Translate this page into:
Diagnostic value of intraluminal stent enhancement in estimating coronary in-stent restenosis

*Corresponding author: Maryam Moradi, Department of Radiology, School of Medicine, Isfahan University of Medical Sciences, Isfahan, Iran. mry.moradi@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Nogourani MK, Moradi M, Khajouei AS, Farghadani M, Eshaghian A. Diagnostic value of intraluminal stent enhancement in estimating coronary in-stent restenosis. J Clin Imaging Sci 2020;10:12.
Abstract
Objective:
In-stent restenosis (ISR) diagnosis is among the most serious complications of patients undergone stent implantation. Although coronary computed tomography angiography (CCTA) has been widely used for ISR assessing, stent narrow lumen and presence of stent’s struts artifacts have limited its efficacy. The use of quantitative techniques may provide more valuable findings for ISR diagnosis. The aim of this study is to assess the predictive value of a quantitative technique of ISR estimation based on stent intraluminal enhancement derived from CCTA.
Materials and Methods:
In the current study, 40 patients with the previous history of coronary artery diseases (CADs) and coronary stent placement who reexperienced CAD symptoms and referred for CCTA were assessed in 2017–2018. Stent intraluminal “enhancement value” (EV) was measured using calcium score and post-contrast images of CCTA. The cutoff point was determined using conventional invasive coronary angiography as the gold standard.
Results:
Total numbers of 58 stents were evaluated, in which stent intraluminal enhancement was assessed in initial, middle, and end sites of stent, achieved cutoff points for more than 50% of ISR were 204, 168, and 204 Hounsfield units, respectively. These cutoff points had diagnostic value of 77.5% for initial part, 86% for midpart, and 81% for end part, respectively.
Conclusion:
The use of quantitative method of stent intraluminal EV for ISR estimation has better diagnostic value in comparison to qualitative techniques that can help better clinical decision making. Moreover, measurements of this method are somewhat easier and also secondary artifacts of stent struts and calcified plaques would be eliminated.
Keywords
Stent
Computed tomography angiography
In-stent restenosis
Enhancement value
INTRODUCTION
While coronary artery stent implantation is a gold standard approach for coronary arteries stenosis causing angina and/or myocardial infarction, in-stent restenosis (ISR) is the main complication of this approach occurring in 20–35% of bare-metal stents and only 5–10% for drug-eluting stents.[1-3]
Coronary angiography is the standard approach for the assessment of ISR incidence and luminal stenosis severity that is an invasive procedure, posing considerable burden and costs in addition to its potential complications. Therefore, the requirement of a non-invasive means for ISR assessing is inevitably useful. Furthermore, gradual increase in number of patients undergoing coronary arteries stent placement better clarifies this requirement to minimize invasive procedures.[4]
ISR diagnosis is the most challenging matter among patients with coronary artery disease (CAD) undergone stenting. Although coronary computed tomography angiography (CCTA) has been widely utilized for coronary arteries assessing, its efficacy for stent evaluation due to artifacts of stent struts, especially beam hardening and its narrow lumen, is still an important issue.[5]
Multidetector CT (MDCT) introduction made a great revolution regarding coronary stent assessment as it could provide high-quality images with satisfactory temporal and spatial resolution. Furthermore, there are limitations including the type of stent (bare metal/drug eluting) and its diameter, stent strut thickness, its angularity, and motional artifacts in quality of these images.[2,3,6,7]
The last versions of CT scans have more rapid imaging reception, higher spatial and temporal resolution and have particular software capable of coronary arteries and stent reconstruction led to less biases. Furthermore, by cardiac rate control and breath holding during imaging, the motion artifacts have been minimized.[6]
Nowadays, most of CCTA reports are based on visual assessing of contrast flow of stent lumen while this method does not have ideal diagnostic value with significant interobserver biases. Quantitative measurements provide more reliable information about the status of stents. Recent studies have presented variety of quantitative methods with acceptable sensitivity and specificity for the diagnosis of ISR.[8-10]
The current study has aimed to provide a quantitative method for ISR estimation, in which predictive value of stent enhancement in CCTA would be evaluated.
