Translate this page into:
Extra-pituitary Cerebral Anomalies in Pediatric Patients of Ectopic Neurohypophysis: An Uncommon Association
-
Received: ,
Accepted: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Context:
Ectopic neurohypophysis (EN) refers to an interrupted, nonvisualized, and thinned out pituitary stalk with ectopic location of the posterior pituitary gland. Concurrent extra-pituitary cerebral and extra-cranial anomalies have been rarely reported in patients of EN.
Aim:
The aim of this study was to evaluate the magnetic resonance imaging (MRI) findings of extra-pituitary cerebral anomalies in pediatric patients of EN.
Settings and Design:
A hospital-based cross-sectional study was conducted in a tertiary care center.
Subjects and Methods:
The study group comprised eight pediatric patients of EN associated with extra-pituitary cerebral or vascular anomalies. Clinical and biochemical assessment was done in all patients.
Results:
Out of the total eight patients with EN, MRI showed interrupted pituitary stalk in five patients (62.5%) and nonvisible pituitary stalk in three patients (37.5%). Ectopic posterior pituitary bright spot was demonstrated in median eminence in six patients (75%), faintly visualized in one patient (12.5%) and nonvisualized in another one patient. Statistical significant association was noted between pituitary gland height and patient's body height with the pituitary gland volume (P < 0.001). Varied extra-pituitary cerebral anomalies encountered in our patients ranged from isolated anomalies such as optic nerve hypoplasia in three patients (37.5%), corpus callosum dysplasia in four patients (50%), agyria-pachygyria complex in two patients (25%), and intracranial vascular anomalies in two patients to syndromic association of tuberous sclerosis in one patient.
Conclusion:
Identifying and reporting of associated extra-pituitary cerebral anomalies in patients with EN are crucial in assessing the overall neurological outcome of such patients.
Keywords
Magnetic resonance imaging
pituitary stalk interruption
posterior pituitary

Introduction
Ectopic neurohypophysis (EN) is characterized by a very thin or interrupted pituitary stalk, hypoplasia or aplasia of anterior pituitary gland, and an ectopic (or absent) posterior pituitary gland. It is also referred as pituitary stalk interruption syndrome. Magnetic resonance imaging (MRI) had been the standard investigation of choice in depicting the anatomical abnormalities of the pituitary gland and hypothalamus.[1] This rare entity of EN has an estimated incidence of 0.5/1,000,000 births.[2]
Like other syndromes, pituitary stalk interruption syndrome is also but rarely associated with a spectrum of midline, extra-pituitary cerebral, vascular, or renal abnormalities. Patients with this syndrome have been reported with variable pituitary endocrine disorders ranging from isolated growth hormone deficiency (IGHD), combined pituitary hormonal deficiency (CPHD), and even panhypopituitarism.[3] These varying degree of anterior pituitary hormonal deficiencies in patients with EN present with a wide range of clinical features from growth failure, central hypothyroidism to underdeveloped secondary sexual characteristics.[4] In EN, the posterior pituitary function is usually preserved.[5] Occasionally, the posterior pituitary hormonal functions may be disturbed depending on the position of the ectopic posterior pituitary gland.[5] The etiology for the development of EN is unclear; however, traumatic birth injury, breech delivery, and genetic factors have been considered.[67]
In this study, we aim to evaluate the associated cerebral anomalies in patients with EN on MRI. The objectives of this study are to describe in detail and classify these extra-pituitary anomalies, correlate them with the clinical features and neurological status of such patients, and discuss the pathogenesis of such anomalies coexisting with EN while reviewing related literature.
Subjects and Methods
After approval from the Institutional Ethics Review Committee, a hospital-based cross-sectional study was conducted. The study group comprised eight pediatric patients presenting to the departments of radiodiagnosis, pediatrics neurology, and endocrinology in a tertiary care center in between March 2015 and September 2016.
Patient selection
Both out- and in-patients of pediatric age group with features of dwarfism, hormonal imbalances, and clinical suspicion of pituitary anomalies were included in this study. Patients with contrast sensitivity or poor renal function were excluded as only contrast-enhanced dynamic MRI studies have been included. Informed consent was obtained from patients/parents/guardian before undergoing MRI scan. Medical records of all patients were prospectively evaluated and analyzed. Besides the chronological age of patients and clinical presentation, history of parental consanguinity, antenatal and perinatal history, and family history of short stature were documented with other biochemical and hormonal parameters.
