Translate this page into:
Iatrogenic Coagulopathy and the Development of Posterior Reversible Encephalopathy Syndrome after L-asparaginase Chemotherapy
Address for correspondence: Dr. Eugenia Rota, Department of Neurology, Guglielmo da Saliceto Hospital, Via Taverna 49, 29121 Piacenza, Italy. E-mail: eugenia.rota.md@gmail.com
-
Received: ,
Accepted: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Posterior reversible encephalopathy syndrome (PRES) is a clinical and radiological syndrome mostly related to hypertension, eclampsia, renal failure, or to chemotherapy and/or immunosuppressive drugs. Although the PRES pathophysiology is multifactorial, hypertension and endothelial dysfunction are hypothesized to be the pivotal factors. Here we report a case of PRES in an adult patient after chemotherapy (Escherichia coli L-asparaginase [L-ASP], daunorubicin, vincristine, and intrathecal methotrexate) for acute lymphoblastic leukemia. The development of the PRES was strictly associated with an iatrogenic coagulopathy induced by L-ASP, which inhibits the biosynthesis of hepatic coagulation factors. The nadir of platelet count, antithrombin III (ATIII) and fibrinogen curve was coincident with the onset of the PRES neurological picture; subsequently, the normalization of the ATIII and fibrinogen levels seemed to parallel the good clinical evolution. This case seems to provide new insights into the PRES pathophysiological mechanisms.
Keywords
Coagulopathy
chemotherapy
L-asparaginase
posterior reversible encephalopathy syndrome

INTRODUCTION
Posterior reversible encephalopathy syndrome (PRES) is a clinical-radiological syndrome characterized by typical neurological and magnetic resonance imaging (MRI) findings.[12] The neurological picture includes a headache, altered mental status, seizures, abnormal visual perception and occasionally, focal neurological signs. Typical MRI findings include bilateral subcortical and cortical edema with a predominant posterior distribution. The most common causes of PRES are hypertension, eclampsia, renal failure, or administration of immunosuppressive agents; it is increasingly being reported in children diagnosed with leukemia after chemotherapy, while its development in adults is very rare.
We report an adult case of acute lymphoblastic leukemia (ALL) where the development of a PRES was associated with an iatrogenic coagulopathy.
CASE REPORT
A 65-year-old Italian woman presented with fatigue, bruising, and slight fever. At anamnesis, she was a smoker and had arterial hypertension. Routine blood tests showed: white blood cells 2050 × 109/L (normal range: 4–10 × 109/L); hemoglobin 8.8 g/dl (normal range: 11–15 g/dl); platelet 21,000 × 109/L (normal range: 140–380 × 109/L); lactate dehydrogenase 610 U/L (normal range: 180–450 U/L); fibrinogen 404 mg/dl (normal range: 160–400 mg/dl). Bone marrow aspirate led to a diagnosis of B-ALL. After a prerun with dexamethasone (30 mg/m2/die) for 1 week, the Italian Pediatric Oncological Hematology Association LLA 2000 protocol was administered as remission induction chemotherapy. Dexametasone was administered along with daunorubicin (DNM), vincristine (VCR), Escherichia coli L-asparaginase (L-ASP), and intrathecal methotrexate (MTX).
The scheduled treatment was modified due to the development of peripheral neuropathy. Consequently, VCR was administered on days 8, 15, and 22, but was stopped on day 29 as there was a remission of symptoms. Eighteen days after having started chemotherapy, the patient developed a severe coagulopathy, as a consequence of L-ASP administration. In detail, antithrombin III (ATIII) was 77% (normal range: 80–120%) and fibrinogen: 89 mg/dl (normal range: 160–400 mg/dl). Therefore, the planned L-ASP infusions on days 18, 21, 24, and 27 were suspended and ATIII and fresh-frozen plasma were infused. On day 30, after the last DNM infusion, the patient became somnolent, had neck pain and stiffness; her blood pressure rose to 160/100 mmHg. She was unresponsive (Glasgow Coma scale [GCS]: 7) and had flaccid paralysis of the right arm and leg. Brain computed tomography (CT) scan showed diffuse hypodense areas in the bilateral cerebral and cerebellar lobes with no intracranial bleeding [Figure 1]. Whereas MRI fluid attenuated inversion recovery and T2-weighted fast spin echo sequences evidenced abnormal signal in the subcortical occipitoparietal white matter and more confluent areas of signal hyperintensity in the bihemispheric frontal region, thalamus and cerebellar hemispheres [Figure 2a and b]. Diffusion-weighted images evidenced signal isointensity or slight hyperintensity of the affected areas with an increase in the apparent diffusion coefficient indicating vasogenic edema [Figure 2c–f].