MATERIALS AND METHODS
Study design and participants
This is a prospective cross-sectional study conducted on 40 patients with a history of coronary stent implantation that because of CAD symptoms, recurrence was referred for CCTA performance to MDCT scan center of Chamran Hospital affiliated at Isfahan University of Medical Sciences in 2017–2018.
Patients with CAD symptoms who had a previous history of stent implantation and have undergone conventional angiography during the 1st month following CCTA were included in the study.
Unavailability of conventional angiography images and patient’s unwillingness of participation in the study were exclusion criteria.
All patients were asked about the presence of renal failure or any hypersensitivity to contrast agents. In addition, all of cardiovascular records of patients including their echocardiography were gathered to enter the required information in the study checklist. Then, patients who met inclusion criteria were prescribed sublingual nitroglycerine (0.4 mg, Dana; Iran) for vascular dilation and also oral beta- blocker to achieve appropriate rate control.
Test method
CCTA was performed using 256-slice MDCT scan (Brilliance TM 256; Philips Medical System) and specific workstation was utilized for their reports. The protocol of imaging was as follows:
The properties as collimation=96–128 mm, detector size=0.625 mm, rotation time=0.27 ms, voltage: 120 kv, and 180–200 mAs.
CCTA was primarily performed without contrast injection with an appropriate field of view to assess coronary arteries calcium score. Then, intravenous contrast (Visipaque 320 mg) with 70–90 cc volume, based on patients’ height and weight, with 5–6 cc/s velocity and using bolus track method was injected. Finally, images were taken in prospective manner and the following reconstructions were sent to Philips workstation to provide CCTA reports.
Intraluminal enhancement of stent was measured by comparing calcium score and post-contrast images. In this regard, intraluminal density of stent was measured through focusing on the region of interest at the initial, middle, and end sites of stents in axial plane using calcium score images and repeat this measurement in post-contrast phase as well. The optimal post-contrast phase with less motion artifact and more resolution was considered for mentioned assessment. The difference between measured density presented by Hounsfield unit (HU) from similar sites derived before and after contrast injection was considered as intraluminal enhancement. To minimize interobserver bias, all of the measurements were performed by a target expert radiologist.
Analysis
Enhancement value (EV)=Post-contrast intraluminal density-calcium score intraluminal density.
Conventional invasive coronary angiography (CICA) was used as the gold standard and all patients CICA images reported only by a target cardiologist. CICA results were presented as patency, non-significant stenosis, and significant stenosis occluded. Due to clinical significance of stenosis, those with reports of patency and non-significant (<50%) were presented in a group and those with significant stenosis (over 50%) and occluded were presented in another group. These results were used for achieving cutoff point of EV in CCTA for differentiating these two groups of patients.
Obtained data were entered into SPSS-20 (IBM-The United States) software. ROC curve was drawn to demonstrate the cutoff point of EV for ISR prediction. In addition, sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of stent enhancement were measured. P < 0.05 was considered as statistically significant level.
RESULTS
Participants
In the current study, a number of 40 patients with total number of 58 stents were assessed. A number of 15 (37.5%) of patients were female and remained 25 ones (62.5%) were male. The mean age of the study population was 60.97 ± 10.55 years old.
Test results
Characteristics of implanted stents are presented in Table 1. Twenty-eight out of 58 cases (41.4%) were implanted in the left anterior coronary artery, 10 (17.2%) in the left circumflex artery, and 16 (27.6%) in the right coronary artery. Nine of stents had <3 mm of diameter, 26 of them had 3 mm of diameter, and other 23 ones had over 3 mm diameter.