In this study, only pediatric patients of EN and associated extra-pituitary cerebral anomaly were included. Those patients with isolated EN without associated extra-pituitary cerebral or vascular anomalies were excluded. Neuro-endrocrinological follow-up was done in all eight patients.
Magnetic resonance imaging protocols
The MRI of brain was obtained in every patient using axial T1-weighted image (WI), T2WI, fluid-attenuated inversion recovery (FLAIR), susceptibility-weighted images (SWIs), diffusion-weighted images sequences using 5 mm slice thickness. Sagittal T1WI, coronal T1WI, and coronal T2WI sequences were obtained with 3 mm slice thickness. Initial nonfat-saturated dynamic pituitary imaging was done in T1WI coronal plane after intravenous administration of 0.1 mmol/kg gadolinium followed by fat suppressed T1W postcontrast images in all three planes with 3 mm slice thickness. Postcontrast sagittal plane magnetization-prepared rapid gradient-echo (MPRAGE) images were also obtained.
Axial T2WIs were obtained with echo time (TE): 80–95, repetition time (TR): 3800–4000, FLAIR-weighted images with TE: 90–95, TR: 9000, and inversion time (TI): 2500 and T1WI with TE: 7–9 and TR: 450–500. Sagittal T1WIs were obtained with TE: 7–9, TR: 450–500, and field of view (FOV) of 230. Coronal T2WIs were obtained with TE: 80–95, TR: 3800–4000, and FOV of 230. Postcontrast dynamic pituitary imaging was obtained repetitively 7–10 times for pituitary gland in T1WI coronal plane with TE: 10–11, TR: 330–340, and FOV of 200–210 with slice thickness of 3 mm.
3D T1-weighted MPRAGE images were obtained with TE: 3.37, TR: 1900, inversion time: 1100, pixel bandwidth: 130, flip angle: 15°, FOV: 220–260, matrix: 224 × 256 with slice thickness of 1 mm.
A 3D-multislab time of flight angiogram (TOF) of cerebral arteries was done in two of our patients after suspicious vascular anomalies spotted on the routine sequences all patients with FOV of 200–220.
To obtain pituitary volumes, targeted sagittal and coronal reformatted images with 1 mm slice thickness were obtained from the 3D set of MPRAGE sequence.
Evaluation
All patients were evaluated clinically for chronological age, height, and sexual development.
Short stature was considered when patient's height was below the third percentile for the child's age according to the standard growth charts for the age and height.[8] Plain radiographs were obtained in all patients for their bone age determination. The plasma levels of growth hormone, prolactin, luteinizing hormone, follicular-stimulating hormone, thyrotropin (T3 and T4), thyroid-stimulating hormone, cortisol, and testosterone were documented.
The MRI of all pediatric patients with EN was evaluated with regard to status of pituitary stalk, thin, or interrupted stalk, location of T1WI bright spot of posterior pituitary gland, and morphology of anterior pituitary gland like hypoplasia or aplasia. Height of anterior pituitary gland was measured. Associated extra-pituitary anomalies of cerebral, cerebellar hemispheres, optic nerves, optic chiasma, and cerebral arteries were noted.
The height and anterior-posterior diameter or length of anterior pituitary gland was measured from the midline sagittal MRI images while width was measured on coronal MPRAGE images. The volume of anterior pituitary gland was derived using the formula for ellipsoid ([length × width × height]/2). This indirect way of pituitary gland volume measurement was used by the most of the previous researchers.[4]
Statistical analysis
Data were presented in terms of mean and percentage. One sample t-test was used for pituitary volume and paired t-test was used for assessing the significance of association between the height of pituitary gland and pituitary volume. Calculations were done using Statistical Package for the Social Science (version 16, SPSS Inc., Chicago, USA).
Results
The mean age of our study sample was 7.37 years ± 3.96 (standard deviation [SD]) with a male:female ratio of 1.7:1. Five of our patients presented with delayed developmental milestones and two with proportionate short stature. Plain radiographs in all eight patients showed delayed bone age with respect to their chronological ages.
