Translate this page into:
Gastroduodenal artery embolization for peptic ulcer hemorrhage refractory to endoscopic intervention: A single-center experience

*Corresponding author: Ambarish Bhat, Department of Radiology, University of Missouri, Columbia, Missouri, United States. bhatap@health.missouri.edu
-
Received: ,
Accepted: ,
How to cite this article: Khazi ZM, Marjara J, Nance M, Ghouri Y, Hammoud G, Davis R, et al. Gastroduodenal artery embolization for peptic ulcer hemorrhage refractory to endoscopic intervention: A single-center experience. J Clin Imaging Sci 2022;12:31.
Abstract
Objective
To determine the efficacy of gastroduodenal artery embolization (GDAE) for bleeding peptic ulcers that failed endoscopic intervention. To identify incidence and risk factors for failure of GDAE.
Materials and Methods
A retrospective review of patients who underwent GDAE for hemorrhage from peptic ulcer disease refractory to endoscopic intervention were included in the study. Refractory to endoscopic intervention was defined as persistent hemorrhage following at least two separate endoscopic sessions with two different endoscopic techniques (thermal, injection, or mechanical) or one endoscopic session with the use of two different techniques. Demographics, comorbidities, endoscopic and angiographic findings, significant post-embolization pRBC transfusion, and index GDAE failure were collected. Failure of index GDAE was defined as the need for re-intervention (repeat embolization, endoscopy, or surgery) for rebleeding or mortality within 30 days after GDAE. Multivariate analyzes were performed to identify independent predictors for failure of index GDAE.
Results
There were 70 patients that underwent GDAE after endoscopic intervention for bleeding peptic ulcers with a technical success rate of 100%. Failure of index GDAE rate was 23% (n = 16). Multivariate analysis identified ≥2 comorbidities (odds ratio [OR]: 14.2 [1.68-19.2], P = 0.023), days between endoscopy and GDAE (OR: 1.43 [1.11-2.27], P = 0.028), and extravasation during angiography (OR: 6.71 [1.16-47.4], P = 0.039) as independent predictors of index GDAE failure. Endoscopic Forrest classification was not a significant predictor for the failure of index GDAE (P > 0.1).
Conclusion
The study demonstrates safety and efficacy of GDAE for hemorrhage from PUD that is refractory to endoscopic intervention. Days between endoscopy and GDAE, high comorbidity burden, and extravasation during angiography are associated with increased risk for failure of index GDAE.
Keywords
Embolization
Peptic ulcer disease
Endoscopy
Gastroduodenal artery
Interventional radiology
INTRODUCTION
Peptic ulcer disease (PUD) is responsible for approximately 40-60% of acute non-variceal upper gastrointestinal (GI) hemorrhage, with duodenal ulcers being the most common culprit.[1-3] The prevalence of PUD has been declining over the past two decades owing to treatment of Helicobacter pylori, judicious non-steroidal anti-inflammatory (NSAID) use, and use of antisecretory medications like proton pump inhibitors (PPIs) or H2- blockers.[1,3] However, acute hemorrhage secondary to PUD continues to pose a significant risk for morbidity and mortality, with the incidence of perforation as high as 30% among patients with hemorrhage secondary to PUD.[4]
Upper GI endoscopic intervention with esophagogastroduodenoscopy (EGD) is currently the gold standard test for treating PUD-related hemorrhage since it has a diagnostic and therapeutic role in its management.[5]Endoscopic hemostasis can be achieved through various means, including mechanical, thermal, or local administration of vasoactive drugs.[5,6] However, recurrent hemorrhage within 30 days after endoscopic intervention for PUD occurs in 5-10% of patients.[7-9] Besides endoscopic intervention, patients with recurrent GI hemorrhage can undergo operative management or transcatheter arterial embolization (TAE) for definitive treatment. In a recent meta-analysis comparing TAE to surgery for hemorrhage from PUD, Tarasconi et al. determined that TAE was a safe and effective treatment option with similar clinical outcomes and a trend toward lower mortality.[10]
Despite emerging data regarding the efficacy of TAE for hemorrhage from PUD, there is a paucity of data assessing the efficacy of gastroduodenal artery embolization (GDAE) and risk factors associated with failure of index GDAE. Therefore, the purpose of the present study is to determine the efficacy of GDAE for bleeding peptic ulcers that failed endoscopic intervention and identify risk factors for failure of index GDAE.
