Translate this page into:
Cystic and Cavitary Lung Lesions in Children: Radiologic Findings with Pathologic Correlation
Address for correspondence: Prof. Kemal Odev, Departments of Radiology, Necmettin Erbakan University Meram Faculty of Medicine, Department of Radiology, 42080 Konya, Turkey. E-mail: kemalodev50@yahoo.com
-
Received: ,
Accepted: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
A number of diseases produce focal or multiple thin-walled or thick-walled air- or fluid-containing cysts or cavitary lung lesions in both infants and children. In infants and children, there is a spectrum of focal or multifocal cystic and cavitary lung lesions including congenital lobar emphysema, congenital cystic adenomatoid malformation, pleuropulmonary blastoma, bronchogenic cyst, pulmonary sequestration, Langerhans cell histiocytosis, airway diseases, infectious diseases (bacterial infection, fungal infection, etc.), hydatid cysts, destroid lung, and traumatic pseudocyst. For the evaluation of cystic or cavitary lung lesion in infants and children, imaging plays an important role in accurate early diagnosis and optimal patient management. Therefore, a practical imaging approach based on the most sensitive and least invasive imaging modality in an efficient and cost-effective manner is paramount. We reviewed the conventional radiographs and computed tomography findings of the most common cystic and cavitary lung lesions in infants and children.
Keywords
Cavitary lung lesions
computed tomography
cystic lung lesions
magnetic resonance imaging
radiograph
INTRODUCTION

Cysts and cavities are commonly seen lesions in the lung, viewed by chest radiograph and chest computed tomography (CT). A lung cyst or cystic airspace is described as a parenchymal space with a well-defined, thin wall (usually less than 2-mm thick). These can present with a relatively thin (≤4 mm) wall. A cystic lesion is an air- or fluid-containing lesion, measuring 1 cm or more in diameter.[12] In contrast, the term cavity is used for an air-containing lesion with a relatively thick wall (greater than 4 mm).
Many infants or children with cystic or cavitary lung lesions have a congenital malformation or known underlying disease (bronchiectasis, infectious diseases, etc) or pulmonary arteriovenous malformations (AVMs).[13] Adults with cystic or cavitary lung lesions with thin and thick walls typically present with neoplasms, obstructive pulmonary diseases, infectious diseases, parasitic disease, pulmonary infarct, septic embolism, collagen vascular diseases (e.g., rheumatoid arthritis), diffuse pulmonary diseases (e.g., Langerhans cell histiocytosis, lymphangioleiomyomatosis), vasculities, and chest trauma.[4]
Because of a wide variation in cystic and cavitary lung lesions in infant and children, the major diagnostic challenge is the differentiation of a cavitated lesion from an abscess or other benign disorders. A single detector CT provides more detailed information concerning chest anatomy and pathology than a chest radiograph. Similar to adults, in children, high-resolution CT (HRCT) is indicated when persistent symptoms of lung disease or abnormal pulmonary function tests are found in the context of normal or nonspecific abnormalities on radiologic images.[5] However, the authors have reported that the risk of radiation-induced damage is greater in children than in adults. Therefore, they advocate the use of low-dose HRCT of the chest in cooperative children in order to obtain further reduction in radiation exposure.[6] Although a single detector CT has wide applications for parenchymal diseases in children, helical- and multi-detector CT (MDCT) have considerable advantages over a single-detector CT. MDCT is particularly useful in the assessment of abnormalities such as pulmonary sequestrations and AVMs.[78]
The purpose of this presentation is to review the radiographic and chest CT appearances of cystic or cavitary lesions in infants and children.