Significant perinatal history was documented among all our cases with three patients (37.5%) having positive history of birth asphyxia, three patients (37.5%) born as intrauterine growth retardation, and two patients (25%) with a history of breech delivery. Hormonal analysis showed IGHD in four patients (50%) and CPHD in the rest. Short stature was noted in two of our patients of CPHD. The MRI revealed interrupted pituitary stalk in five patients (62.5%) and nonvisible pituitary stalk in three patients (37.5%). T1WI hyperintense posterior pituitary bright spot was demonstrated in median eminence in six patients (75%), faintly visualized in one patient (12.5%), and nonvisualized in another one patient (12.5%).
The mean height of anterior pituitary gland was 2.64 mm ± 0.85 (SD), where five patients (62.5%) had anterior pituitary height <3 mm. The volume of anterior pituitary gland varied from 28.56 mm3 to 120.6 mm3 with a mean volume of 71.25 mm3 ± 3.58 (SD). The pituitary gland volumes in our study sample were significantly smaller (P = 0.001) than published norms according to age keeping same standards in the method of calculation.[9] There was a statistically significant correlation between the height of pituitary gland and anterior pituitary gland volume with P = 0.001. Statistical significance was also obtained between the patient's body height and volume of pituitary gland with P = 0.009.
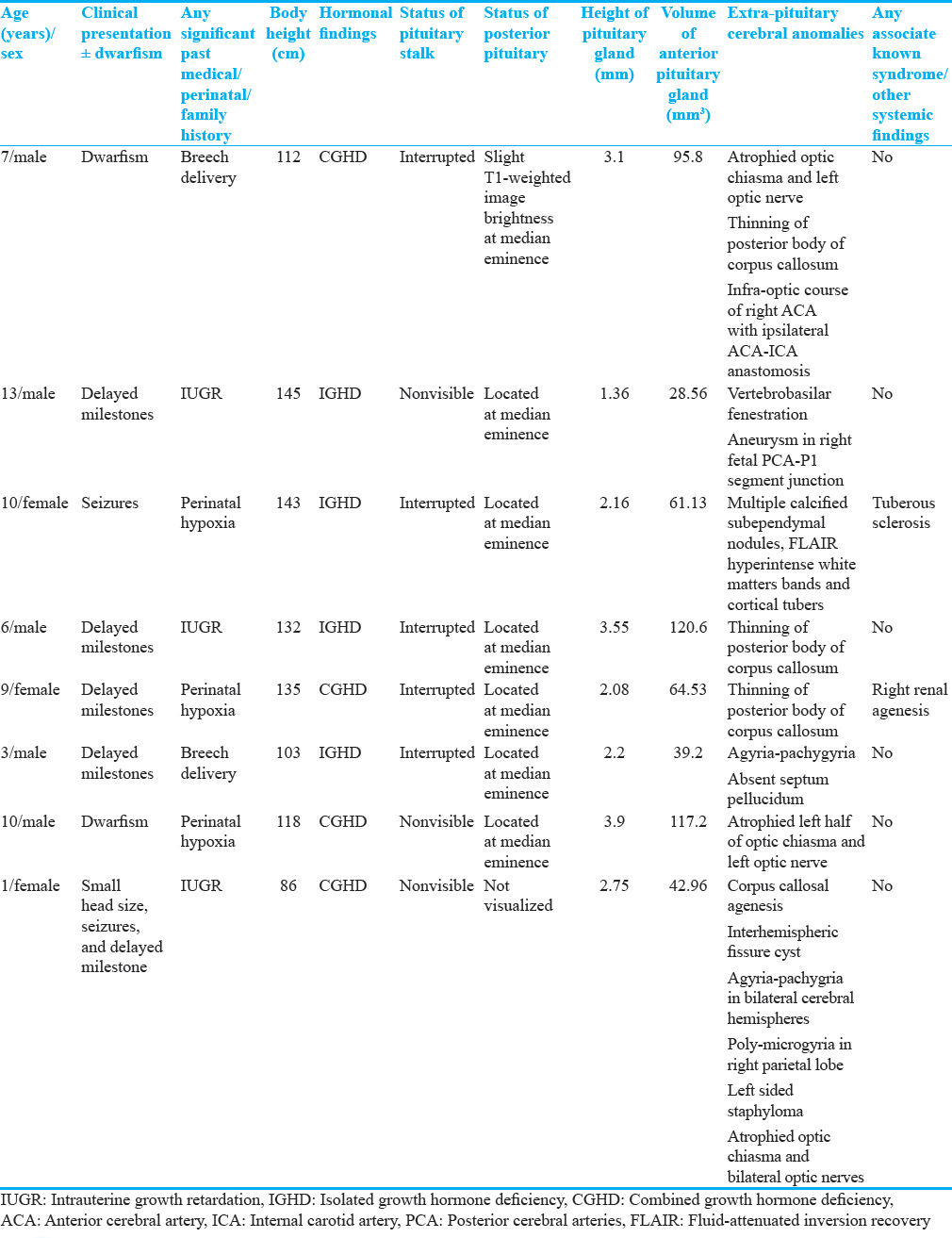
Uncommon extra-pituitary cerebral anomalies were identified as optic chiasma and optic nerve hypoplasia [Figure 1] in three patients (37.5%), agenesis or dysplasia of corpus callosum [Figure 2] in four patients (50%), agyria-pachygyria complex [Figures 3 and 4] in two patients (25%), and tuberous sclerosis [Figure 5], absent septum pellucidum [Figure 3], and polymicrogyria [Figure 4] in one patient each (12.5%). Staphyloma was noted in only one patient (12.5%) [Figure 4].
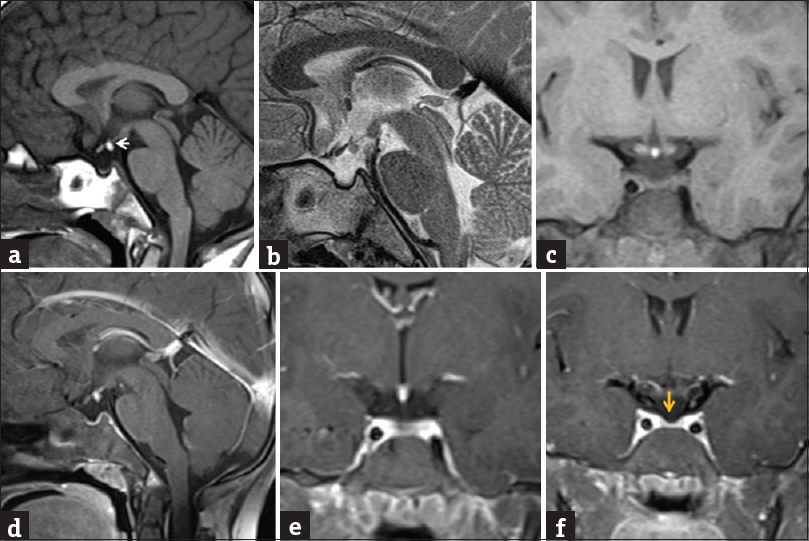
- A 13-year-old dwarf male presented with delayed developmental milestone. Noncontrast sagittal T1-weighted image, sagittal T2-weighted image, and coronal T1-weighted images (a-c) showed invisible pituitary stalk with ectopic location of T1-weighted image bright and T2-weighted image hypointense posterior pituitary in lower most portion of median eminence (←arrow). Postgadolinium fat-saturated sagittal T1-weighted image and coronal T1-weighted images (d-f) showed nonvisualized pituitary stalk with thinned out anterior pituitary gland (yellow ↓ arrow).