MATERIALS AND METHODS
Patient selection
A retrospective review of all cases that failed endoscopic therapy for hemorrhage from bleeding PUD and subsequently underwent GDAE from January 2011 to December 2019 at a tertiary academic institute was analyzed for this study. Information regarding demographics, endoscopic findings, angiographic findings, endovascular intervention, post-embolization outcomes, and the mortality rate was identified using the database maintained by the Division of Interventional Radiology (IR) and the hospital’s electronic medical record system. Failure of endoscopic therapy was defined as the inability to control hemorrhage following at least two different endoscopic techniques (injection, mechanical, or thermal therapy). Patients that underwent GDAE for arteriovenous malformation, prior treatment with Yttrium 90 or traumatic events were excluded. Pediatric patients that underwent GDAE were excluded. This study was approved by the local institutional review board prior to data collection and analysis which waived the need for informed consent.
Patient demographics and comorbidities
Basic patient demographics, including age and sex, were recorded in the database. Preangiographic comorbidities such as diabetes mellitus, hypertension, hyperlipidemia, coronary artery disease, congestive heart failure, chronic obstructive pulmonary disease, chronic kidney disease, history of PUD, history of prior or current malignancy, history of acute pancreatitis, history of thromboembolic events, smoking history, NSAID use, and anticoagulation therapy were recorded. Smoking history was noted as yes if patients were current or former smokers.
Endoscopic findings and number of days between endoscopy and GDAE were recorded. Specifically, Forrest classification identified during endoscopy was recorded as Forrest class I: active bleeding, Forrest class II: evidence of recent bleeding, and Forrest class III: lesion without evidence of bleeding. Preangiography hemoglobin level was recorded for each patient. Lastly, the length of hospital stay after embolization was also recorded.
Embolization technique
Consent was obtained prior to the procedure. The technique used for GDA embolization has been described previously.[11] A celiac angiogram was performed using a 5 Fr catheter. If contrast extravasation was noted, embolization was performed distal and proximal to the extravasation [Figure 1]. If not, prophylactic embolization of the GDA was performed based on the location of the ulcer as described in the endoscopic report or if the ulcer was treated using a radio-opaque metallic endoclip, this was used as a marker to plan the level of embolization. A microcatheter and 0.018” coils were used for embolization in all cases [Figure 2]. Proximal embolization was performed as close as possible to the origin of the GDA. A superior mesenteric artery angiogram was performed to ensure there were no alternative collateral branches back-feeding into the GDA, prior to the conclusion of the procedure.
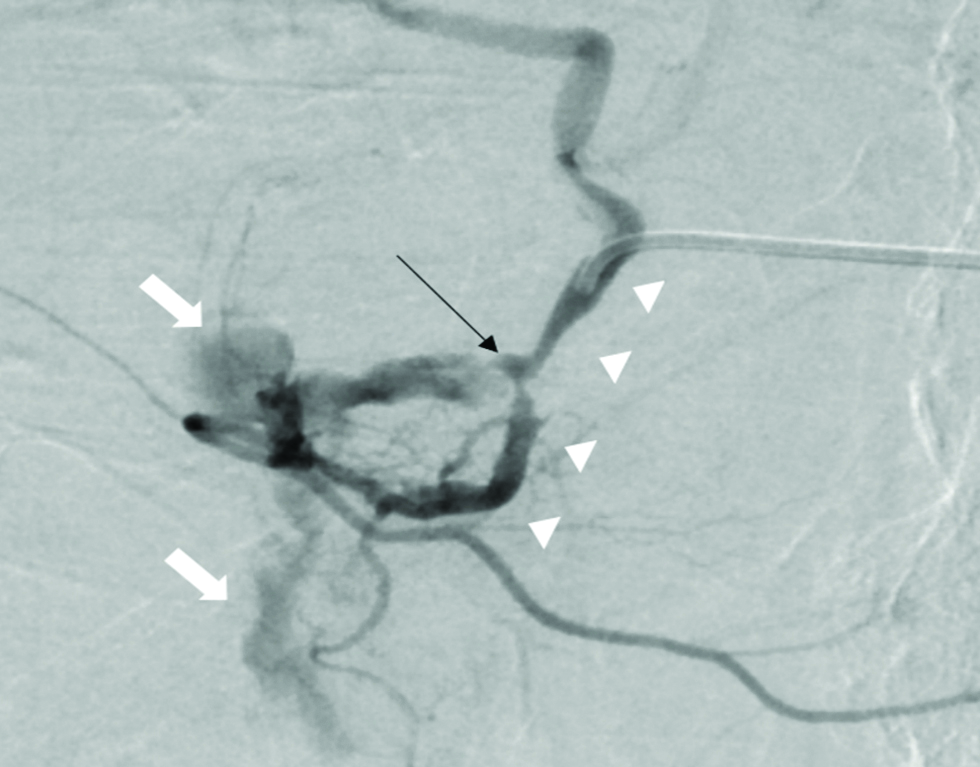
- Selective angiogram of the gastroduodenal artery (white arrowheads) in a 69-year-old male, showing the pseudoaneurysm (black arrow) in the mid segment of the gastroduodenal artery and associated extravasation of contrast (white block arrows).