| Parameter | Total | ISR <50% | ISR 50%–100% | ISR 100% |
|---|---|---|---|---|
| Number of stents | 58 | 39 (67.24%) | 16 (27.58%) | 3 (5.17%) |
| Location of stents | ||||
| Left anterior descending artery | 24 (41.4) | 15 (41.7) | 9 (56.3) | 0 |
| Left circumflex artery | 10 (17.2) | 5 (13.9) | 2 (12.5) | 1 (33.3) |
| Right coronary artery | 16 (27.6) | 12 (33.3) | 3 (18.8) | 1 (33.3) |
| Other | 8 (13.8) | 6 (11.1) | 2 (12.5) | 1 (33.3) |
| Stent diameter (mm) | ||||
| <3 | 9 (15.5) | 7 (17.94) | 2 (12.5) | 0 |
| 3 | 26 (44.8) | 20 (51.28) | 6 (37.5) | 0 |
| >3 | 23 (39.6) | 12 (30.76) | 8 (50) | 3 (100) |
| Length (mm) | 17.22±8.33 * | 21.74±10.46* | 25.87±12.52 * | 27.33±4.61 * |
| Segment | ||||
| Origin | 2 (3.4) | 1 (2.6) | 1 (6.3) | 0 |
| Proximal | 27 (46.6) | 21 (53.8) | 6 (37.5) | 0 |
| Proximal-middle | 2 (3.4) | 1 (2.6) | 1 (6.3) | 0 |
| Middle | 23 (39.7) | 15 (38.5) | 7 (43.8) | 1 (33.3) |
| Middle-distal | 1 (1.7) | 0 | 0 | 1 (33.3) |
| Distal | 3 (5.2) | 1 (2.6) | 1 (6.3) | 1 (33.3) |
Among stents, 39 ones (67.24%) were patent or had ISR of <50%, 16 ones (2931%) had over 50% stenosis, and 3 ones (3.44%) were completely occluded.
The cutoff point for EV was measured in three sites of stents in CCTA images that were achieved based on CICA findings. These cutoff points were 204, 168, and 204 HU for initial, middle, and end part of stents, respectively, which show more than 50% of ISR. Mentioned cutoff points are presented in Table 2. Considering no statistical difference among these cutoff points (use 168 or 204) (P = 0.4), 168 HU was considered as the most valuable cutoff for the diagnosis of over 50% ISR due to its higher sensitivity and PPV [Figure 1].
| Index | Cutoff point | Sensitivity (%) | Specificity (%) | Accuracy (%) | Likelihood ratio+ | AUC | 95% CI | P |