CONGENITAL LUNG DISEASES
Congenital Lobar Emphysema
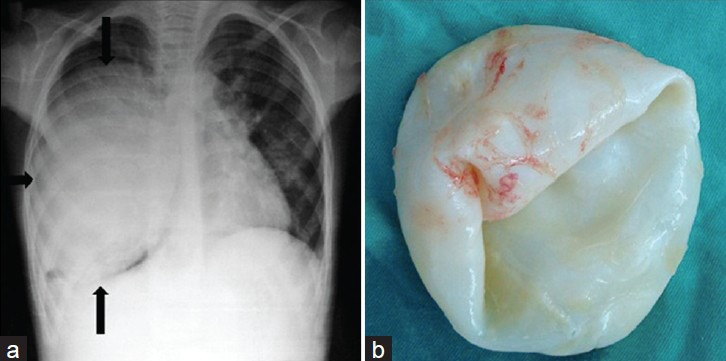
Congenital lobar emphysema (CLE) is characterized by over-distension and air-trapping in the affected lobe, concomitant compression of the remaining lung tissue, and displacement of the mediastinum by herniation of the emphysematous lobe across the anterior mediastinum into the opposite side of the chest.[389] There is controversy as to whether the condition is developmental or acquired. Deficiency of the cartilage wall suggests a developmental cause.[10] Murray[11] concluded that CLE could be secondary, congenital, or acquired and that many conditions causing bronchial obstruction could be the causative factor, including congenitally defective cartilage and acquired bronchial mucus plugs. Radiographic features include a translucency on the affected side, often with mediastinal displacement and herniation of the affected lobe across the mediastinum. The ipsilateral lobes are compressed, as may be the contralateral lung too. Hyperlucency of the affected lobe and herniation to the contralateral side are the most common radiographic findings [Figure 1].[3910] CT scans of CLE have been reported to be helpful in looking for an abnormal bronchus or focal bronchial obstruction as the underlying etiology.[1213] Overinflation of the affected lobe and compressive atelectasis of other lobes are the most common CT findings [Figure 1].[391013]

- Congenital lobar emphysema in a 6-month-old infant. a) Chest radiograph of a neonate shows a marked overinflation of the left upper lobe, which herniated across the midline (white arrows). There is contralateral shift of the heart and mediastinum and widening of the ribs spaces on the left. b) CT scan (lung window) at the level of the carina shows hyperaeration of the left lobe (black arrows), with attenuation of the vascular markings. The mediastinal structures are shifted to the right side. c) Photograph of the specimen shows upper lobe (thin arrows) and the lower lobe (thick arrow).
Congenital Cystic Adenomatoid Malformation
Congenital cystic adenomatoid malformation (CCAM) is usually observed in neonates because of respiratory distress and may occasionally be observed in older children or adults with recurrent infection.[10141516] This malformation consists of adenomatoid proliferation of bronchioles that form cysts instead of normal alveoli. This disorder has been classified into three types based on clinical, gross, and microscopic criteria.[381014] There is considerable controversy over classification and nomenclature regarding these pulmonary malformations. Recently, the new term congenital pulmonary airway malformations (CPAM)[8] has been recommended over the previously used term CCAM.[17]
Imaging findings of CCAMs often correlate with underlying histopathological characteristics. The typical imaging findings of type 1 CPAM are one or more large air-filled cystic lesions [Figure 2].[31819] Early radiographs may show a water-density mass if the cysts are filled with retained fetal lung fluid.[3] After birth, air can enter lesions as CPAM communicate with each other and air-filled cysts; air-fluid levels in the cyst or a combination of these findings can result. Conversely, fluid accumulation may transform air-filled cysts into fluid-filled cysts.[14] In the presence of infection, there may be adjacent alveolar consolidation. Multiple cystic lesions can cause mediastinal shift and herniation of the affected lobe.[3] Type 2 CPAM usually consists of an air-filled multicystic mass or focal area of consolidations [Figure 3]. Type 3 CPAM tends to be seen as homogeneous soft tissue density mass due to microscopic cysts that can be identified only at histologic evaluation. Type 4 CPAM consists of large air-filled cysts of distal acinar origin.[31819] Differential diagnosis of Type 1 or Type 2 CPAM in a neonate includes congenital diaphragmatic hernia, pulmonary sequestration, bronchogenic cyst, CLE, and other bronchopulmonary foregut malformations.[314]

- Type I congenital cystic adenomatoid malformation (CPAM) in a 15-year-old girl. (a) Chest radiograph shows multiple air-filled cysts with septa occupying the entire left hemithorax, with mediastinal shift to the contralateral side. (b) CT scan (lung window) shows air-filled, thin-walled spaces of varying size. (c) Specimen demonstrates an expansile, septated, fluid-filled cystic mass.