- Angiogram of the gastroduodenal artery in a 69-year-old after coil (white arrow heads) embolization, with resolution of extravasation.
Outcomes
The primary outcome assessed was the failure of index GDAE. For this study, failure of GDAE was defined as the need for re-intervention (repeat embolization, endoscopy, or surgery) to treat rebleeding or all-cause mortality within 30 days after GDAE.
Research ethics standards compliance
This original research was completed under an institutional review board-approved protocol. The IRB number was 2004777. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Statistical analysis
Descriptive statistics were used to report patient demographics, medical comorbidities, endoscopic findings, angiographic findings, and post-embolization outcomes. Multiple binomial logistic regression analyzes with backward stepwise elimination were performed to identify independent risk factors associated with the need for the failure of index GDAE. Due to the sample size of the present study, patient comorbidities were grouped as patients that only had one comorbidity versus those with ≥2 comorbidities for multivariate analyzes. NSAID use, pre-embolization anticoagulation therapy, pre-embolization hemoglobin, days to angiography after endoscopy, length of GDA embolized (in mm), Forrest classification of peptic ulcer, and angiographic finding of extravasation were included in the multivariate analyses. The Hosmer-Lemeshow goodness of fit test was also performed for each multivariate model to assess for appropriate calibration with fitness defined as P > 0.1. All statistical analysis were performed using R-statistical software (www.r-project.org) with statistical significance defined as P > 0.05.
RESULTS
Patient characteristics
There were 70 patients (30% female) with an average age of 63 (±14.9) years that underwent GDAE after failed endoscopic interventions for bleeding secondary to PUD. A detailed list of patient characteristics and comorbidities is highlighted in Table 1. Technical success was noted in 100% of patients. Endoscopic findings such as Forrest classification of peptic ulcer and angiographic findings such as presence of extravasation, mean length of GDA embolized, mean fluoroscopy time, and mean fluoroscopy dose are reported in Table 2. The meantime between endoscopy and angiography was 1.54 ± 3.38 days.
| Patient characteristics | n (%) |
|---|---|
| Mean age, in years (SD) | 63 (14.9) |
| Female sex | 21 (30) |
| Diabetes mellitus | 19 (27.1) |
| Hypertension | 37 (52.9) |
| Hyperlipidemia | 21 (30) |
| Coronary artery disease | 10 (14.3) |
| Congestive heart failure | 9 (12.9) |
| Chronic obstructive pulmonary disease | 12 (17.1) |
| Chronic kidney disease | 8 (11.4) |
| Malignancy | 26 (37.1) |
| Acute pancreatitis | 10 (14.3) |
| Peptic ulcer disease | 26 (37.1) |
| Smoking history | 17 (24.3) |
| History of venous thromboembolism | 18 (25.7) |
| Non-steroidal anti-inflammatory drug use | 22 (31.4) |
| Anti-coagulation therapy | 11 (15.7) |
| Endoscopic findings | n (%) |
|---|---|
| Forrest classification | |
| Type 1: Active bleeding | 17 (24.3) |
| Type 2: Recent bleeding | 17 (24.3) |
| Type 3: Lesion without bleeding | 36 (51.4) |
| Angiographic findings | |
| Pre-embolization hemoglobin level, in g/dL | 9.6 (2.6) |
| Angiographic evidence of bleeding | |
| Extravasation | 13 (18.6) |
| GDA pseudoaneurysm | 8 (11.4) |
| Normal vascular anatomy | 49 (70) |
| Length of GDA embolized, in mm (SD) | 35.27 (17.04) |
| Fluoroscopy time, in mins (SD) | 21.3 (11.9) |
| Fluoroscopy dose, in µGym (SD) | 1456.2 (1448.5) |
Failure of index GDAE
Failure of index GDAE was noted in 22.9% (n = 16) of patients. Of those that failed index GDAE, 12 patients underwent repeat intervention. The all-cause mortality rate within 30 days after index GDAE was 8.6% (n = 6/70) [Table 3]. The mean length of hospital stay was 9.2 ± 13.7 days. There were no procedure-related complications.
