound imaging. On MRI, in comparison with normal breast parenchyma, AlloDerm® is hyperintense on T2-weighted imaging and isointense on T1-weighted imaging and demonstrates mild enhancement. To the best of the authors’ knowledge, this is the first multimodality imaging description of AlloDerm® used in postmastectomy patients. The conformation of AlloDerm® at surgical placement and the degree of host cell migration and neoangiogenesis are factors to take into consideration when performing diagnostic evaluations; and, familiarity with the various imaging appearances of AlloDerm® can be helpful to exclude residual or recurrent disease.
Keywords
AlloDerm®
breast reconstruction
imaging
mastectomy
radiology
INTRODUCTION

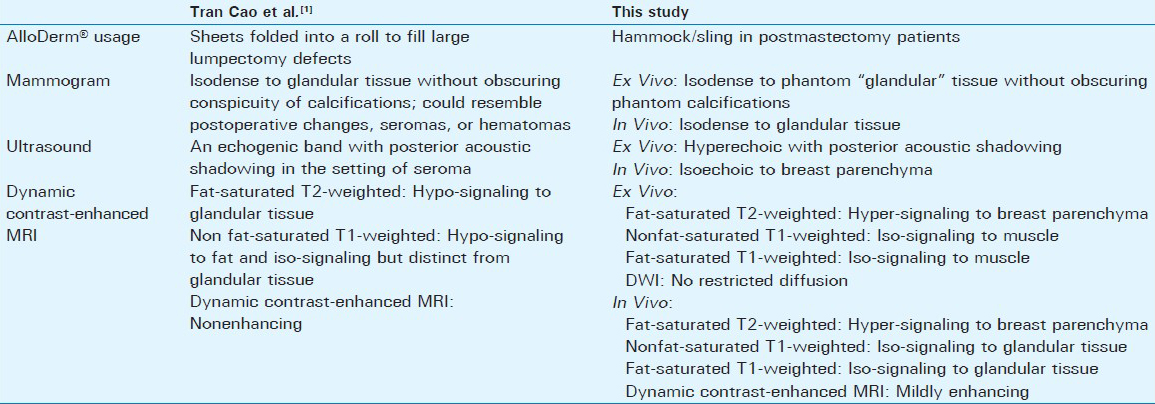
AlloDerm® (LifeCell Corp.; Branchburg, NJ) is an acellular dermal matrix originating from cadaveric dermis, which is processed to remove antigenic epitopes and cells, creating an immunologically inert scaffold.[12345] The matrix [Figure 1] contains collagen, elastin, hyaluronic acid, fibronectin, proteoglycans, growth factor receptors, and vascular channels, which allow for host cell migration and angiogenesis.[23] In current practice, large lumpectomy cavities have been filled with AlloDerm®.[1] In postmastectomy patients where the pectoralis major muscle provides inadequate coverage for the tissue expander/implant, an AlloDerm® sling described by Breuing and Warren[4] is created by sewing it to the inferior edge of the pectoralis major muscle and along the chest wall at the location of the inframammary fold to construct a subpectoral-sub-AlloDerm®pocket.[4] In the United States, over 75% of implant-based reconstruction is done using dermal matrices (all name brands); of that group over 75% is specifically AlloDerm®. AlloDerm® has also been used to improve cosmesis of the breast contour. Complications associated with AlloDerm® including infection, hematoma formation, and flap necrosis are similar to those seen with standard submuscular reconstruction.[6] A systematic review by Sbitany and Serletti,[6] found a higher rate of postoperative seromas in patients undergoing reconstruction with acellular dermal matrix compared with those without. Colwell et al.,[7] found no significant difference in the total overall costs between single-stage implant reconstruction with AlloDerm® and tissue-expander reconstruction without AlloDerm®.

- Photograph of a single sheet of prehydrated AlloDerm®. Once hydrated, the AlloDerm® can be folded, rolled, and layered. Dermal matrices other than AlloDerm® such as porcine derived matrices are similar in appearance.