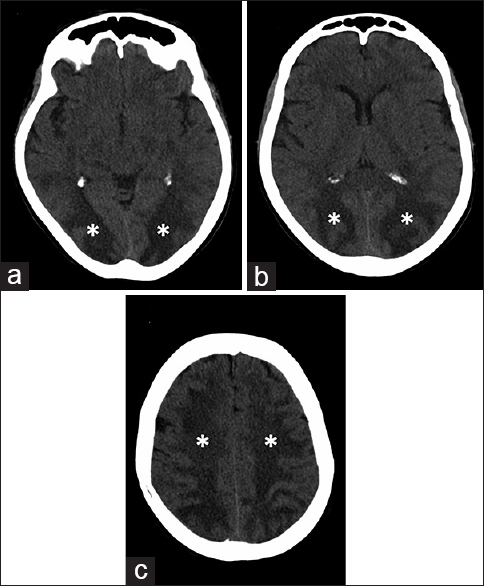
- 65-year-old Italian woman, after chemotherapy (Escherichia coli L-asparaginase, daunorubicin, vincristine, intrathecal methotrexate) for a B-acute lymphoblastic leukemia, developed a comatose state. Brain axial computed tomography scan at the level of (a) occipital lobe, (b) parietal lobe and (c) centrum semiovale of frontal lobes showed subcortical white matter hypodensity (*).
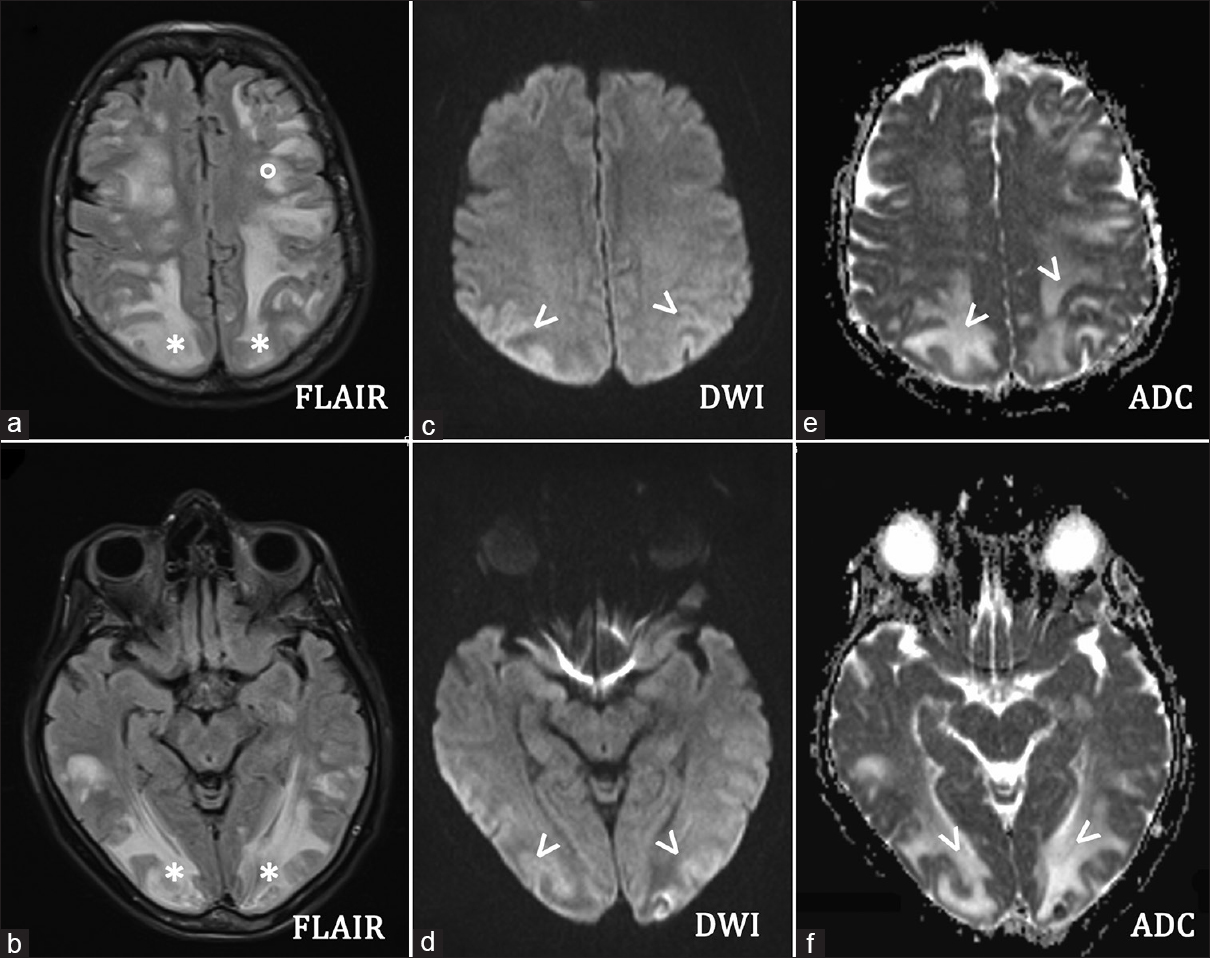
- 65-year-old Italian woman, after chemotherapy (Escherichia coli L-asparaginase, daunorubicin, vincristine, intrathecal methotrexate) for a B-acute lymphoblastic leukemia, developed a comatose state Brain axial magnetic resonance imaging (a and b) fluid-attenuated inversion recovery sequences showed increased signals in the subcortical occipital (*) and frontal (°) white matter. Brain axial (c and d) diffusion weighted imaging revealed light hyperintensity of the affected areas (arrows) with an increased apparent diffusion coefficient value (arrows) (e and f), indicating vasogenic edema. Diffusion-weighted imaging should always be combined with the study of the apparent diffusion coefficient map that allows us to differentiate the vasogenic oedema (increased signal in apparent diffusion coefficient, i.e., posterior reversible encephalopathy syndrome, as in the present case) from the cytotoxic edema (decreased signal in apparent diffusion coefficient, i.e., ischemic lesion).