| Post-embolization events | n (%) |
|---|---|
| Length of hospital stay, in days (SD) | 9.2 (13.7) |
| Failure of index GDA embolization | 16 (22.9) |
| Re-intervention for rebleeding | 12 (17.1) |
| Repeat embolization | 2 (4.3) |
| Repeat endoscopy | 5 (7.1) |
| Surgical intervention | 5 (7.1) |
| All-cause mortality | 6 (8.6) |
Risk factors for failure of index GDAE
Multivariate analysis identified ≥2 comorbidities (odds ratio [OR]: 14.2, 95% confidence interval [CI]: 1.68-19.2, P = 0.023), each day between endoscopy and GDAE (OR: 1.43, 95% CI: 1.11-2.27, P = 0.028), and extravasation during angiography (OR: 6.71, 95% CI: 1.16-47.4, P = 0.039) as independent predictors of index GDAE failure [Table 4].
| Risk factors | Odds ratio [95% confidence interval] | P-value |
|---|---|---|
| Age | 1.01 [0.96-1.06] | 0.79 |
| Male sex | 1.21 [0.22-7.39] | 0.822 |
| Comorbidities ≥2 | 14.2 [1.69-19.2] | 0.02* |
| NSAID use | 1.48 [0.24-9.30] | 0.668 |
| Anticoagulation therapy | 6.53 [0.55-94.6] | 0.139 |
| Pre-embolization Hb (drop by 1 g/dL) | 0.77 [0.46-1.15] | 0.2411 |
| Days to angiography after endoscopy (increase by 1 day) | 1.43 [1.11-2.27] | 0.028* |
| Length of GDA embolized | 0.99 [0.94-1.04] | 0.699 |
| Forrest classification (reference: Type 3- Lesion without bleeding) | ||
| Type 1: Active bleeding | 4.76 [0.75-36.9] | 0.107 |
| Type 2: Recent bleeding | 0.51 [0.02-6.63] | 0.627 |
| Angiographic finding (reference: normal vascular anatomy) | ||
| Extravasation | 6.71 [1.16-47.4] | 0.039* |
| Pseudoaneurysm | 3.57 [0.15-60.1] | 0.377 |
*Significant finding (P > 0.05), Hb: hemoglobin, GDA: Gastroduodenal Artery
DISCUSSION
The present study assessed the rate of clinical success after GDAE for patients with hemorrhage due to PUD that was refractory to endoscopic interventions. The study found that failure of initial GDAE rate was 22.9% (n = 16) and the all-cause mortality rate within 30 days after initial GDAE was 8.6%. Additionally, the study determined that time between endoscopy and GDAE (in days), high comorbidity burden, and extravasation during angiography were associated with increased odds of failure of initial GDAE.
With advances in endoscopic tools and techniques, most patients with hemorrhage secondary to PUD achieve hemostasis without requiring subsequent interventions. Additionally, the incidence of rebleeding after achieving initial endoscopic hemostasis is low due to concurrent use of high-intensity PPI therapy and appropriate treatment of Helicobacter pylori infection after endoscopy.[12-14] However, when the initial endoscopic intervention is not sufficient, patients often require a second endoscopic procedure, surgery or TAE. In such scenarios, surgical options are associated with high mortality rates ranging from 20 to 40%. Such patients that require re-intervention tend to have higher comorbidity indices and poorly tolerate general anesthesia.[15] As TAE is a minimally invasive procedure and does not require general anesthesia, it has garnered a lot of interest in management of these patients. In a study comparing outcomes after surgery and TAE for bleeding from PUD refractory to endoscopic intervention, Sverden et al. determined that patients undergoing TAE had a lower risk of mortality, complications, and length of hospital stay.[16] However, compared to surgery, TAE was associated with a higher risk of rebleeding.[16] Similarly, a recent meta-analysis comparing surgery to TAE for non-variceal upper GI bleeding refractory to endoscopic intervention identified a lower incidence of complications and a trend toward improved mortality rate after TAE compared to surgery.[10] However, the incidence of rebleeding was higher in the TAE group.[10] In the present study, the all-cause mortality rate within 30 days after GDAE for bleeding PUD was 8.6%.
