Translate this page into:
Imaging Diagnosis of Neonatal Anemia: Report of Two Unusual Etiologies
Address for correspondence: Dr. Shabnam Bhandari Grover, E 81, Kalkaji, New Delhi - 110 019, India. E-mail: shabnamgrover@yahoo.com
-
Received: ,
Accepted: ,
This is an open‑access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Anemia in neonatal period is rare, with the common causes being Rh and ABO blood group incompatibility, hemorrhagic disease of newborn, congenital hemolytic anemia, hemoglobinopathies, and TORCH (toxoplasmosis, rubella, cytomegalovirus, herpes virus) infections. Congenital leukemia and infantile osteopetrosis (OP) are among the rare causes of neonatal anemia. A review of the literature shows approximately 200 reported cases of congenital leukemia. Articles describing the imaging features of congenital leukemia are still rarer. Infantile OP, another rare disorder with a reported incidence of 1 in 250,000 has characteristic imaging features, which are diagnostic of the disease. We report a case each, of two rare diseases: Congenital leukemia and infantile osteopetrosis. Additionally, our report highlights the radiological and imaging features of congenital leukemia and infantile OP and their crucial role in arriving at an early diagnosis.
Keywords
Congenital leukemia
infantile osteopetrosis
neonatal anemia
radiological and imaging features
INTRODUCTION

Anemia in the neonatal period is often a complex problem owing to the unique blood picture during this period, the normal variation in hematological parameters, and also because in no other period of life is anemia known to occur due to such varied causes. The common causes of neonatal anemia are rhesus factor (Rh) and blood group system (A, B, AB, and O) incompatibility, hemorrhagic disease of newborn, congenital hemolytic anemia, hemoglobinopathies, and TORCH (toxoplasmosis, rubella, cytomegalovirus, herpes virus) infections.[1] Congenital leukemia and osteopetrosis (OP) are among the very rare causes of neonatal anemia. Approximately 200 cases of congenital leukemia have been reported. The incidence rate is between 4.3 and 8.6 per million live births. The neonates with this condition have poor prognosis, with 24 months survival rate of only 23%.[23] The majority of patients (80%) described have acute myeloid leukemia (AML), while 20% have acute lymphoid leukemia (ALL).[2] The clinical presentations of congenital leukemia include pallor, hepatosplenomegaly, ecchymosis, recurrent infections, and sepsis.[3] Although there are reports describing the clinical and hematological profile of congenital leukemia, there are no reports in literature emphasizing the radiological features. OP is a rare disorder caused by defective osteoclastic resorption with the infantile (autosomal recessive (AR)) type having an incidence of 1 in 250,000 births. Infantile OP presents in neonates with clinical features of marrow failure, anemia, infections, and fractures.[45]
We report two cases, one of congenital leukemia and the other of OP, both rare diseases. We highlight the radiological and imaging features of congenital leukemia and infantile OP and their crucial role in an early diagnosis.
CASE REPORTS
Case 1
A 3-month-old male child presented with history of pallor, abdominal distension, petechial rashes, recurrent respiratory infections, and repeated hospitalization since birth. Records of the previous hospitalization revealed persistent anemia and thrombocytopenia. Routine hemogram at our hospital revealed anemia (Hb 6.3 g/dl), leukocytosis (total leukocyte count (TLC)-83,000 cells/mm3), and thrombocytopenia (platelet count-35,000 cells/mm3). Skeletal survey (X-ray) requisitioned to rule out congenital infections revealed diffuse osteopenia and prominent calcific zone at metaphyseal ends of the long bones [Figure 1]. Radiograph of the dorsolumbar spine showed osteopenia with prominent vertebral end plates [Figure 2]. Abdominal sonography revealed hepatomegaly and increased hepatic echogenicity [Figure 3]. The infant showed no facial dysmorphisms suggestive of chromosomal disorder and karyotyping was negative for chromosomal anomalies. The serum biochemical parameters including the liver and renal function tests were within normal limits. The TORCH serology test for toxoplasmosis, rubella, cytomegalovirus, and herpes virus that includes enzyme-linked immunosorbent assay for detection of immunoglobulin IgG antibodies was found to be negative. The radiological and sonographic abnormalities seen in an anemic neonate with negative TORCH serology, and normal serum biochemical parameters suggested leukemic infiltration. Magnetic resonance imaging (MRI) of the lumbosacral spine and pelvis, revealed abnormal marrow signal intensities in bilateral iliac bones [Figure 4]. Bone marrow biopsy from the iliac bones revealed marrow infiltration with immature lymphoblasts and diagnosis of acute lymphoblastic leukemia was confirmed [Figure 5]. The infant was started on induction chemotherapy and is on regular follow-up.

- Case 1: 3-month-old male child presenting with symptoms of pallor, abdominal distension, petechial rashes, and recurrent respiratory infections later diagnosed with congenital leukemia. X-ray of both knees and legs, shows diffuse osteopenia with prominent metaphyseal zones (arrows).