Breast imaging after reconstruction with AlloDerm® is typically obtained for diagnostic evaluation when there is concern of a postoperative complication, or a clinical area of concern such as a palpable abnormality or pain. Radiologists unfamiliar with the imaging features of AlloDerm® may find it diagnostically challenging. Buck et al.,[8] reported a case of a patient with a new palpable, fixed mass in her breast after mastectomy. Surgical excision of the mass demonstrated a foreign body giant cell infiltrate secondary to the acellular dermal matrix, without evidence of recurrent tumor.[8]
A literature search yielded a single preliminary report describing the radiologic appearances of AlloDerm® in breast conservation therapy patients where sheets of AlloDerm® were folded or rolled to fill larger defects.[1] A brief description of the imaging appearance of the postmastectomy AlloDerm® sling was described by Dialani as isointense to glandular tissue on T1W imaging and lacking enhancement with gadolinium.[9] The objective of this manuscript is to provide imaging features of AlloDerm® in both primary and reconstructive breast surgeries, particularly in the postmastectomy patient.
Ex vivo imaging of Alloderm®
Ex vivo imaging of AlloDerm® (after hydrating in normal saline for 30 min as per usage instructions) was performed with mammography [Figure 2], ultrasound [Figure 3], and magnetic resonance imaging (MRI) [Figure 4] to illustrate the imaging features prior to host cell migration and angiogenesis.
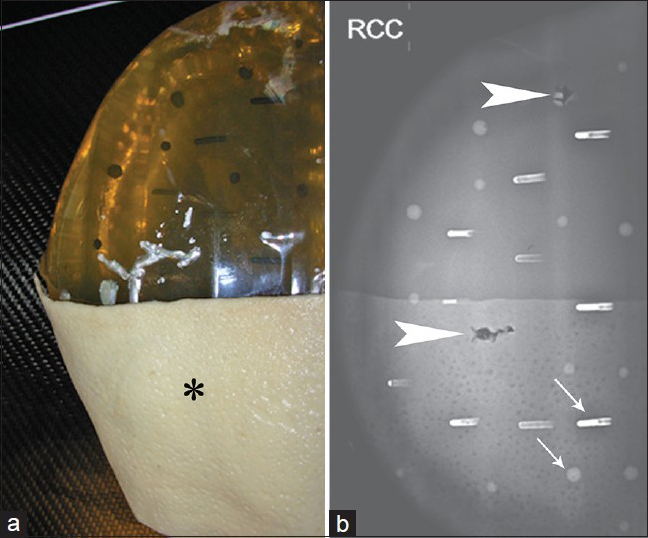
- Mammogram of hydrated ex vivo AlloDerm®. (a) A single sheet of AlloDerm® (asterisk) was placed on the medial aspect of a breast phantom, and (b) a right craniocaudal view obtained shows the AlloDerm® is isodense to “glandular” tissue; several air/AlloDerm® interfaces are visible. There are simulated calcifications (arrows) and two biopsy tracts (arrowheads) in the phantom.
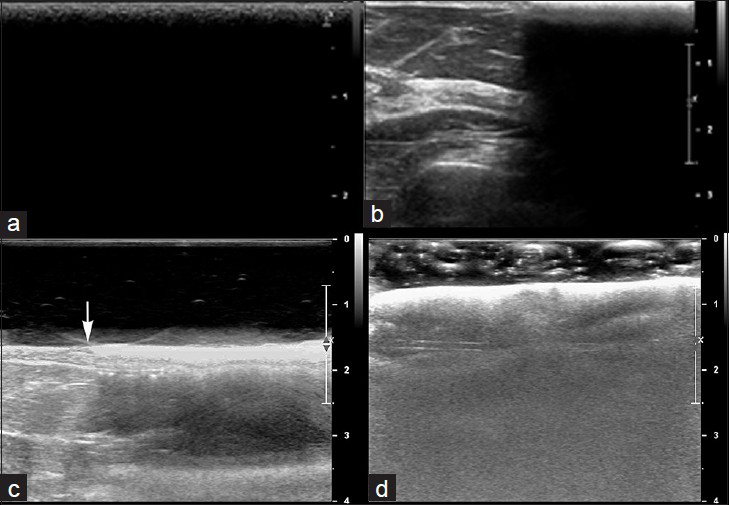
- Ultrasound of hydrated ex vivo AlloDerm®. (a) Transverse view shows a single sheet of AlloDerm® overlying the skin in the field of view entirely. (b) Transverse view of partially visualized AlloDerm® adjacent to normal breast parenchyma, shows the AlloDerm® is hyperechoic to normal breast parenchyma and demonstrated posterior acoustic shadowing. (c) Additional transverse view of normal breast with and without AlloDerm® on top of the breast (junction denoted with an arrow) was performed using a standoff gel pad reveals the hyperechoic surface of the AlloDerm®. Artifacts presumably caused by the microairbubbles within the acellular AlloDerm® can be seen deep to the AlloDerm®. (d) AlloDerm® immersed in sonographic gel also shows these artifacts.