- Type II congenital cystic adenomatoid malformation (CPAM) in a 10-month-old infant. a) Chest radiograph shows an area of hyperlucency (arrow) in the right lower lobe. b) CT scan (lung window) demonstrates the presence of several small cysts (arrow) in the right lower lobe.
Pleuropulmonary Blastoma
The authors believe that this shows a histologic resemblance to the common developmental neoplasms of childhood, such as Wilms’ tumor, hepatoblastoma, retinoblastoma, and medulloblastoma. As with these other pediatric malignancies, most causes of pleuropulmonary blastoma (PPB; 94%) present in children less than 6 years of age.[2021] These pathologic types of PPB are recognized based on their pathologic features: Type 1 PPB is an entirely cystic lesion and indistinguishable from non-neoplastic cysts in both clinical and imaging features. Type 2 PPB is characterized by cystic lesion and solid mass. The air-filled cystic spaces are obliterated by a solid mass and mixed-pattern primitive sarcoma. Type 3 PPB occurs as a purely solid high-grade sarcoma.[22]
Bronchogenic Cyst
Bronchogenic cysts (BCs) are congenital lesions thought to result from abnormal budding of the embryonic foregut. The most frequent symptoms are pain, cough, fever, respiratory distress, or dyspnea. They are usually found in the mediastinum or pulmonary parenchyma and, less commonly, cysts may be found in the neck, pericardium, pleura, diaphragm, or abdominal cavity.[32324] Intrapulmonary BCs represent approximately 15-20% of all bronchogenic cysts and usually occur in the lower lobes. These are observed not only in infants and children but also in adults.[2425] Intrapulmonary bronchogenic cysts are usually sharply defined, solitary, non-calcified, round, or oval opacities confined to a single lobe. These can present as a homogeneous water density, an air- filled cyst, or with an air-fluid level. The cyst can rupture into the trachea, pericardial cavity, or pleural cavity. Infection of the cyst may lead to surrounding acinar shadow. An air-filled cyst or one with an air-fluid level may be present if a complicating tracheo-bronchial connection develops [Figure 4].[325] At CT, the precise anatomic location of the cyst can be defined. CT attenuation value of uncomplicated bronchogenic cysts varies from water density (0-20 HU) to high density (80-90 HU).[2325] The high CT attenuation of bronchogenic cysts on unenhanced CT scans is caused by hemorrhage, proteinaceous mucus, calcium, or calcium oxalate.[3232426]

- Intrapulmonary bronchogenic cyst in a 12-year-old boy. (a) Chest radiograph shows a cystic lesion (arrow) with air-fluid level in the right upper lobe. (b) CT scan (lung window) demonstrates thin-walled cystic lesion (arrow) with an air-fluid level, consistent with the cyst in the upper lobe. (c) An air-fluid level is seen in the cystic mass (arrow) with low signal intensity in the dependent layer on axial T1-weighted MR image. (d) Cystic mass shows high signal intensity (arrow) in the dependent area on T2-weighted MR image.