- Case 1: 3-month-old male child presenting with symptoms of pallor, abdominal distension, petechial rashes, and recurrent respiratory infections later diagnosed with congenital leukemia. X-ray of dorsolumbar spine shows osteopenia with prominent vertebral end plates (arrows).

- Case 1: 3-month-old male child presenting with symptoms of pallor, abdominal distension, petechial rashes, and recurrent respiratory infections later diagnosed with congenital leukemia. Ultrasound abdomen shows enlarged liver of 7.52 cm in craniocaudal span with diffuse and homogeneously increased echogenicity (arrow).

- Case 1: 3-month-old male child presenting with symptoms of pallor, abdominal distension, petechial rashes, and recurrent respiratory infections later diagnosed with congenital leukemia. Magnetic resonance imaging (MRI) of the pelvis in coronal plane using short tau inversion recovery (STIR) sequence, shows increased marrow signal intensities in both iliac bones (R × L) (arrows).
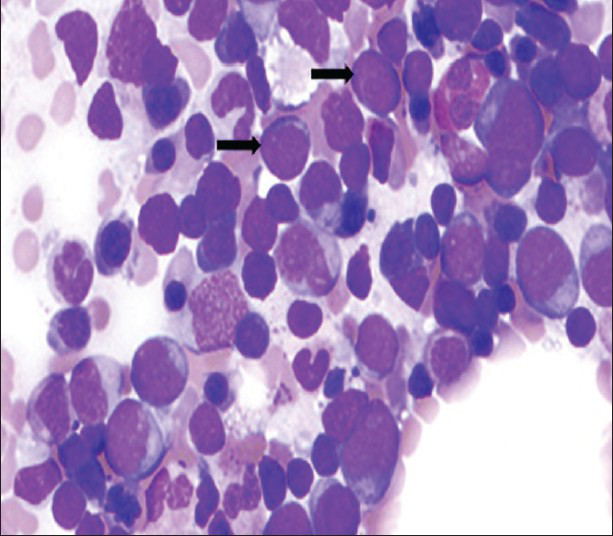
- Case 1: 3-month-old male child presenting with symptoms of pallor, abdominal distension, petechial rashes, and recurrent respiratory infections later diagnosed with congenital leukemia. Bone marrow aspirate from the iliac bone (Leishman stain, 40 ×) shows numerous immature lymphoblasts (black arrows).
Case 2
A 3-month-old male infant was also brought with features of pallor, recurrent respiratory infections, and abdominal distension since birth. Records of previous hospitalization revealed persistent anemia (hemoglobin (Hb) 8.1 g/dl) and thrombocytopenia (platelet count-23,000 cells/mm3). Skeletal survey was requisitioned for this infant at our hospital to exclude congenital infections. Radiographs in this patient revealed diffusely increased bone density with loss of medullary cavity in the long bones [Figures 6 and 7]. A ‘bone within bone’ appearance was also noted in the ends of long bones [Figure 6]. Radiograph of dorsolumbar spine showed increased density of the vertebral body with central linear lucency giving the appearance of ‘sandwich vertebra’ [Figure 7]. Radiograph of the skull revealed marked sclerosis of the skull base [Figure 8]. Computed tomography (CT) of the brain done with adequate body lead shielding and using minimal dose, did not reveal any hydrocephalus or cranial nerve compression [Figure 9]. The radiological features were characteristic for OP. Attempted bone marrow aspiration revealed a dry tap. Bone biopsy revealed disorganized bony trabaculae with obliteration of marrow spaces, which confirmed the diagnosis of OP [Figure 10].

- Case 2: 3-month-old male child presenting with symptoms of pallor, abdominal distension, and recurrent respiratory infections later diagnosed with osteopetrosis. Frontal radiograph of right femur shows diffuse increase in bone density with flared metaphysis (arrow). A “Bone within a bone” appearance is also observed (curved arrow).

- Case 2: 3-month-old male child presenting with symptoms of pallor, abdominal distension, and recurrent respiratory infections later diagnosed with osteopetrosis. Lateral radiograph of spine reveals sclerosis of vertebral bodies (curved arrow) with relative central lucency (arrows); a characteristic “sandwich vertebra” appearance.

- Case 2: 3-month-old male child presenting with symptoms of pallor, abdominal distension, and recurrent respiratory infections later diagnosed with osteopetrosis. Frontal radiograph of skull shows increased bone density with prominent sclerosis of skull base (arrows).

- Case 2: 3-month-old male child presenting with symptoms of pallor, abdominal distension, and recurrent respiratory infections later diagnosed with osteopetrosis. Noncontrast computed tomography (NCCT) head shows sclerosis of skull base (Solid arrow). Brain parenchyma and ventricular system are normal.