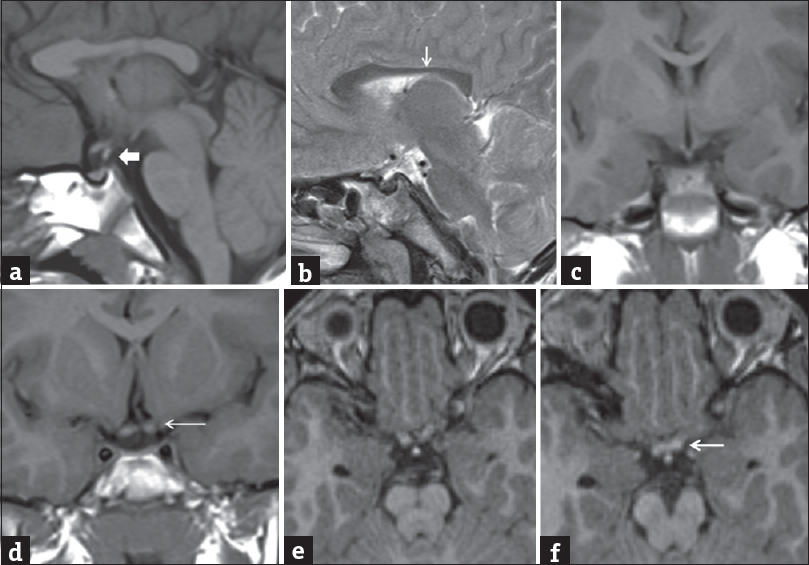
- A 7-old-year male presented with delayed developmental milestone with seizures. Noncontrast sagittal T1-weighted image (a) and sagittal T2-weighted image (b) showed interrupted pituitary stalk (block ← arrow) with faintly visualized slight T1-weighted image hyperintense posterior pituitary bright spot in median eminence. Thinning and irregular contour of posterior body of corpus callosum were noted (↓arrow). Noncontrast coronal T1-weighted images (c and d) and axial magnetization-prepared rapid gradient-echo (e and f) showed atrophied optic chiasma and left optic nerve (←arrow).
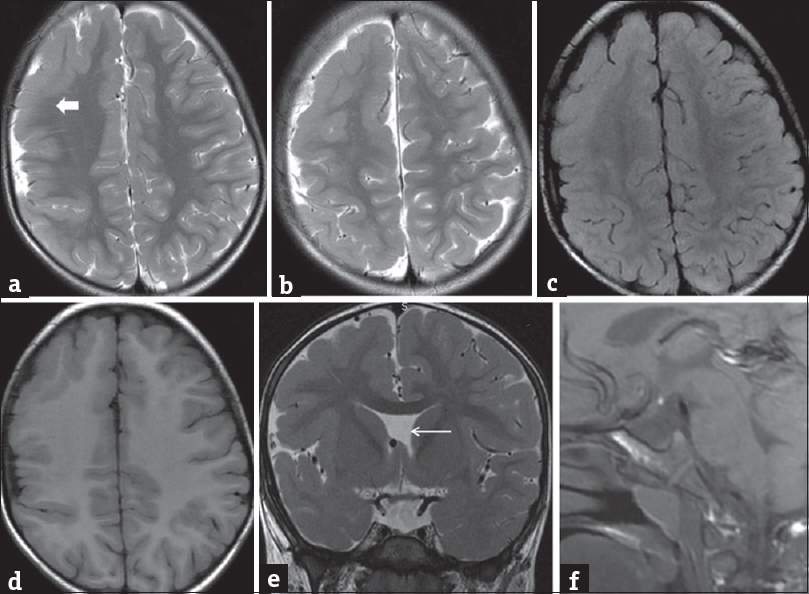
- A 3-year-old male patient presented with repeated episodes of seizures. Axial T2-weighted image (a and b), fluid-attenuated inversion recovery (c), and T1-weighted (d) images showed agyria-pachygyria along the right cerebral hemispheric cortices with indistinct gray-white matter differentiations (block ← arrow). Coronal T2-weighted image (e) showed the absence of septum pellucidum (←arrow). Sagittal T1-weighted image (f) showed invisible pituitary stalk with ectopic T1 bright posterior pituitary in median eminence.

- A 1-year-old female presented with delayed developmental milestone. Axial T2-weighted (a-c) and coronal T2-weighted (d) images showed left orbital globe staphyloma (thin white ← arrow) with corpus callosal agenesis with a larger interhemispheric fissure cyst. Agyria-pachygria noted in bilateral cerebral cortices (thicker white ← arrow) with polymicrogyria in the right occipital lobe (block → arrow). Coronal T1-weighted image (e) and sagittal T1-weighted image (f) images show interrupted pituitary stalk with nonvisualized posterior pituitary T1 bright spot in normal or ectopic location (red ← arrow).