MR imaging appearance is dependent on the cyst's content, specifically the presence and amount of mucus or other proteinaceous material. If the fluid within the bronchogenic cyst is of a signal intensity similar to that of cerebrospinal fluid and is mainly serous, it will appear at a very low signal intensity on T1-weighted images and at very high signal intensity on T2-weighted images [Figure 4].[2325] However, many bronchogenic cysts may contain large amounts of proteinaceous material. Such lesions have a characteristic appearance, with high signal intensity on T1-weighted images. This appearance must be differentiated from lesions that contain fat, which also have a bright signal intensity on T1-weighted images.[25] The differential diagnosis of intraparenchymal bronchogenic cysts must include acquired cystic lesions, such as a lung abscess, a hydatid cyst, infection with nocardia, an infected bulla, lobar emphysema, fungal diseases, and tuberculosis, especially when the lesions manifest as air-filled or have an air-fluid level.[2426]
Pulmonary Sequestration
A pulmonary sequestration (PS) is defined as a segment of lung parenchyma that receives its blood supply from the systemic circulation and that does not communicate with the tracheobronchial tree. It includes intralobar (ILPS) and extralobar (ELPS) forms. ILPS is a segment of pulmonary tissue that shares the visceral pleural covering as the normal, adjacent lung tissue. ELPS is an entirely separate segment of pulmonary tissue that is invested in its own pleural layers.[327] ILPS is more commonly diagnosed in later childhood or even in adulthood. However, ELPS is usually diagnosed in early life.[3] ILPS is usually located in the posterior basal segment of one of the lower lobes. Focal bronchiectasis, areas of atelectasis, cavitation, and multiple cystic areas may also be recognized within an ILPS. ELPS is usually found as well-defined, solid retrocardiac masses in the cardiophrenic angle. The main diagnostic feature that must be identified by imaging in either type of PS is the feeding systemic arterial vessels [Figure 5].[3] MDCT and MR angiography are useful for prospective evaluation of pulmonary sequestration in both the pediatric and adult population. CT has the advantage of being able to show the pulmonary parenchymal abnormality as well as the arterial and venous anatomy, all in a single examination [Figure 5]. The main disadvantage of CT is the presence of radiation risks.[27]

- Intralobar pulmonary sequestration in a 9-year-old girl. (a) Chest radiograph shows soft tissue opacity (arrow) adjacent to the left hemidiaphragm. (b) Contrast-enhanced CT (mediastinal window) shows a heterogenous opacity with small cystic lesions (arrow) in the left lung. (c) Multi-detector CT angiography with maximum intensity projection reveals the presence of an abnormal artery (arrows) arising from the thoracic descending aorta.
DIFFUSE LUNG DISEASES
Langerhans Cell Histiocytosis
Histiocytosis is a group of diseases with different clinical courses, formally termed as Hand-Schüller–Christian syndrome, Letterer–Siwe disease, and eosinophilic granuloma (EG). These were later grouped as histiocytosis X. In 1987, histiocytosis was classified as Langerhans cell histiocytosis (LCH).[28] Isolated pulmonary LCH is very rare in children, due to two main reasons. First, there is a clear association between cigarette smoking and pulmonary LCH, though pulmonary LCH occurs in a very small percentage of smokers.[2930] Second, pulmonary LCH begins in childhood and remains clinically occult. The first symptoms are often a spontaneous pneumothorax or are characteristic of a prolonged lung disease, such as chronic cough and dyspnea.[30] The radiographic pattern in LHC is characterized by nodules, with a cavitating nodule in the early stages. In the later stages, some interstitial fibrosis and thin-walled cysts may develop [Figure 6]. CT findings include cystic lesion [Figure 6] and are either stable or progress with other lesions, such as nodules, suggesting the active process. Lesions are distributed diffusely throughout the lung and predominantly in the upper and middle lung zones. Spontaneous pneumothorax is a frequent complication occurring in 20-30% of the cases [Figure 6].[31]
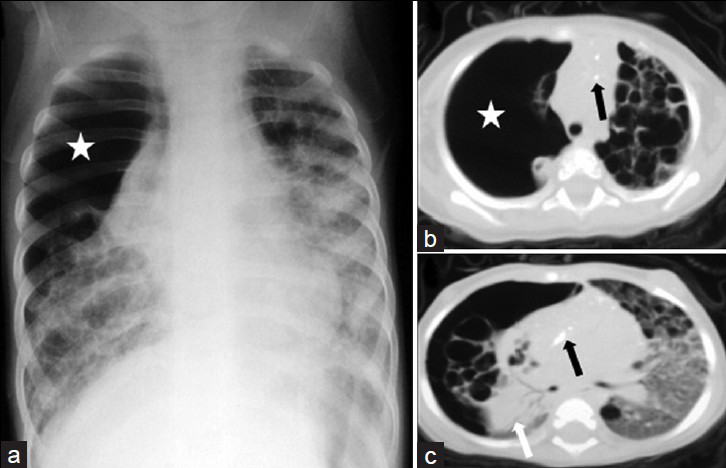
- Langerhans cell histiocytosis in a 10-month-old infant. (a) Chest radiograph shows a large pneumothorax (*) associated with collapse of the right lung and bilateral ring and linear opacities, and ground glass attenuation. CT scans (b) (mediastinal window) and (c) (lung window) show bilateral numerous thick- and thin-walled lung cysts. There is evidence of diffuse ground glass attenuation on the left lung and multiple punctate calcifications (black arrows) within the mediastinum. Note the large pneumothorax (*) on the right lung with almost complete collapse of the right lung (white arrow).