- Case 2: 3-month-old male child presenting with symptoms of pallor, abdominal distension, and recurrent respiratory infections later diagnosed with osteopetrosis. Bone marrow biopsy (H and E stain, ×20) shows, disorganized bony trabaculae and obliteration of marrow spaces (black arrows).
DISCUSSION
Leukemia is a bone marrow infiltrative hematopoietic malignancy causing anemia, thrombocytopenia, hepatosplenomegaly, and occasionally lymphadenopathy. Although leukemia is one of the commonest childhood malignancies, neonatal leukemia is rare posing diagnostic challenge to the physician and hematologist with some cases being diagnosed only on post-mortem.[23] In such cases, the radiological features guide the clinician in reaching the final diagnosis. Skeletal changes are seen in 40-75% cases of leukemia.[6] The various radiological findings on conventional imaging include diffuse osteopenia, metaphyseal lucent bands, metaphyseal dense bands, periosteal reaction, and focal lytic lesions.[6] In our first case, the infant had anemia and thrombocytopenia, serum biochemistry was normal and TORCH serology was negative. However, diffuse osteopenia with prominent calcific metaphyseal zones was documented, which aroused the suspicion of leukemia.
MRI in this infant showed marrow infiltration in the iliac bones and biopsy from the same site led to the final diagnosis of leukemia.
MRI of the bone marrow in leukemia has important clinical and prognostic significance and it further helped to clinch the diagnosis in our case. MRI also has a role in detecting relapse following treatment.[7]
OP is a genetically heterogeneous disorder classified as AR and autosomal dominant (AD) types. The AR variety, also known as congenital or infantile or malignant OP, occurs in infancy and has a rapid downhill course due to severe bone marrow failure. AD variety also known as benign or tarda variety is detected in older children, adolescents, and adults and has a relatively benign course.[5] Infantile OP manifests in the first few months of life, usually with features of severe bone marrow failure. Clinical presentation includes hepatosplenomegaly and failure to thrive, while hematological manifestations are anemia and thrombocytopenia.[5] The increase in bone density due to defective osteoclastic resorption can, however, paradoxically weaken the bone, resulting in a predisposition to fractures. They fracture easily because the bones have disorganized structure and fail to remodel along lines of stress.[5]
Our second infant presented with features of bone marrow failure such as anemia, thrombocytopenia, and hepatosplenomegaly (due to extra medullary hematopoiesis). Correlating the clinical presentation and radiological features of diffusely increased bone density, marked skull base sclerosis, bone within bone appearance in long bones, the radiological diagnosis was infantile OP.
The radiological appearance in OP is characteristic and diagnosis can be made with confidence on the skeletal survey itself. Cranial CT and MRI are additional investigations to rule out intracranial complications like cranial nerve palsies, ventriculomegaly, atrophy, tonsillar herniation, vertebral artery compression, etc., which are attributable to the skull base sclerosis.[45] Cranial CT did not reveal hydrocephalus, foraminal compression, or any other abnormalities in our infant.
Both reported infants had similar clinical presentation of failure to thrive, hepatosplenomegaly, hematological anemia, and thrombocytopenia since birth. In the first infant, the radiological features of osteopenia with prominent metaphyseal bands, increased hepatic echogenicity on sonography, and altered marrow signal of iliac bone on MRI, are all explained by leukemic infiltration of bone marrow, liver, and spleen. In the second infant with OP, the radiological manifestations of bony sclerosis with “bone within bone” appearance was characteristic of OP. Bone sclerosis in OP, encroaches the medullary cavity, causing marrow failure and pancytopenia, whereas extramedullary hematopoiesis manifests clinically and radiologically as hepatosplenomegaly. However, the hepatic and splenic echogenicity in extramedullary hematopoiesis are normal, in contradiction to the increased echogenicity seen in infiltrative disorders like leukemia.
CONCLUSION
We report two cases where the infants aged 3 months, showed similar clinical and hematological presentations of recurrent infections, anemia, thrombocytopenia, and hepatosplenomegaly since birth. The characteristic radiological and imaging features with appropriate clinical correlation were valuable in arriving at an accurate diagnosis of congenital leukemia and osteopetrosis (OP). Leukemia and OP should be considered in the differential diagnosis of neonatal anemia, in spite of these being rare entities. Our report emphasizes the role of radiology in diagnosing two very rare causes of neonatal anemia namely congenital leukemia and OP. The importance of skeletal survey and skeletal MRI in the workup of such cases is highlighted by our case report.
Available FREE in open access from: http://www.clinicalimagingscience.org/text.asp?2013/3/1/58/124079
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- Clinico-hematological profile of congenital leukemia. indian J Pediatr. 2004;71:927-8.
- [Google Scholar]
- Congenital leukemia: A diagnostic dilemma. Indian J Med Paediatr Oncol. 2008;29:41-3.
- [Google Scholar]
- Osteopetrosis- A rare cause of anemia: Review of literature. Indian J Pathol Microbiol. 2009;52:363-7.
- [Google Scholar]
- Myeloproliferative and Similar Disorders. In: Adam A, Dixon AK, eds. Grainger & Allison's Diagnostic Radiology-A textbook of medical imaging Vol 2. (5th ed). Philadelphia: Churchill Livingstone Elsevier; 2008. p. :1777-8.
- [Google Scholar]
- Clinical significance of magnetic resonance imaging of bone marrow in patients with leukemia. J Tongji Med Univ. 2001;21:242-5.
- [Google Scholar]






