Translate this page into:
Enhancement patterns in contrast mammography – A pictorial essay

*Corresponding author: Johannes Peters, Department of Surgery, Royal Darwin Hospital, Darwin, Australia. johannesp99@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Peters G, Lynch AM, Peters J. Enhancement patterns in contrast mammography – A pictorial essay. J Clin Imaging Sci 2021;11:63.
Abstract
Contrast-enhanced mammography (CEM) is a new technology in breast imaging and cancer detection. It has been shown to have a comparable performance to breast MRI. Currently, there is no independent BI-RADS lexicon available for CEM. This pictorial essay will demonstrate the use of breast MRI descriptors according to the BI-RADS breast MRI lexicon, to describe enhancement patterns for recombined CEM images. The authors recommend using enhancement pattern descriptors already in use for breast MRI when reporting CEM studies, to promote uniformity of interpretation and reporting.
Keywords
Contrast enhanced mammography
Contrast mammography
Mammography
INTRODUCTION
Contrast-enhanced mammography (CEM) is a new technology in breast imaging. The accuracy in detection of breast cancer in CEM is greater than conventional mammography, ultrasound (US) alone, and the US in combination with conventional mammography.[1] With a sensitivity of 93-98% and specificity of greater than 90% for detection of breast cancer, CEM has comparable performance to that of breast MRI.[1-5]
TECHNIQUE
CEM uses a dual-energy X-ray technique combined with intravenous administration of an iodinated contrast agent. 100 mL of non-ionic low-osmolar iodinated contrast agent: iohexol 350 mg I/mL Omnipaque (GE Healthcare) is injected two minutes prior to the acquisition of paired low-energy (23–32kVp) and high-energy (45–49kVp) standard mammogram images using a power injector at a rate of 3 mL/s.[5,6] Low-energy images have appearances similar to conventional digital mammograms and are used for unenhanced image interpretation. Recombined images are created by subtracting the low-energy images from high-energy images, allowing signal from the background breast tissue to be canceled out and areas of contrast uptake to be highlighted.[6] The optimal window for obtaining contrast-enhanced images of the breast is between 2 and 8 min post-contrast agent injection. During this imaging window, a standard bilateral two-view mammogram is obtained. Several breast pathologies such as lobular cancers, mucinous, tubular cancers, and ductal carcinoma in situ show characteristically slow contrast enhancement patterns.[7] For this reason, the authors suggest adding a minimum of two additional views (MLO views) at the end of the imaging acquisition as “late phase images” to check for breast pathology with slow enhancement patterns.
The displayed images were acquired and processed using Hologic Selenia® Dimensions® I-View software.
The average glandular dose of CEM has been reported between 1.2 to 1.8 times of a full-field digital mammogram.[8]
These values still fall below the guidelines recommended 3mGy average glandular dose exposure for breast imaging.[5]
ENHANCEMENT PATTERNS IN CONTRAST MAMMOGRAPHY
There is no independent BI-RADS lexicon for CEM available at present. The authors suggest that breast MRI descriptors according to the BI-RADS breast MRI lexicon are used to describe enhancement patterns for the recombined CEM images [Table 1].[9]
| Masses | |
|---|---|
| Shape |
|
| Margins |
|
| Internal Enhancement characteristics |
|
| Non-mass enhancement | |
| Distribution |
|
| Internal enhancement |
|
| Focus | A tiny dot of enhancement that does not clearly represent a space-occupying lesion or mass and does not clearly show a mass on pre-contrast imaging |
Enhancement Patterns of Masses:

- 38-year-old woman presented with a palpable lump in the right upper inner breast. Mammography including CEM was performed for further workup. (a) Right craniocaudal low energy image showed a well circumscribed oval mass lesion. (b) A right craniocaudal recombined image (CEM) showed a mass with homogeneous enhancement, a mammographic biopsy under tomosynthesis guidance confirmed a fibroadenoma on histopathology.

- 51-year-old woman with a palpable lump in the left outer central breast. Mammography including CEM was performed for further workup. (a) A left mediolateral oblique and (b) left craniocaudal low energy image showed a round mass lesion with irregular margins. (c) The left mediolateral oblique recombined and (d) left craniaocaudal CEM images showed a mass with heterogeneous contrast enhancement. The lesion was a Grade 3 invasive ductal, basal phenotype cancer on excisional biopsy.

- 80-year-old woman with a palpable lump in the left upper outer breast. (a) The left mediolateral oblique low energy image revealed an irregular mass lesion with spiculated margins. (b) The left mediolateral oblique early phase recombined image displayed a mass with rim enhancement (c) The late phase of the left mediolateral oblique recombined image showed centripetal enhancement, highly suggestive of a malignant process. Lumpectomy of the lesion confirmed an invasive ductal carcinoma not otherwise specified, grade 3 on histopathology.