HRCT demonstrates cystic airspaces, which usually measure less than 10 mm in diameter. In some cases, cysts are the only abnormality visible on HRCT, but in the majority of cases, small nodules (usually smaller than 5 mm in diameter) are also present.[31]
AIRWAY DISEASES
Congenital Bronchial Atresia
Bronchial atresia results from local obliteration or stenosis of a segmental, subsegmental, or lobar bronchus at or near its origin.[32] This rare anomaly usually involves the left lobe and segment bronchi of the right lobe, middle lobe, and occasionally lower lobe. Radiographic features include a hilar mass and overinflation of the peripheral lung, often associated with an opaque round mucocele in the bronchial tree just distal to the obstruction. The dilated bronchi contain retained secretions.[1029] CT scan is helpful in demonstrating the precise anatomic location of the bronchial atresia and a hilar mass. The dilated bronchi beyond the atresia are completely opaque or show air-fluid levels or occasionally may be purely air-filled. Magnetic resonance imaging (MRI) is also useful for demonstration of the anatomic features.[103233]
Congenital Bulla
A bulla is an emphysematous space within the lungs having a diameter of more than 1 cm in the distended state, but may reach a considerable size. A large bulla can cause mediastinal displacement and compressive effects on the affected lung.[343536] Bullae may be single or multiple and may represent a localized abnormality (focal paraseptal emphysema) or, more commonly, part of generalized emphysema. On chest radiograph, a bulla appears as an avascular hyperlucent area, usually separated wholly or partially from the remaining lung by a thin curvilinear wall [Figure 7]. Occasionally, the wall is completely absent. The main complication of bulla is pneumothorax, infection, and hemorrhage. CT allows accurate assessment of the number, size, and location of bulla [Figure 7].[34]

- Congenital bulla in a 6-year-old girl. (a) Chest radiograph shows unilateral hyperlucency affecting the entire right lung (white arrows). There is a reduction in vascularity on the right lung. (b) CT scan (lung window) demonstrates a large bulla (star) on the right side causing considerable compression of the mediastinum and the adjacent right upper lobe.
Bronchiectasis
Bronchiectasis is the permanent dilatation of bronchi and is associated with inflammation. Saccular or cystic bronchiectasis is characterized by progressive dilatation of the airways, which end in a cystic, saccular, or grape-like cluster.[3738] Although the chest radiograph may demonstrate more severe forms of bronchiectasis, it is frequently normal. In milder cases of bronchiectasis, chest radiograph shows streaky linear opacities in the distribution of the bronchi and the thickened bronchial walls. In advanced cystic bronchiectasis, multiple thin-walled ring shadows that may contain fluid levels are present. Although the lower lobes are the most frequent sites of bronchiectasis, multiple lobes are often affected.[3738] The signs of bronchiectasis on HRCT depends on the morphologic type of bronchiectasis. Specific abnormalities found on HRCT include dilatation of an airway lumen, signet ring sign, lack of tapering of an airway toward the periphery, varicose constructions along airways, and ballooned cysts at the end of a bronchus [Figure 8].[38].

- Cystic bronchiectasis in a 12-year-old girl. HRCT shows bilateral diffuse cystic bronchiectasis (black arrows).