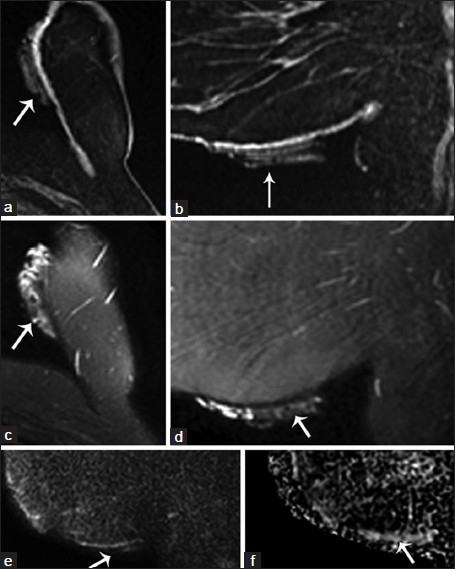
- MRI of hydrated ex vivo AlloDerm®. Noncontrast MRI imaging with technique meeting ACR-accreditation standards was performed on a GE Signa HD 1.5T Magnet (Waukesha, WI). A folded sheet of AlloDerm® was taped to the inferomedial aspect of the left breast (arrows). Fat saturated T1-weighted 3D spoiled gradient-recalled echo (SPGR) (a) axial and (b) sagittal reformatted images show AlloDerm® exhibiting hypointense-to-isointense T1 signal compared with glandular breast tissue. Fat-suppressed T2-weighted (c) axial and (d) sagittal images of AlloDerm® demonstrate hyperintense T2 signal with heterogeneity which is likely related to the folded configuration of the AlloDerm®; and (e) sagittal diffusion-weighted imaging (DWI) and (f) apparent diffusion coefficient (ADC) images of AlloDerm® show no restricted diffusion.
In vivo imaging of Alloderm® in breast reconstruction
Intraoperative photos illustrate how AlloDerm® can be used in reconstruction of the breast [Figure 5]. The imaging features of AlloDerm® in three patients are described in Figures 6–8. Table 1 summarizes the imaging features of AlloDerm® in our patients compared with those in the study by Tran Cao et al.[1]
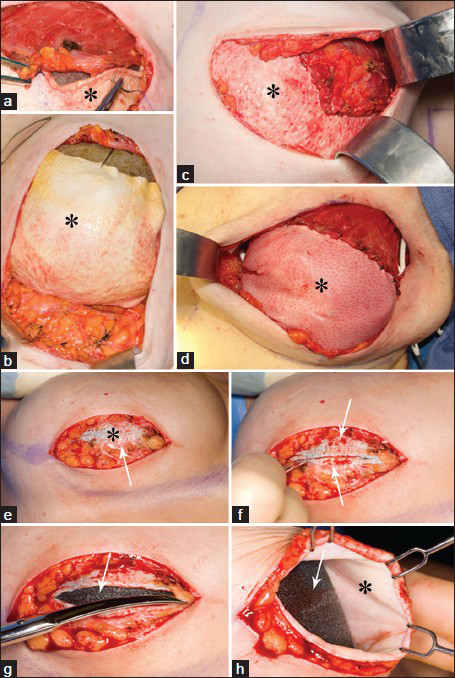
- Intraoperative photos of AlloDerm® (asterisk) placement and suturing initially (a-d) and (e-h) 4 -months later. (a, b) With the tissue expander in place, the AlloDerm® is shown sewn to the pectoralis major muscle above and the inframammary fold below. (c and d) The AlloDerm® is checked for contour and a “hand in glove” fit to the overlying skin envelope. (e-h) Four months later, at a second stage, the patient undergoes implant exchange with removal of the tissue expander. Just deep to the subcutaneous fat the incorporated AlloDerm® is seen (e, asterisk). Bleeding after incising the AlloDerm® demonstrates host incorporation and vascularization (e and f, arrow). The tissue expander (g and h, arrow) is exposed, and the undersurface of the incorporated AlloDerm® can be visualized (h, asterisk).