|---|---|---|---|---|---|---|---|---|
| PCD initial | 500 | 79.49 | 63.16 | 74.14 | 2.15 | 0.69 | 0.53–0.85 | 0.404 |
| EV initial | 204 | 87 | 57.89 | 77.5 | 2.07 | 0.73 | 0.60–0.84 | |
| PCD mid | 402 | 94.87 | 63.16 | 84.45 | 2.57 | 0.81 | 0.68–0.90 | 0.763 |
| EV mid | 168 | 92.3 | 73.68 | 86 | 3.5 | 0.85 | 0.68–0.90 | |
| PCD end | 386 | 97.44 | 42 | 79.31 | 1.68 | 0.72 | 0.56–0.88 | 0.113 |
| EV end | 204 | 87 | 68 | 81 | 2.7 | 0.81 | 0.68–0.90 |
EV: Enhancement value, PCD: Post-contrast density
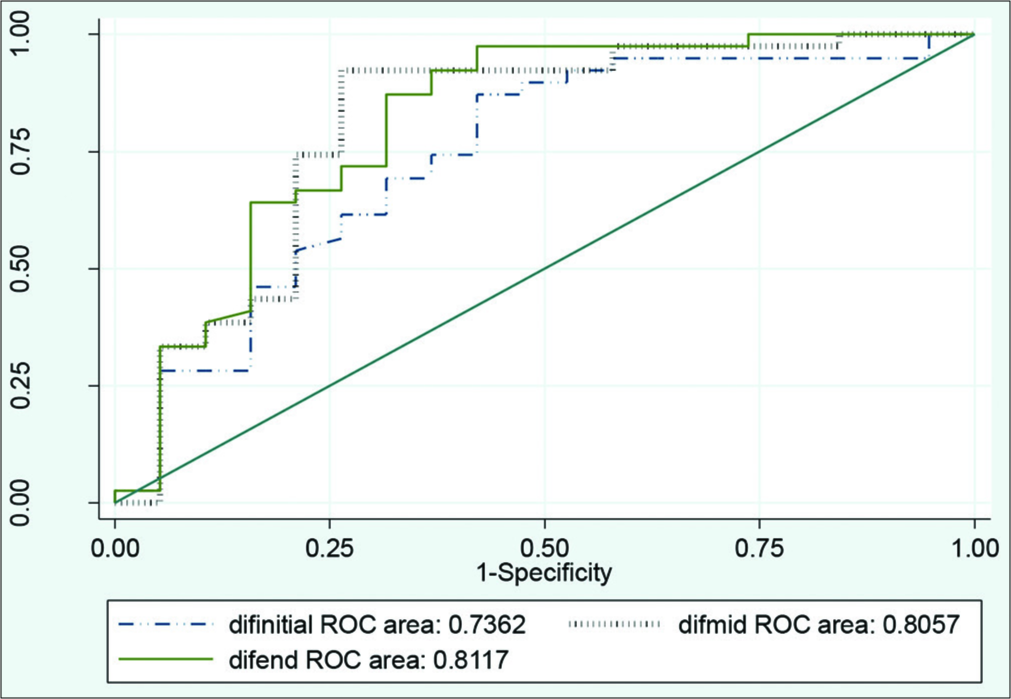
- Comparison of diagnostic enhancement value in three sites of initial, middle, and end of stents.
ISR was once measured based on stent intraluminal density just in post-contrast phase and then measured again using EV for comparison this method.
Table 2 shows validity, specificity, PPV, and diagnostic value based on stent intraluminal EV and also based on stent intraluminal density in post-contrast phase for ISR diagnosis, mentioned as post-contrast density (PCD). Comparison of PPV of EV method versus PCD showed superiority of EV method.
Considering area under curve (AUC) of all three above figures, EV is superior to PCD regarding its better predictive value [Figure 2].
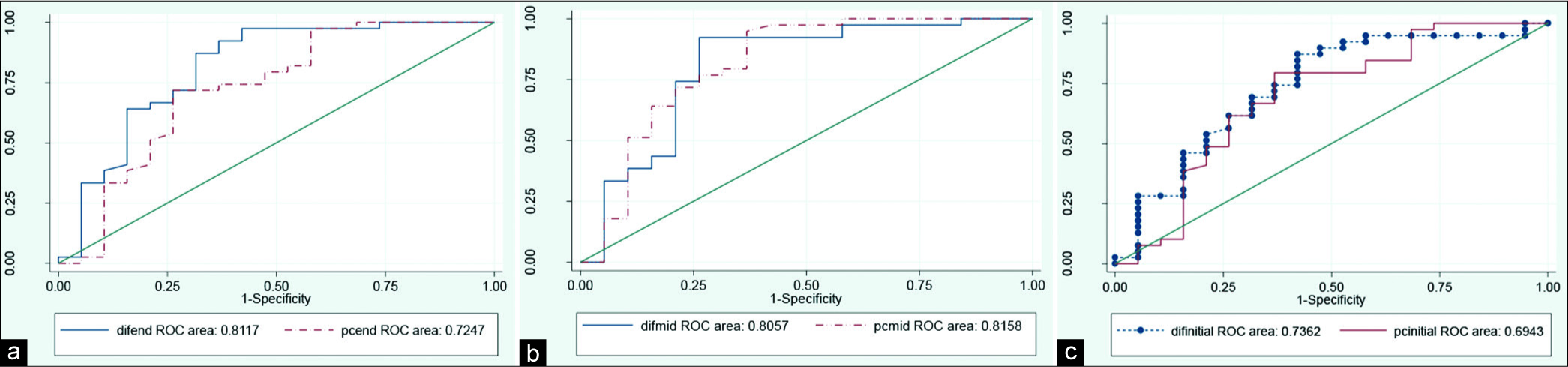
- Comparison of diagnostic value of in-stent restenosis diagnosis using enhancement value versus post-contrast density; (a) initial part of stent, (b) middle part of stent, and (c) end part of stent.
As stent diameter may have impact on intraluminal density, ISR diagnostic value in EV method was assessed before and after stent diameter adjustment. Maximal 4–7% of decrease in three points of initial, middle, and end part of stent AUC were found that was negligible.