Cystic Fibrosis
Cystic fibrosis (CF) is a complex inherited disorder of infants, children, and young adults. This autosomal recessive condition is the most common lethal inherited disorder in the caucasian population. The pathogenesis of CF due to an abnormality in exocrine gland function involves multiple organ systems. The lung, however, bears the brunt of pathologic damage and accounts for the majority of morbidity and mortality. Due to recurrent and persistent pulmonary infection, patients with CF develop chronic restrictive and obstructive lung disease.[3940] Normal chest radiographs may be present early in the disease. Moreover, the chest radiographic features overlap with many other disorders, particularly those characterized by inflammatory or destructive changes of the airways.[3940] The earlist radiographic sign of CF in infants and children is hyperinflation due to mucus plugging of small bronchioles. Atelectasis, especially of the right upper lobe, is common in infancy. HRCT enables the definition of specific and more clinical relevant pathologic changes in CF, such as bronchiectasis, peribronchial thickening, mucus plugging, hyperinflation, and mosaic perfusion [Figure 9].[3940] However, the risks of radiation exposure always remain a concern when using HRCT imaging. Additionally, HRCT is necessary in assessing clinical progression and response to therapy.[4041]

- Cystic fibrosis in a 15-year-old girl with an abnormal sweat chloride test.(a, b) HRCT shows bilateral bronchial wall thickening and cystic bronchiectasis. Note the cylindrical bronchiectasis with mucous impaction (white arrows) and scattered patchy consolidations.
Congenital Bronchiectasis (Williams-Campbell Syndrome)
This malformation is a rare disorder characterized by a deficiency of cartilage in subsegmental bronchi, leading to distal airway collapse and bronciectasis. However, the syndrome has been described in children with recurrent pneumonia and broncho-obstructive symptoms. CT demonstrates bilateral cylindrical or cystic bronchiectasis distal to the third-generation bronchi with hyperinflation of the lung. The trachea and central bronchi remain normal in caliber, a distinguished feature of this disease.[42]
PARASITIC DISEASES
Parasitic diseases, such as paragonimiasis and echinococcosis, can be characterized by multiple thin-walled cysts.[1] Paragonimiasis is a parasitic disease caused by the trematode Paragonimus westermani or other species of Paragonimius. Paragonimiasis is endemic in certain areas of East and Southeast Asia. Typical findings on radiographs are a patch-air space consolidation with or without cysts, ring shadows, subpleural linear opacities, and bilateral pleural effusions. The characteristic CT features are round cystic lesions (5-15 mm) filled either with fluid or gas with consolidation.[43]
Hydatidosis is a parasitic infection of the lung and other organs by the larval stage of the tapeworm Echinococcus granulosus.[44] Synchronous pulmonary and hepatic hydatid disease may occur in 4-25% of the cases.[45] Most intact lung hydatid cysts (HCs) are asymptomatic and are incidentally discovered on routine radiologic examination. Compared to adults, pediatric patients may show symptoms in the early phase of disease due to compression of adjacent structures or perforation of the cyst.[4647]
Radiographically, intact cysts (closed cyst) are seen as well-demarcated, spherical, homogeneous single [Figure 10] or multiple masses [Figure 11] surrounded by normal lung tissue. The cysts are usually in the lower lobes. An intact cyst is indistinguishable from other nodular lesions, such as granulomas, abscess, bronchogenic cysts, arteriovenous aneurysms, and solitary metastases.[4448] Unlike in adults, in children, cysts may grow faster in the lungs than in the liver due to decreased elasticity of the lungs.[46] Calcification of cysts in the lung parenchyma is extremely rare.[47] Hydatid cysts may rupture spontaneously or due to trauma[49], and expanding cysts may reach a bronchiole and erode into it. A communication with a bronchial tree shows a variety of characteristic radiographic signs, such as the water-lily sign [Figure 12a, b], the sign of the rising sun [Figure 12c, d], a crescent, a meniscus sign, a whirl sign, or an empty sign [Figure 12e, f]. Chest radiograph may be helpful in displaying some signs, such as the water-lily or meniscus sign. However, it is impossible to describe the entire morphology of the disease with only a plain film. CT is helpful in showing the water density in the intact cysts [Figure 11], but, in complicated cysts, increased density can be confused with mass lesions. Complicated or infected cysts may demonstrate air-bubbles or air-fluid level within the cyst and ring enhancement on contrast-enhanced CT [Figure 13]. Superinfection of ruptured HCs with bacteria can change the appearance of the cyst on radiograph and CT. Complicated cysts are also difficult to differentiate from other cystic lesions, such as abscess, hematoma, or congenital cysts.[44]
- Lung hydatid cyst in a 10-year-old boy. a) Chest radiograph shows a well circumscribed, masslike lesion (black arrow) on the right lung and displacement of the mediastinum to the left. b) Photograph of the specimen.