Despite the efficacy of TAE for PUD refractory to endoscopic intervention, there is a risk of recurrent bleeding requiring re-intervention. In a retrospective review, Loffroy et al. reported an incidence of rebleeding within 30 days after TAE refractory to the endoscopic intervention of 28%.[17] Recently, Kaminskis et al. compared clinical outcomes in high-risk patients that underwent prophylactic TAE after endoscopy for PUD with endoscopy alone. A lower rate of rebleeding was observed in patients that underwent prophylactic TAE.[18] These findings underscore the importance of prompt angiographic intervention in patients’ refractory to endoscopic management to reduce the risk of recurrent bleeding.
In the present study, GDAE failure was observed in 23% of cases. Identifying predictors of GDAE failure may allow physicians to stratify higher-risk patients for close observation following GDAE. Our results demonstrate ≥2 comorbidities, days between GDAE and endoscopy, and extravasation during angiography were independent predictors of index GDAE failure. Other studies have also identified coagulopathy or multiple comorbidities as risk factors for GDAE failure.[15,19] There are limited studies exploring the importance of time to angiography and extravasation during angiography as crucial risk factors for clinical success. In a series of 54 patients with bleeding duodenal ulcer, Lee et al. identified a lower incidence of mortality and need for ICU care among patients that underwent early TAE.[20] The association between early TAE and mortality requires further investigations. In consideration of the impact of extravasation during angiography on clinical outcomes after TAE, Choi et al. reported the incidence of clinical failure and 30-day all-cause mortality was significantly higher in patients with extravasation noted during angiography.[21] Interestingly, endoscopic evidence of active or recent bleeding as assessed by Forrest classification was not an independent risk factor for post-embolization transfusion or failure of initial GDAE in the present study. In support of this notion, Loffroy and colleagues did not find a significant difference in clinical success, need for re-intervention, 30-day complications, and mortality; when comparing patients with bleeding ulcers (Forrest class I or II) and non-bleeding ulcers (Forrest class III) post-TAE.[22] Additionally, the lack of significance of Forrest classification suggests that angiographic success for GADE is independent of the severity of bleed based on endoscopic findings.
Limitations
The primary limitations of this study include its retrospective nature and small sample size. The absence of a control group precluded the ability to establish causation. Furthermore, while this study identified time to GDAE and extravasation during angiography as risk factors for GDAE failure, the small sample size prevented stratification of patients based on pre-embolization coagulopathy. Additionally, data on preprocedure nutrition and conditioning was limited. Lastly, the retrospective nature and small sample size prevented any evaluation of anatomical variance, which may have influenced time to sub-selective canalization, presence of collateral vessels, or proximity to other structures. These considerations will be the topic of future study.
CONCLUSION
The study demonstrates the safety and efficacy of GDAE for hemorrhage due to PUD that is refractory to endoscopic intervention. Days between endoscopy and GDAE, high comorbidity burden, and extravasation during angiography are associated with increased risk for failure of index GDAE.
Research highlights
Gastroduodenal artery embolization (GDAE) for bleeding peptic ulcers is safe and effective to control hemorrhage in the setting of PUD refractory to endoscopic intervention
Days between endoscopy and GDAE, high comorbidity burden, and extravasation during angiography are associated with failure of index GDAE.
Prompt notification of possible persistent hemorrhage in the setting of PUD after endoscopy can help improve patient outcomes.
Other factors such as length of GDA embolized, Forrest classification of the ulcer, and pre-embolization hemoglobin level is not associated with failure of index GDAE.
Declaration of patient consent
Institutional Review Board (IRB) permission obtained for the study.
Financial support and sponsorship
Nil.
Conflict of interest
There are no conflicts of interest.