DISCUSSION
Stent implantation is one of the old and effective treatments of coronary artery stenosis while ISR is among the most significant complications of this approach.[11,12] Incidence of restenosis is in association with factors including the type of stent (metallic or drug eluting), stent diameter and length, and the individual’s atherosclerotic factors such as diabetes.[3,4] ISR diagnosis is made based on CICA. Although it is still the gold standard method of ISR diagnosis, its potential complications caused considerable trend toward non-invasive techniques.
Electron beam CT was the first non-invasive modality for stent status assessment introduced in 1995. In this method, it used images for flow-related analysis which showed flow limiting ISR indirectly. This method did not outlive due to its low spatial resolution, unavailability of direct stent lumen visualization, and inability for diagnosis non-obstructive intimal hyperplasia.[13]
In the 1st year of the 2000s, 4-slice CT scans were utilized, in which distal contrast runoff was the criterion of stent patency. These types of CTs were eliminated as well due to its low spatial and temporal resolution. Moreover, this technique was also affected by collateral vessels. By 16-slice CT scans introduction, stent lumen observation was possible and new windows of non-invasive stent luminal status assessment were opened toward physicians.[2] This superiority is while factors including type of stent, its strut thickness, luminal diameter, and angle and motional artifacts can affect new CT scan modalities efficacy in negative manner. New generations of CT scans (e.g., 64, 128, and 320 slice) provide higher number of cuts with less thickness obtained in fewer time and they have some software for coronary artery and stent reconstruction with specific filters such as sharp kernel, therefore, they can better visualize stent’s lumen and provide more concise information about luminal status. Furthermore, new modalities provide reconstructed images with more clarified view of stents.[13,14]
Qualitative stent patency assessment regarding intraluminal contrast flow has considerable interobserver bias, for narrow lumens in special. Quantitative method for luminal stenotic status estimation has better diagnostic values that have recently made them of great interest. In recent studies, various quantitative methods for ISR assessment such as corrected coronary opacification (CCO), remodeling index, lesion length, non-calcified lesion volume, and stent restenosis index (SRI) were introduced.[8-10]
In most of quantitative methods mentioned above such as CCO introduced by Gao et al., in 2014,[9] or SRI presented by Yoshimura et al., in 2015,[8] coronary artery intraluminal density before and after stent was considered for patency assessment. Considering the fact that post-stent coronary artery intraluminal density would be affected by collateral vessels blood flow, direct stent luminal assessment seems superior.
SRI quantitative method was presented by Yoshimura et al., in which coronary artery intraluminal density before and after stent was measured and their difference based on luminal diameter correction was considered as stent stenotic status.[8] In the current study, we used EV method and presented our outcomes following pre- and post-stent diameter adjustment. Regarding ROC curve, diagnostic values were not considerably improved. Therefore, we concluded that stent diameter is negligible but further studies to assess the impact of stent diameter on EV assessment are recommended.
The other quantitative method introduced by Kitagawa et al. utilized intraluminal density difference of proximal to stent site with stent itself. They presented 70 HU as the reliable cutoff point for over 50% of ISR.[15]
Dr. Makoto Amanuma from Japan raised a novel method of ISR assessment, in which software for CT angiography subtraction has been used. The new method was accompanied with abilities including increased ISR diagnostic value and possibility of assessing stents with even <2.5 mm of diameter. In subtraction method, the final image will be achieved through comparison of images before and after contrast injection causing elimination of stent strut artifacts and calcified plaques. This superiority would be achieved in EV method introduced in our study as well.[16] Furthermore, EV method gives us qualitative information with easier manner.
Considering this fact that basis of the most of the previous recommended quantitative methods for stent assessment was performed in post-contrast phase and this phase may be affected by confounding factors such as stent strut blooming artifacts and its plaques, we have selected EV method which is achieved through comparison of with and without contrast phases. Therefore, the mentioned bias may be eliminated.
Furthermore, in the current study, post-contrast intraluminal HU for the assessment of ISR was compared with EV as well. Statistical comparison of two techniques in initial, middle, and end sites showed no statistical differences, while PPV of EV method was considerably superior to post-contrast phase.