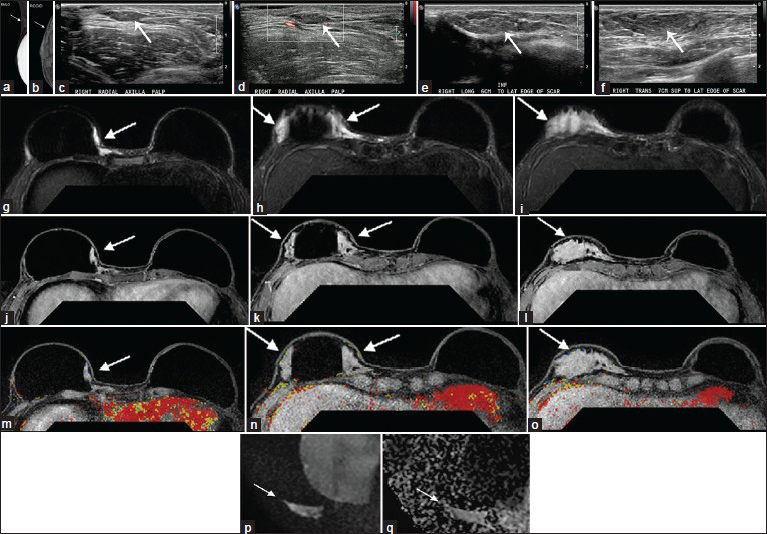
- Case 1: 36-year-old female with bilateral skin sparing mastectomies and implants. Mammogram: (a) Right mediolateral oblique (MLO) and (b) right craniocaudal (CC) implant displaced views demonstrate multiple variable-sized circumscribed masses (arrows) that are isodense to normal glandular tissue and most prominent along the lateral aspect of the implant and the axillary tail. Targeted Ultrasound: (c), without and (d) with color flow in the right axilla at the palpable area of concern, demonstrate multiple isoechoic, vague parallel hypoechoic to isoechoic masses with smooth margins (c and d, arrows). There is a small amount of vascular flow within the axillary mass. (e and f) Transverse and longitudinal sonographic views of the right breast demonstrate some of the masses resembling fatty lobules extending approximately 6 cm inferior to the lateral edge of the mastectomy scar to 7 cm superior to the lateral edge of the scar (e and f, arrow). Similar findings continue to extend toward the axilla adjacent to the implant (images not shown). MRI: Fat-suppressed T2-weighted images (g-i) at three different axial slice locations (superior to inferior) show there is an elongated slightly lobulated area of increased T2 signal (g-i, arrows) in the right breast, (j-l) postgadolinium images at the same locations show no appreciable enhancement (j-l, arrows) corresponding to the location of the AlloDerm® sling adjacent to an intact silicone implant, and, (m-o) post-gadolinium with CAD overlay (m-o) show no kinetic enhancement associated with the AlloDerm®. (p) Diffusion-weighted image and (q) apparent diffusion coefficient map demonstrate mildly increased signal without evidence of restricted diffusion (arrows). The MRI findings related to the AlloDerm® extended from the hammock/sling into the axilla where it was palpable.