- A 10-year-old female patient presented with repeated episodes of seizures. Axial fluid-attenuated inversion recovery (a) image showed abnormal fluid-attenuated inversion recovery hyperintense white matters lesions in bilateral deep periventricular white matters with focal cortical tuber in left parietal lobe. T1-weighted image (b) shows T1 isointense to slight hyperintense subependymal nodules (←arrow), showed blooming on susceptibility weighted imaging (c) (yellow ← arrow) suggesting calcified subependymal nodules of tuberous sclerosis. Fat saturated sagittal T1-weighted image and coronal T1-weighted images (d-f) showed interrupted pituitary stalk (block ← arrow) and ectopic neurohypophysis in median eminence.
Rare vascular anomalies were noted in two of our patients (25%) with EN. One patient had infraoptic course of right anterior cerebral artery (ACA) arising from cavernous segment of the right internal carotid artery (ICA) associated with coexistence of normal ipsilateral A1 segment of right ACA [Figure 6]. Another patient had vertebrobasilar fenestration with bilateral fetal posterior cerebral arteries (PCA) with a lobulated aneurysm at the junction of right fetal PCA – hypoplastic P1 segment of right PCA [Figure 7].

- Volume rendering images (a and b) and source images (c and d) of time of flight angiogram showed infra-optic course of the right anterior cerebral artery, which arises from the medial aspect of cavernous segment of right internal carotid artery (↑arrow) associated coexistence of normal ipsilateral A1 segment of right anterior cerebral artery which representing anomalous anastomosis between internal carotid artery and anterior cerebral artery (same patient of Figure 3).

- Source image (a) of time of flight angiogram showed fenestration in vertebrobasilar junction (thin ↑ arrow). Volume rendering images (b and c) showed vertebrobasilar fenestration (←arrow) with fetal origin of bilateral posterior cerebral artery and a lobulated aneurysm at the junction of right fetal posterior cerebral artery-hypoplastic P1 segment of right posterior cerebral artery (block ← arrow) (same patient of Figure 1).
Table 1 shows the detailed MRI findings in eight pediatric patients of EN and associated extra-pituitary anomalies.
Discussion
The anterior and posterior pituitary glands are different from each other both embryologically and histologically. Embryologically, anterior pituitary grows upward from the ectoderm of the stomodeum (Rathke's pouch), whereas the posterior pituitary grows as a downward evagination from neuroectoderm of the diencephalon.[10] Hypothalamus controls the endocrine function of pituitary gland through the hypothalamic-hypophyseal portal axis.[6] Normally, posterior pituitary gland is easily identified as a hyperintense bright spot on T1W MR images. The presence of phospholipids containing the arginine vasopressin-neurophysin complex in neurosecretory vesicles of posterior pituitary gland is attributed for the T1WI bright signal.[10] In normal subjects, the presence of T1WI posterior pituitary bright signal frequency varies from 90% to 100%[7] and to as low as 63%.[11] Age-related decline in the rate of posterior pituitary T1WI bright signal on MRI is approximately 1% per year between 7 months and 85 years of age.[11]
There are various theories describing the etiopathogenesis of EN though the exact cause remains unestablished. While some opine that EN could result from defective neuronal migration during embryogenesis, another possible pathophysiologic mechanism cited is transection of the pituitary stalk due to some injury. Injuries to pituitary stalk can be from vascular insults, perinatal anoxia, or compression injuries which also result in reorganization of the proximal neurons of the posterior pituitary gland.[12]
EN or pituitary stalk interruption syndrome was first reported by Fujisawa et al., in 1987.[13] EN has a male predominance pointing toward X-linked inheritance.[45] It has been generally reported as sporadic cases and in majority of the cases, the etiology is unknown.[6714]
In EN, normal T1WI bright spot is absent in the sella and located at the median eminence or along interrupted infundibular stalk. Differentiation of T1WI bright EN from dermoid tumor or lipoma can be confirmed by obtaining fat suppressed T1W MR images.[1516]
Previous literatures reported various extra-pituitary cerebral anomalies in patients of EN as craniofacial dysmorphism, central maxillary incisor tooth, septo-optic dysplasia, optic nerve hypoplasia, corpus callosal dysgenesis, vascular anomalies, cellular migration disorder, persistent craniopharyngeal canal, Kallmann syndrome, microcephaly, cerebellar atrophy, vermian dysplasia, Chiari malformation type-1, ophthalmic abnormality, Pallister-Hall syndrome, Dubowitz syndrome, and Worster-Drought syndrome.[451114171819] However, in our limited sample, we did not find any case with posterior fossa or facial anomalies. Previous literatures suggested coexistence of EN with periventricular heterotopias because of sharing a common underlying causative mechanism of abnormal neuronal migration.[20] The presence of pachy/poly/micro-gyria complex as associated anomaly in 3 of our patients also indirectly supports the theory of neuronal migration and cleavage anomaly as plausible explanation in the etiopathogenesis for EN.