- A 40-year-old woman presented with lumpy breasts and underwent a mammography study including CEM. (a) The right mediolateral oblique and (b) craniocaudal recombined mammographic images showed a mass with subtle rim enhancement. (c) The mass with subtle rim enhancement seen on contrast mammography corresponds to a cyst on ultrasound. This was confirmed with an ultrasound guided cyst aspiration of the lesion which collapsed entirely after aspiration.
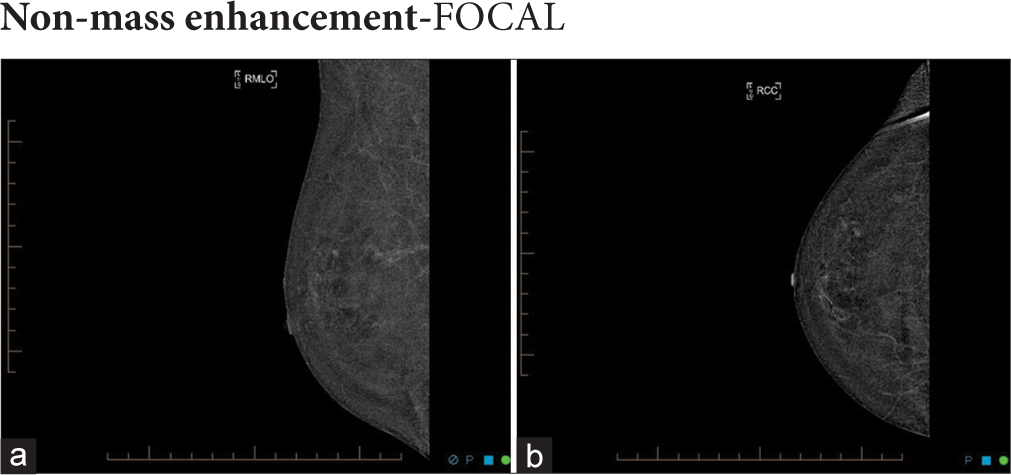
- 50-year-old woman with right breast pain. (a) The right mediolateral oblique and (b) craniocaudal recombined images show no suspicious mass or non-mass enhancement. There is a tiny focus of contrast uptake in the right upper outer breast only, too small for further characterization.

- A 57-year-old woman presented with left bloody nipple discharge. (a) Left mediolateral oblique and (b) craniocaudal recombined images showed smooth linear non-mass enhancement with homogeneous internal enhancement pattern. The lesion was confirmed to be an intraductal papilloma on core biopsy.

- 66-year-old patient presented for a screening mammogram. She had a strong family history of breast cancer. The (a) right mediolateral oblique low energy image showed pleomorphic microcalcification in segmental distribution. (b) Right mediolateral oblique low energy image: Pleomorphic microcalcification in segmental distribution, magnified. (c) Right mediolateral oblique recombined image: Segmental non-mass enhancement with clustered ring internal enhancement pattern, Histopathology: High grade DCIS.

- A 77-year-old woman presented with discomfort in the left inner breast. (a) The left craniocaudal low energy image showed pleomorphic microcalcification in regional distribution. (b) Left craniocaudal low energy magnification image: Pleomorphic microcalcification in regional distribution, magnified. (c) Left craniocaudal low energy view, magnified: Pleomorphic microcalcification in regional distribution.

- 39-year-old patient with strong family history of breast cancer. A bilateral mammogram including CEM was performed. (a) The left and (b) right mediolateral oblique recombined CEM images showed multiple regions of non-mass enhancement with non-specific morphological features, similar in both breasts in keeping with normal, moderate background enhancement.

- 42-year-old woman with right breast pain. (a) Right mediolateral oblique and (b) craniocaudal recombined CEM images show diffuse non-mass enhancement in keeping with mild to moderate, normal background enhancement.
CONCLUSION
It is important to recognize enhancement patterns in CEM. This pictorial essay provides examples and guidance on how to classify these patterns. We recommend using enhancement pattern descriptors already in use for Breast MRI when reporting CEM studies, to promote uniformity of interpretation and reporting.
Declaration of patient consent
Patients consent not required as patients identities are not disclosed or compromised.
Financial Support and Sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Dual-energy contrast-enhanced digital mammography: Initial clinical results of a multireader, multicase study. Breast Cancer Res. 2012;14:R94.
- [CrossRef] [PubMed] [Google Scholar]
- Contrast-enhanced spectral mammography vs. mammography and MRI-clinical performance in a multi-reader evaluation. Eur Soc Radiol. 2017;27:2752-64.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical utility of contrast-enhanced spectral mammography as an adjunct for tomosynthesis-detected architectural distortion. Clin Imaging. 2017;46:44-52.
- [CrossRef] [PubMed] [Google Scholar]
- Assessing tumor extent on contrast-enhanced spectral mammography versus full-field digital mammography and ultrasound. Clin Imaging. 2017;46:78-84.
- [CrossRef] [PubMed] [Google Scholar]
- Breast cancer: Update on imaging modalities. S Afr Radiogr. 2020;58:33-6. Available from: https://sar.org.za/index.php/sar/article/view/589/419 [Last accessed on 2021 Sep 12]
- [Google Scholar]
- Contrast-enhanced mammography: A systematic guide to interpretation and reporting. AJR Am J Roentgenol. 2019;212:222-31.
- [CrossRef] [PubMed] [Google Scholar]
- Large non-enhancing breast cancer on breast magnetic resonance imaging: A case report. Cureus. 2018;10:e2332.
- [CrossRef] [Google Scholar]
- Radiation exposure of contrast-enhanced spectral mammography compared with full-field digital mammography. Invest Radiol. 2014;49:659-65.
- [CrossRef] [PubMed] [Google Scholar]
- ACR BI-RADS® magnetic resonance imaging In: ACR BI-RADS® Atlas. Breast Imaging Reporting and Data System. Reston, VA: American College of Radiology; 2013.
- [Google Scholar]