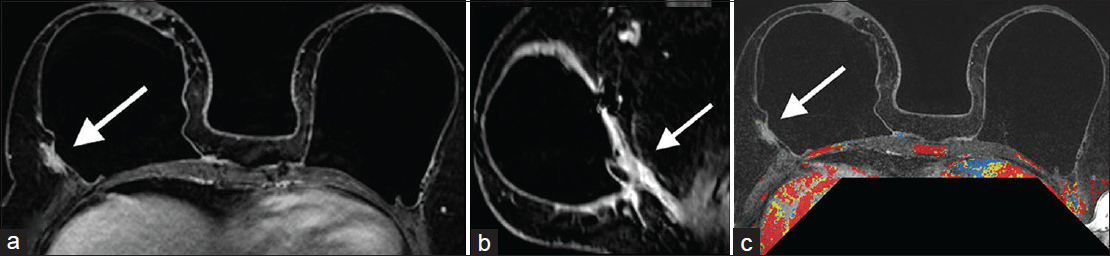
- Case 2: 46-year-old female with bilateral prophylactic mastectomies and bilateral subpectoral implants. MRI: Contrast-enhanced (a) axial, (b) reformatted sagittal, and (c) postgadolinium with CAD overlay images demonstrate mildly increased prominence and enhancement (arrows) posterior and lateral to an intact right implant. This corresponded to the location and configuration of the AlloDerm® hammock/sling.
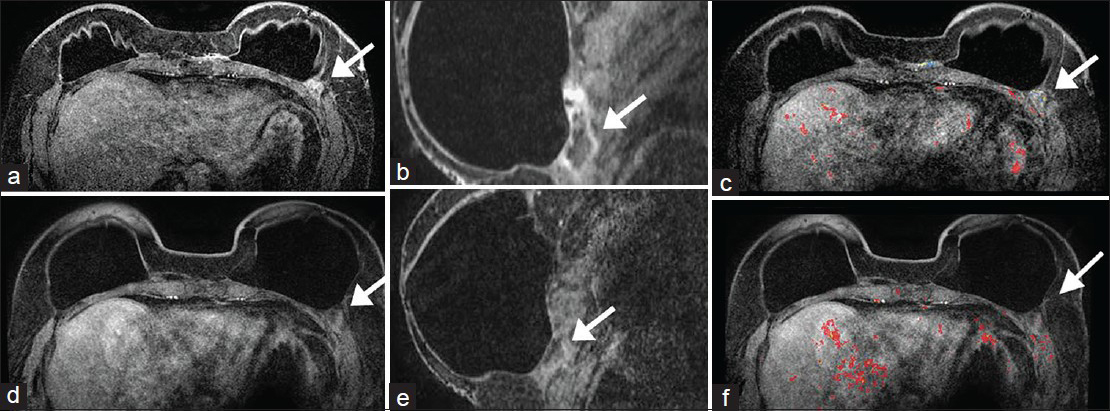
- Case 3: 58-year-old female with bilateral mastectomies and implants. Prior MRI: Contrast-enhanced a) axial, b) reformatted sagittal of the left breast, and c) postgadolinium with CAD overlay MR images demonstrate an area of minimal non-mass enhancement in the location of the AlloDerm® (arrows) in the inframammary fold posterior and lateral to the implant. No worrisome enhancement kinetics is seen. Follow-up MRI 16 months later: There are intact implants and less conspicuity and prominence of the non-mass enhancement (d–f, arrows). This would be in keeping with interval incorporation of AlloDerm® into the host.
Case 1
A 36-year-old female, who 2 years prior underwent bilateral skin sparing mastectomies and implants for left breast invasive ductal carcinoma, presented with palpable nodularity along the upper outer aspects of her reconstructed right breast. A diagnostic mammogram and targeted ultrasound demonstrated multiple masses corresponding to the area of palpable concern.
Mammogram
Right mediolateral oblique (MLO) and right cranial-caudal (CC) implant displaced views demonstrated multiple variable-sized circumscribed masses that were isodense to normal glandular tissue and most prominent along the lateral aspect of the implant and the axillary tail [Figure 6a and b].