Electroencephalography documented diffuse theta-delta slowing of the background activity. No cerebrospinal fluid analysis was performed because of a platelet count of 33,000/μl (normal range: 150,000–450,000/μl), which normalized on day 41. Diuretic and anti-edemigen therapy was started and the patient's cognitive status progressively improved, leading to her recovering consciousness (GCS: 15 on day 45). A control brain MRI at 14 days after the clinical onset of the neurological picture [Figure 3a and b] evidenced persistent subcortical and cortical hyperintensity in the bilateral parietal, occipital, and frontal lobes, which were no longer present at a further MRI control 1 month later [Figure 3c and d]. Blood tests showed that coagulopathy had improved and did not evidence any other remarkable findings.
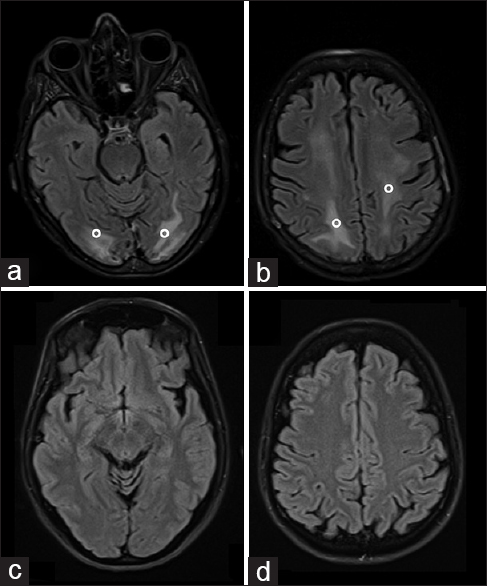
- 65-year-old Italian woman, after chemotherapy (Escherichia coli L-asparaginase, daunorubicin, vincristine, intrathecal methotrexate) for a B-acute lymphoblastic leukemia, developed a comatose state Brain axial magnetic resonance imaging fluid-attenuated inversion recovery sequences performed 14 days later showed persistently increased signals (°) in the subcortical occipital (a) and frontal (b) white matter, that disappeared 1 month later (c and d).