References
- Diagnosis and treatment of peptic ulcer disease. Am J Med. 2019;132:447-56.
- [CrossRef] [PubMed] [Google Scholar]
- The acute upper gastrointestinal bleed. Surg Clin North Am. 2018;98:1047-57.
- [CrossRef] [PubMed] [Google Scholar]
- Perforated peptic ulcer - an update. World J Gastrointest Surg. 2017;9:1-12.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Management of nonvariceal upper gastrointestinal bleeding: Guideline Recommendations from the International Consensus Group. Ann Intern Med. 2019;171:805-22.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnosis and management of nonvariceal upper gastrointestinal hemorrhage: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2015;47:a1-46.
- [CrossRef] [PubMed] [Google Scholar]
- Outcomes and role of urgent endoscopy in high-risk patients with acute nonvariceal gastrointestinal bleeding. Clin Gastroenterol Hepatol. 2018;16:370-7.
- [CrossRef] [PubMed] [Google Scholar]
- Risk factors associated with rebleeding in patients with high risk peptic ulcer bleeding: Focusing on the role of second look endoscopy. Dig Dis Sci. 2016;61:517-22.
- [CrossRef] [PubMed] [Google Scholar]
- Predictors of peptic ulcer rebleeding after scheduled second endoscopy: Clinical or endoscopic factors? Endoscopy. 2006;38:726-29.
- [CrossRef] [PubMed] [Google Scholar]
- Transcatheter arterial embolization versus surgery for refractory non-variceal upper gastrointestinal bleeding: A meta-analysis. World J Emerg Surg. 2019;14:3.
- [CrossRef] [PubMed] [Google Scholar]
- Emergency transcatheter arterial embolization for patients with acute massive duodenal ulcer hemorrhage. World J Gastroenterol. 2012;18:4765-70.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Risk factors determining the need for second-look endoscopy for peptic ulcer bleeding after endoscopic hemostasis and proton pump inhibitor infusion. Endosc Int Open. 2016;4:E255-262.
- [CrossRef] [PubMed] [Google Scholar]
- Evidence-based clinical practice guidelines for peptic ulcer disease 2015. J Gastroenterol. 2016;51:177-94.
- [CrossRef] [PubMed] [Google Scholar]
- Asia-Pacific Working Group consensus on non-variceal upper gastrointestinal bleeding. Gut. 2011;60:1170-7.
- [CrossRef] [PubMed] [Google Scholar]
- Embolization of acute nonvariceal upper gastrointestinal hemorrhage resistant to endoscopic treatment: Results and predictors of recurrent bleeding. Cardiovasc Intervent Radiol. 2010;33:1088-1100.
- [CrossRef] [PubMed] [Google Scholar]
- Transcatheter arterial embolization compared with surgery for uncontrolled peptic ulcer bleeding: A population-based cohort study. Ann Surg. 2019;269:304-9.
- [CrossRef] [PubMed] [Google Scholar]
- Arterial embolotherapy for endoscopically unmanageable acute gastroduodenal hemorrhage: Predictors of early rebleeding. Clin Gastroenterol Hepatol. 2009;7:515-23.
- [CrossRef] [PubMed] [Google Scholar]
- Endoscopic hemostasis followed by preventive transarterial embolization in high-risk patients with bleeding peptic ulcer: 5-year experience. World J Emerg Surg. 2019;14:45.
- [CrossRef] [PubMed] [Google Scholar]
- Upper gastrointestinal hemorrhage and transcatheter embolotherapy: Clinical and technical factors impacting success and survival. J Vasc Interv Radiol. 2001;12:1263-71.
- [CrossRef] [PubMed] [Google Scholar]
- Early angiographic embolization is more effective than delayed angiographic embolization in patients with duodenal ulcer bleeding. J Gastroenterol Hepatol. 2012;27:1670-4.
- [CrossRef] [PubMed] [Google Scholar]
- Relationship between angiography timing and angiographic visualization of extravasation in patients with acute non-variceal gastrointestinal bleeding. BMC Gastroenterol. 2020;20:426.
- [CrossRef] [PubMed] [Google Scholar]
- A comparison of the results of arterial embolization for bleeding and non-bleeding gastroduodenal ulcers. Acta Radiol. 2011;52:1076-82.
- [CrossRef] [PubMed] [Google Scholar]