Targeted ultrasound
Ultrasound without and with color flow at the palpable area of concern in the right axilla demonstrated multiple, vague parallel hypoechoic to isoechoic masses with smooth margins [Figure 6c and d]. There was a small amount of vascular flow within the axillary mass. In the right breast some of the masses resembled fatty lobules extending approximately 6 cm inferior to the lateral edge of the mastectomy scar to 7 cm superior to the lateral edge of the scar [Figure 6e and f]. Similar findings continued to extend toward the axilla adjacent to the implant.
MRI
Subsequent breast MRI to further characterize the masses in the right breast and axilla was performed. Fat-suppressed T2-weighted images at three different axial slice locations (superior to inferior) showed an elongated area of increased T2 signal in the reconstructed right breast [Figure 6g–i]. Postgadolinium images at the same locations showed no appreciable enhancement corresponding to the location of the AlloDerm® sling adjacent to an intact silicone implant [Figure 6j–l]. Postgadolinium with computer aided detection (CAD) overlay [Figure 6m–o] images also showed no areas of kinetic enhancement corresponding to the areas of AlloDerm®. Diffusion-weighted image [Figure 6p] and apparent diffusion coefficient map [Figure 6q] demonstrated mildly increased signal without evidence of restricted diffusion. The MRI findings related to the AlloDerm® extending from the hammock/sling into the axilla where it was palpable.
Follow-up
Review with the plastic surgeon confirmed that this location and configuration corresponded to the AlloDerm® sling. Therefore, this was given a BIRADS 2 (Breast Imaging Reporting and Data System) benign finding and the patient is currently doing well without evidence of recurrent breast cancer.
Case 2
A 46-year-old female with bilateral prophylactic mastectomies and bilateral subpectoral implants presented to her plastic surgeon 9-months after surgery with pain in her left breast. Breast MRI was ordered for further diagnostic evaluation after targeted ultrasound revealed no correlative finding.
MRI
Contrast-enhanced axial [Figure 7a], reformatted sagittal [Figure 7b], and postgadolinium with CAD overlay [Figure 7c] MRI demonstrated mildly increased prominence and enhancement posterior and lateral to an intact right implant. This corresponded to the location and configuration of the AlloDerm® hammock/sling. No abnormality was noted in the left breast to correspond to the patient's area of pain. There was nothing indicating malignancy on the MRI.
Follow-up
The pain and nodularity resolved and the patient is currently doing well with no complaints regarding her reconstruction.
Case 3
A 58-year-old female who underwent a right mastectomy for invasive ductal carcinoma 8 years prior and a left mastectomy for high grade ductal carcinoma in situ 2 years prior and subsequent bilateral implants presented with an area of cellulitis and clinically presumed fat necrosis in the right breast. Breast MRI done 16 months earlier demonstrated intact implants, mild enhancement along the left inframammary fold in the location of the AlloDerm®, and no areas suspicious for malignancy in either breast.
Prior MRI
Contrast-enhanced axial [Figure 8a], reformatted sagittal image of the left breast [Figure 8b], and postgadolinium with CAD overlay [Figure 8c] MRI demonstrated an area of minimal non-mass enhancement in the location of the AlloDerm® (arrows) in the inframammary fold posterior and lateral to the implant on the left. No worrisome enhancement kinetics were seen. During the intervening time, the patient continued to have a mild tenderness over the medial aspect of her left breast. A breast MRI was again performed.
Follow-up MRI 16 months later
The images showed intact implants and less conspicuity and prominence of the non-mass enhancement [Figure 8d–f, arrows]. This would be in keeping with interval incorporation of AlloDerm® into the host.
DISCUSSION
Given the differences in AlloDerm® usage in this study and the one by Tran Cao et al.,[1] there are both similarities and variations in imaging features. The earlier manuscript described AlloDerm® folded into a roll to fill large lumpectomy defects; this manuscript describes AlloDerm® used as a hammock/sling in postmastectomy patients. Both manuscripts describe the mammographic appearance of AlloDerm® as isodense to glandular tissue. The variability of sonographic features of AlloDerm® between the two manuscripts and between the ex vivo and in vivo cases here probably reflects the continuum of vascularization and incorporation of AlloDerm® into the host. In particular, the distinct contrast between the in vivo and ex vivo sonographic features suggests more posterior acoustic shadowing when there is less incorporation into the host. Similarly, varying enhancing MRI features shown in our patients compared with those of Tran Cao et al.[1] can be attributed to differences in AlloDerm® use and the elapsed time of incorporation into the host. We believe that MRI enhancement of AlloDerm® varies over time, with little if any enhancement of the matrix initially, as demonstrated by Tran Cao et al.,[1] to similar enhancement to adjacent tissue after full incorporation into the host.