Remission induction therapy induced complete B-ALL remission and she was given consolidation therapy after the development of the PRES. At time of writing, the patient is in complete remission.
DISCUSSION
PRES may be induced by several drugs during remission induction chemotherapy for ALL. Only three other adult cases of PRES that developed after the administration of L-ASP treatment have been reported to date.
Although the pathophysiology of the PRES is multifactorial,[2] hypertension and endothelial dysfunction are hypothesized to lead to the formation of vasogenic edema. Along with the vasculature damage induced by hypertension itself, the collapse of brain vasculature autoregulatory functions seems to involve in the PRES development.[3]
The incidence of PRES has increased since the protocol for chemotherapy was modified to include more frequent L-ASP administration. L-ASP inhibits the biosynthesis of hepatic coagulation factors, such as antithrombin and fibrinogen, thereby favoring thromboembolic and hemorrhagic events. Although the mechanism underlying PRES during L-ASP is still unclear, microthrombosis or direct endothelial damage have been hypothesized to play a role.[1] In-vitro experiments suggest that chemotherapy drugs, including L-ASP, can induce expression of tissue factor (TF) and a procoagulant activity on endothelial cells, where the injury to endothelium could contribute to hypercoagulability.[4] L-ASP carries neurotoxicity which seems to be even more pronounced with E. coli L-ASP, as in the case reported herein.[5]
It is known that tumor necrosis factor (TNF) affects endothelial permeability and shows procoagulant activity on tumor-associated neovasculature, mainly via induction of cell surface expression of TF on endothelial tissue.[6] ATIII may reverse endothelial cell permeability induced by TNF, affecting actin cytoskeletal filaments and vascular endothelial cadherin expression (gap-junction).[6] Tumor cell lines with high TF expression on cell surface may favor localized vascular permeability. Interestingly, ALL cells bear high TF expression.[7]
Indeed, our patient showed a patent coagulopathy, with ATIII and fibrinogen reduction, which preceded the PRES development. The nadir of ATIII and fibrinogen curve was coincident with the onset of the PRES neurological picture [Figure 4]. Subsequently, the normalization of the ATIII and fibrinogen levels seemed to parallel the clinical evolution. The extensive subcortical/cortical hyperintensity in the bilateral parietal, occipital, and frontal lobes, may be consistent with L-ASP-induced brain endothelial damage, perhaps precipitated by DNM toxicity. Indeed, the effects of the other drugs of the protocol may have contributed to the PRES development in our patient; nevertheless, the jatrogenic coagulopathy induced by L-ASP seems to be the pivotal pathophysiological factor, on the basis of the time course of both coagulopathy and PRES clinical evolution. Moreover, other natural occurring anticoagulants, such as protein C and S, are reduced after ASP treatment and may contribute to PRES development.[8]
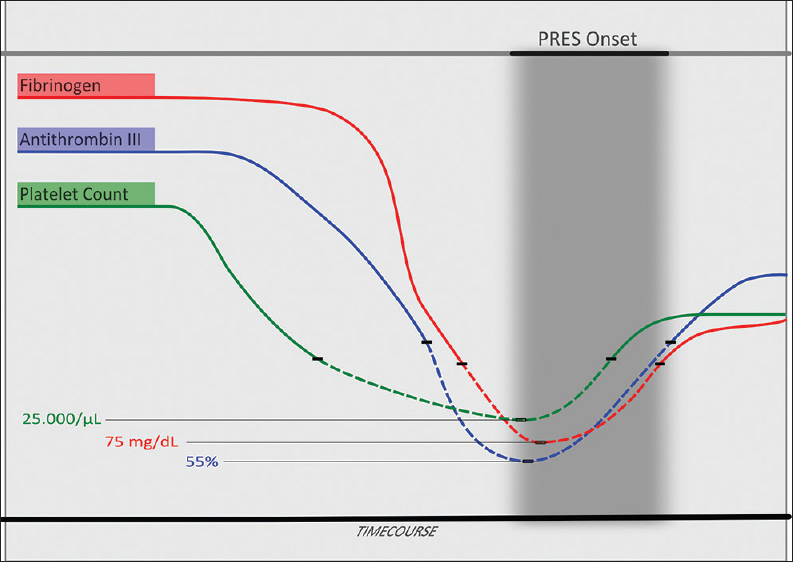
- 65-year-old Italian woman, after chemotherapy (Escherichia coli L-asparaginase, daunorubicin, vincristine, intrathecal methotrexate) for a B-acute lymphoblastic leukemia, developed a comatose state Fibrinogen, antithrombin III and platelet count time course in relation to the posterior reversible encephalopathy syndrome clinical onset. The dashed line indicates the values under the lower limit of normality for each parameter (fibrinogen: 160–400 mg/dl; antithrombin III: 80–120%; platelets: 150,000–450,000/μl). The nadir of antithrombin III and fibrinogen curve was coincident with the onset of the posterior reversible encephalopathy syndrome neurological picture.