Growth hormone deficiency (GHD) is a common endocrine cause of short stature. The occurrence of IGHD has been estimated at one per 4000 persons.[19] GHD may be idiopathic or due to secondary causes such as tumor, surgery, or irradiation to the sellar region. Although neonatal hypoglycemia has been commonly described in association with EN,[1921] in our sample, we did not encounter any case with a history of hypoglycemia. Nonvisualization of the pituitary stalk is associated with variable degree of lack of anterior pituitary function; however, some anterior pituitary function had always been reported to be preserved.[12] In EN, posterior pituitary hormone deficiency appears to be uncommon suggesting that this ectopically located neurohypophyseal tissue functions normally to produce antidiuretic hormone.[22] This is also reflected in the MRI findings as preserved signal of posterior pituitary in majority (6 out of 8) of our patients.
In our study, four patients with CPHD had more extra-pituitary cerebral abnormalities than those with only IGHD, in keeping with similar findings obtained by Ultmann et al., and Hamilton et al.[1219] EN has been found in up to 43% of children presenting with short stature with GHD[2324] while in our study sample, two patients of EN had CPHD with proportionate short stature.
Some studies on the contrary have proposed perinatal hypoxia or breech delivery as a consequence rather than a cause of EN.[24] In our study sample, 3 patients had a history of perinatal hypoxia and two had a history of breech delivery.
Recent studies favor a genetic basis for EN and are supported by rare familial causes of EN.[1825] We did not find any positive family history among our patients.
To the best of our knowledge, no previous literature previously reported an association of EN with tuberous sclerosis, but we have encountered a patient of EN with tuberous sclerosis.
Rare vascular anomaly like infra-optic course of ACA with ipsilateral ACA-ICA anastomosis was encountered in 1 of our patient with EN, previous literature described association of EN or GHD with aplasia or hypoplasia of ICA and its branches.[26]
In long-standing patients of idiopathic central diabetes insipidus, T1WI posterior pituitary bright signal intensity is absent, which occurs due to failure to synthesize, transport, or store neurosecretory granules in posterior pituitary gland. Thickening of infundibular stalk is usually found in patients of central diabetes insipidus.[27] In our study sample, 1 patient of EN showed moderate loss of posterior pituitary T1WI bright signal while in another, the posterior pituitary T1WI bright signal was nonvisible. While pituitary stalk is not visualized in patients with pituitary stalk transection and in patients with chronic central diabetes insipidus, postcontrast MRI shows normal enhancement along pituitary stalk.
Limitation
Our study sample included only eight patients of EN with concomitant extra-pituitary cerebral anomalies. We look forward doing a prospective study in the future including a larger sample size.
Conclusion
In the present study, the association of extra-pituitary cerebral anomalies in patients of EN have been highlighted which support and emphasize the genetic cause and primary neuronal migration abnormalities involved with this rare disorder rather than traumatic or vascular accidents as the possible etiology.
Radiologist and clinicians should be cautious to look for features of associated extra-pituitary anomalies during evaluation of patients with EN, which are actually significant to determine the overall neurological outcome of these patients and explain further neurological signs and symptoms.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Available FREE in open access from: http://www.clinicalimagingscience.org/text.asp?2017/7/1/19/206660
References
- MR imaging of the pituitary stalk: Size, shape, and enhancement pattern. AJR Am J Roentgenol. 1992;159:375-7.
- [Google Scholar]
- 17q21.31 microdeletion in a patient with pituitary stalk interruption syndrome. Eur J Med Genet. 2011;54:369-73.
- [Google Scholar]
- Long-term evolution of endocrine disorders and effect of GH therapy in 35 patients with pituitary stalk interruption syndrome. Horm Res. 2005;64:266-73.
- [Google Scholar]
- Hypothalamic-pituitary dysfunction in growth hormone-deficient patients with pituitary abnormalities. J Clin Endocrinol Metab. 1991;73:79-83.