Differentiating AlloDerm® from abscess, recurrence, postoperative seroma/hematoma, fat necrosis, capsular contraction, or even extracapsular implant rupture is not always straightforward. In these settings, the imaging features of AlloDerm® are nonspecific with AlloDerm® being considered in the differential when it can be corroborated with the operative notes. In our practice, postmastectomy and postreconstructed breast concerns are addressed on a case-by-case basis. Almost always, targeted ultrasound is the initial imaging modality of choice. For cases where fat necrosis is a differential consideration, mammograms may also be obtained to look for expected calcifications or for fat density masses. For breast reconstructions with a transverse rectus abdominus myocutaneous flap or deep inferior epigastric perforator flap, an initial mammographic approach is used when patients present with a clinical area of concern. Ultrasound and mammograms are certainly the most cost-effective way for evaluation in these instances. Many of the bilateral postmastectomy patients are initially referred to MRI because of lack of breast parenchyma, the exquisite soft tissue characterization afforded by MRI, and ability to simultaneously evaluate implant integrity. In those cases where conventional diagnostic workup with mammogram and ultrasound remains equivocal, MRI is often performed, mainly because the conformation and configuration of AlloDerm® relative to the rest of the reconstructed breast can be better assessed. Through all our cases, we have found that the most helpful information comes from clinical history, correlation with the operative note, and direct discussion with the clinician or surgeon. In most cases, if there is no strong clinical suspicion and the patient is able to return for short-term follow-up, biopsy can thus be obviated.
We suspect there is a continuum of imaging features of AlloDerm® as it is incorporated into the host. A prospective longitudinal study at various time points of AlloDerm® integration would be helpful.
CONCLUSION
AlloDerm® imaging characteristics are not unique and can mimic both benign and malignant features. The variation in appearances can be confounded by the extent of incorporation of AlloDerm® into the host at the time of imaging. Familiarity of some of the appearances of AlloDerm® in the postmastectomy reconstructed breast may help to obviate the need for immediate biopsy.
ACKNOWLEDGMENT
The authors thank Sonia Watson, PhD, for her editorial assistance in preparation of the manuscript.
Available FREE in open access from: http://www.clinicalimagingscience.org/text.asp?2014/4/1/19/131641
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- A preliminary report on the clinical experience with AlloDerm in breast reconstruction and its radiologic appearance. Am Surg. 2010;76:1123-6.
- [Google Scholar]
- The use of AlloDerm in postmastectomy alloplastic breast reconstruction: Part I. A systematic review. Plast Reconstr Surg. 2011;127:2232-44.
- [Google Scholar]
- Immediate bilateral breast reconstruction with implants and inferolateral AlloDerm slings. Ann Plast Surg. 2005;55:232-9.
- [Google Scholar]
- Inferolateral AlloDerm hammock for implant coverage in breast reconstruction. Ann Plast Surg. 2007;59:250-5.
- [Google Scholar]
- Acellular dermis-assisted prosthetic breast reconstruction: A systematic and critical review of efficacy and associated morbidity. Plast Reconstr Surg. 2011;128:1162-9.
- [Google Scholar]
- Retrospective review of 331 consecutive immediate single-stage implant reconstructions with acellular dermal matrix: Indications, complications, trends, and costs. Plast Reconstr Surg. 2011;128:1170-8.
- [Google Scholar]
- Diagnostic dilemma: Acellular dermis mimicking a breast mass after immediate tissue expander breast reconstruction. Plast Reconstr Surg. 2009;124:174-6e.
- [Google Scholar]
- MR imaging of the reconstructed breast: What the radiologist needs to know. Insights Imaging. 2012;3:201-13.
- [Google Scholar]