Eventually, the current study achieved remarkable successful outcomes in assessing ISR, for lumens with <3 mm diameter in special, using 256-slice CT scan. This achievement occurred due to high velocity, better spatial and temporal resolution, and using specific reconstruction filters.
Limitations
Inaccessibility of data about the type of stent (stent brand) used for patients in their previous angiographic treatment is the most significant limitations of the current study.
Furthermore, we need larger sample volume for the evaluation of the effect of stent diameter and length and native coronary artery which contains stent on EV method.
CONCLUSION
The use of quantitative method of intraluminal stent enhancement for ISR estimation has better diagnostic value in comparison to qualitative and subjective methods that can help better clinical decision-making. Moreover, measurements of this method are somewhat easier and also secondary artifacts of stent strut and calcified plaques would be eliminated through EV method.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- A randomized comparison of coronary-stent placement and balloon angioplasty in the treatment of coronary artery disease. Stent restenosis study investigators. N Engl J Med. 1994;331:496-501.
- [CrossRef] [PubMed] [Google Scholar]
- CT imaging of coronary stents: Past, present, and future. ISRN Cardiol. 2012;2012:139823.
- [CrossRef] [PubMed] [Google Scholar]
- Coronary in-stent restenosis: Predisposing clinical and stent-related factors, diagnostic performance and analyses of inaccuracies in 320-row computed tomography angiography. Int J Cardiovasc Imaging. 2016;32(Suppl 1):105-15.
- [CrossRef] [PubMed] [Google Scholar]
- High coronary calcium score and post-procedural CK-MB are noninvasive predictors of coronary stent restenosis. Clin Interv Aging. 2017;12:399-404.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnostic accuracy of coronary in-stent restenosis using 64-slice computed tomography: Comparison with invasive coronary angiography. J Am Coll Cardiol. 2007;49:951-9.
- [CrossRef] [PubMed] [Google Scholar]
- Performance of dual-source CT with high pitch spiral mode for coronary stent patency compared with invasive coronary angiography. J Geriatr Cardiol. 2016;13:817-23.
- [Google Scholar]
- 64-multislice detector computed tomography coronary angiography as potential alternative to conventional coronary angiography: A systematic review and meta-analysis. Eur Heart J. 2007;28:3042-50.
- [CrossRef] [PubMed] [Google Scholar]
- New quantitative method to diagnose coronary in-stent restenosis by 64-multislice computed tomography. J Cardiol. 2015;65:57-62.
- [CrossRef] [PubMed] [Google Scholar]
- Coronary in-stent restenosis: Assessment with corrected coronary opacification difference across coronary stents measured with CT angiography. Radiology. 2015;275:403-12.
- [CrossRef] [PubMed] [Google Scholar]
- Coronary CT angiography-derived quantitative markers for predicting in-stent restenosis. J Cardiovasc Comput Tomogr. 2016;10:377-83.
- [CrossRef] [PubMed] [Google Scholar]
- Sirolimus-eluting and paclitaxel-eluting stents for coronary revascularization. N Engl J Med. 2005;353:653-62.
- [CrossRef] [PubMed] [Google Scholar]
- High-definition computed tomography for coronary artery stent imaging: A phantom study. Korean J Radiol. 2012;13:20-6.
- [CrossRef] [PubMed] [Google Scholar]
- Multidetector CT for visualization of coronary stents. Radiographics. 2006;26:887-904.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of coronary stents using multidetector CT. Egypt J Radiol Nucl Med. 2016;47:793-801.
- [CrossRef] [Google Scholar]
- Usefulness of measuring coronary lumen density with multi-slice computed tomography to detect in-stent restenosis. Int J Cardiol. 2008;124:239-43.
- [CrossRef] [PubMed] [Google Scholar]
- Subtraction CTA Boosts In-Stent Restenosis Evaluation. 2019. Available from: https://www.auntminnie.com/index.aspx?sec=log&itemid=109751 [Last accessed on 2019 June 26]
- [Google Scholar]