Another hypothesis is that L-ASP induces liver toxicity in ALL, facilitating hyperammonemia, which, in turn, could lead to PRES.[9] Therefore, in our opinion, hyperammonemia should be systematically screened for in patients presenting encephalopathy during chemotherapy, particularly in those treated with ASP.
PRES should be recognized as a life-threatening complication of L-ASP. Prompt diagnosis and treatment are mandatory to avoid progression to ischemia, infarction, and even death.[10] Furthermore, as suggested by the present case, the coagulation factors should be strictly monitored and, in the presence of reduced ATIII, replacement therapy should be considered, particularly when PRES is suspected.
CONCLUSION
The case reported here shows that a PRES may develop after chemotherapy due to ALL (E. coli L-ASP, DNM, VCR, intrathecal MTX), in strict temporal association with an iatrogenic L-ASP induced coagulopathy. This implies that iatrogenic coagulopathy plays a pivotal role in the pathophysiology of PRES and suggests the need for the monitoring of coagulation factors, particularly in the presence of neurological symptoms.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors would like to thank Mrs. Barbara Wade for her linguistic advice and Dr. Ilaria Iafelice, MD, for her assistance in the bibliographical research.
Available FREE in open access from: http://www.clinicalimagingscience.org/text.asp?2016/6/1/7/177553
REFERENCES
- Posterior reversible encephalopathy syndrome in an adult patient with acute lymphoblastic leukemia after remission induction chemotherapy. Int J Hematol. 2012;95:204-8.
- [Google Scholar]
- A reversible posterior leukoencephalopathy syndrome. N Engl J Med. 1996;334:494-500.
- [Google Scholar]
- Gender differences in cerebral blood flow velocity and autoregulation between the anterior and posterior circulations in healthy children. Pediatr Res. 2005;58:574-8.
- [Google Scholar]
- Increased levels of tissue factor activity and procoagulant phospholipids during treatment of children with acute lymphoblastic leukaemia. Br J Haematol. 2010;148:582-92.
- [Google Scholar]
- Immunosuppressive treatments in Crohn's disease induce myelodysplasia and leukaemia. Am J Hematol. 2010;85:634.
- [Google Scholar]
- Induction of permeability across endothelial cell monolayers by tumor necrosis factor (TNF) occurs via a tissue factor-dependent mechanism: Relationship between the procoagulant and permeability effects of TNF. Blood. 2002;100:1334-9.
- [Google Scholar]
- The expression of tissue factor antigen and activity on the surface of leukemic cells. Leuk Res. 1993;17:103-11.
- [Google Scholar]
- Effects of dose-reduced Medac L-asparaginase on coagulation in trial ALL-BFM 2000. Klin Padiatr. 2003;215:321-6.
- [Google Scholar]
- Hyperammonemic encephalopathy after induction chemotherapy for acute lymphoblastic leukemia. J Pediatr Hematol Oncol. 2009;31:955-6.
- [Google Scholar]
- Clinical and radiological spectrum of posterior reversible encephalopathy syndrome. J Cerebrovasc Endovasc Neurosurg. 2013;15:206-13.
- [Google Scholar]