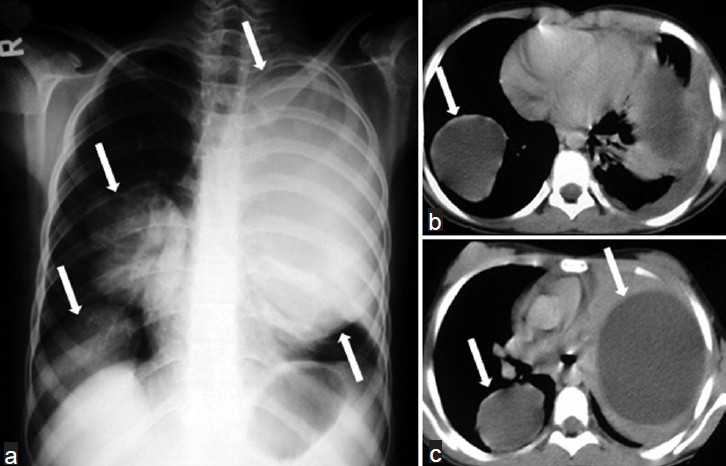
- Multiple lung HCs in a 10-year-old girl. a) Chest radiograph shows multiple well-defined masses of increased opacity in both lung fields (white arrows). b-c) CT scans reveal intact hydatid cysts as unilocular water-density lesions (white arrows).
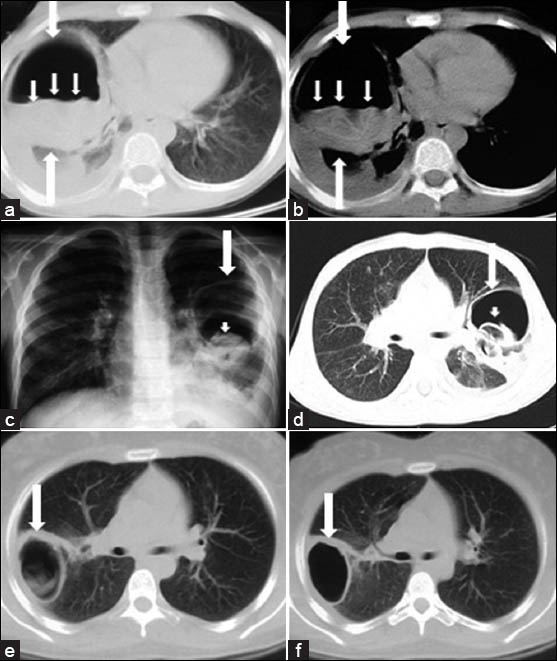
- Radiologic signs of ruptured lung HC in children. a-b) CT scans show detached endocystic membranes (short arrows) floating within the fluid content of a large cyst (water-lily sign) (white arrows). c) Chest radiograph and d) CT scan show a ruptured hydatid cyst (white arrows) and the collapsed parasitic membranes in the dependent part of the pericystic cavity (sign of the rising sun) (arrowhead). e) CT scan shows a ruptured hydatid cyst and (f) follow-up CT scan obtained two months after the initial CT scan shows that the cyst contents are expectorated completely (empty cyst sign) (white arrow).