- [Google Scholar]
- Pituitary stalk interruption syndrome: A clinical-biological-genetic assessment of its pathogenesis. J Clin Endocrinol Metab. 1997;82:3450-4.
- [Google Scholar]
- Hypopituitarism and other disorders of the growth hormone-insulin like growth factor-1 axis. In: Lifshitz F, ed. Pediatric Endocrinology. New York: Informa Healthcare USA Inc; 2007. p. :66-99.
- [Google Scholar]
- Disorders of growth hormone/insulin like growth factor secretion and action. In: Sperling M, ed. Pediatric Endocrinology. Philadelphia: Saunders; 2008. p. :254-334.
- [Google Scholar]
- 2002. National Center for Health Statistics. National Health and Nutrition Examination Survey. Clinical Growth Charts. Hyattsville, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; Available from: http://www.cdc.gov/nchs/about/major/nhanes/growthcharts/clinical_charts.htm
- In vivo assessment of pituitary gland volume with magnetic resonance imaging: The effect of age. J Clin Endocrinol Metab. 1990;71:505-8.
- [Google Scholar]
- Histochemical characterisation and functional significance of the hyperintense signal on MR images of the posterior pituitary. Am J Neuroradiol. 1989;10:1079-83.
- [Google Scholar]
- Developmental disorders of the hypothalamus and pituitary gland associated with congenital hypopituitarism. Best Pract Res Clin Endocrinol Metab. 2008;22:191-206.
- [Google Scholar]
- Pituitary stalk and ectopic hyperintense T1 signal on magnetic resonance imaging. Implications for anterior pituitary dysfunction. Am J Dis Child. 1993;147:647-52.
- [Google Scholar]
- Transection of the pituitary stalk: Development of an ectopic posterior lobe assessed with MR imaging. Radiology. 1987;165:487-9.
- [Google Scholar]
- Developmental abnormalities of the posterior pituitary gland. Endocr Dev. 2009;14:83-94.
- [Google Scholar]
- Molecular effects of novel mutations in Hesx1/HESX1 associated with human pituitary disorders. Development. 2001;128:5189-99.
- [Google Scholar]
- Posterior pituitary ectopia: Another hint toward a genetic etiology. AJNR Am J Neuroradiol. 2000;21:1116-8.
- [Google Scholar]
- Genetics of growth hormone deficiency. J Clin Res Pediatr Endocrinol. 2009;Suppl 1:8-14.
- [Google Scholar]
- Growth hormone deficiency with ectopic neurohypophysis: Anatomical variations and relationship between the visibility of the pituitary stalk asserted by magnetic resonance imaging and anterior pituitary function. J Clin Endocrinol Metab. 1999;84:2408-13.
- [Google Scholar]
- MR imaging in idiopathic growth hormone deficiency. AJNR Am J Neuroradiol. 1998;19:1609-15.
- [Google Scholar]
- Ectopic posterior pituitary lobe and periventricular heterotopia: Cerebral malformations with the same underlying mechanism? AJNR Am J Neuroradiol. 2002;23:1475-81.
- [Google Scholar]
- Evidence of a congenital midline brain anomaly in pituitary dwarfs: A magnetic resonance imaging study in 101 patients. Pediatrics. 1994;93:409-16.
- [Google Scholar]
- Frequency and variation of the posterior pituitary bright signal on MR images. AJNR Am J Neuroradiol. 1989;10:943-8.
- [Google Scholar]
- Clinical, radiographic, and pathological features of symptomatic Rathke's cleft cysts. J Neurosurg. 1991;74:535-44.
- [Google Scholar]
- Pituitary cysts in childhood evaluated by MR imaging. AJNR Am J Neuroradiol. 2005;26:2144-7.
- [Google Scholar]
- MR findings in growth hormone deficiency: Correlation with severity of hypopituitarism. AJNR Am J Neuroradiol. 1998;19:1495-9.
- [Google Scholar]
- Aplasia of right internal carotid artery and hypopituitarism. Pediatr Radiol. 1999;29:586-8.
- [Google Scholar]
- Posterior lobe of the pituitary in diabetes insipidus: MR findings. J Comput Assist Tomogr. 1987;11:221-5.
- [Google Scholar]