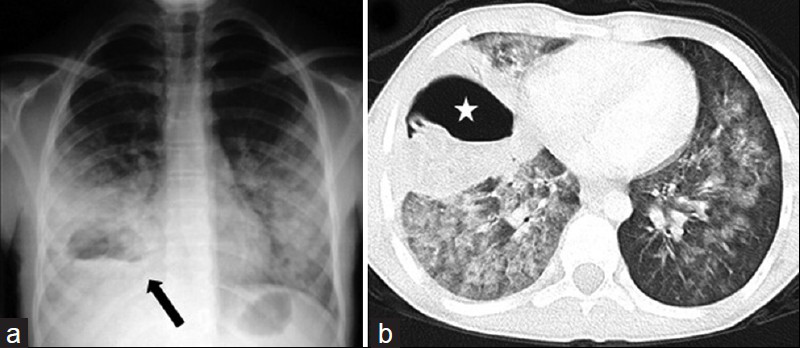
- Hydatid abscess in a 13-year-old boy. a) Chest radiograph shows a ruptured hydatid cyst (arrow) obscured by surrounding pneumonitis and an air-fluid level mimicking abscess. Note that bacterial infection may become widespread throughout the left lung. b) CT scan (lung window) reveals the hydatid abscess (star) with an air-fluid level in the right lung, but retains no pathognomonic features of hydatid disease. Radiologic findings may suggest the hydatid origin of the abscess in the proper clinic setting.
INFECTIOUS DISEASES
Bacterial Infections
Suppurative lung parenchymal complications in children include cavitary necrosis or cavitary pneumonia, pulmonary gangrene, lung abscess, pneumatocele, and bronchopleural fistula.[5051] Necrotizing pneumonia is well-known in the adult population. However, this complication has rarely been reported in children.[50] In children, chest radiographic findings of necrotizing pneumonia are less sensitive than CT.[50] The authors report that necrotizing pneumonia was identified with chest radiographs in only 41% of the cases detected by CT.[50]
Staphylococcus aureus, Pseudomonas aeruginosa, Klebsiella, and anaerobic bacteria commonly result in the development of thick- or thin-walled, air-filled cystic lesions.[150] Pneumatoceles are thin-walled cystic lesions commonly seen in infants and children as a sequelae of staphylococcal pneumonia [Figure 14].[150] Moreover, pneumatoceles can be caused by other bacteria or by hydrocarbon aspiration.[52] Gram-negative, anaerobic bacteria, and, occasionally, streptococcus pneumonia, are responsible for the development of lung abscess [Figure 15]. Abscess may be identified on plain radiographs, but CT may help discern underlying lung lesions predisposing to the development of the abscess or facilitate radiologic intervention. CT image demonstrates the thick-walled cavity containing mobile, central fluid occurring in the midst of an area of consolidated lung. An air-filled level is often apparent on the CT scan, even when it is not evident on the chest radiograph [Figure 15].[53]
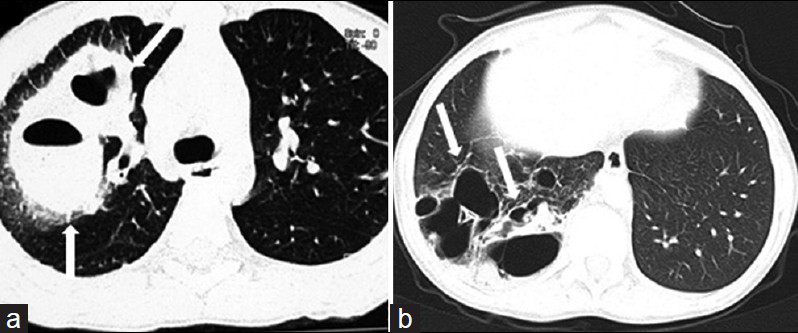
- Pneumatoceles developing in 13-year-old boy with staphylococcal pneumonia. a) CT scan shows thick-walled pneumatoceles (white arrows) in the early phase of pneumonia in the right upper lobe. b) In another patient, follow-up CT scan obtained 20 months after initial infection shows numerous residual pneumatoceles (white arrows) in the right lower lobe.
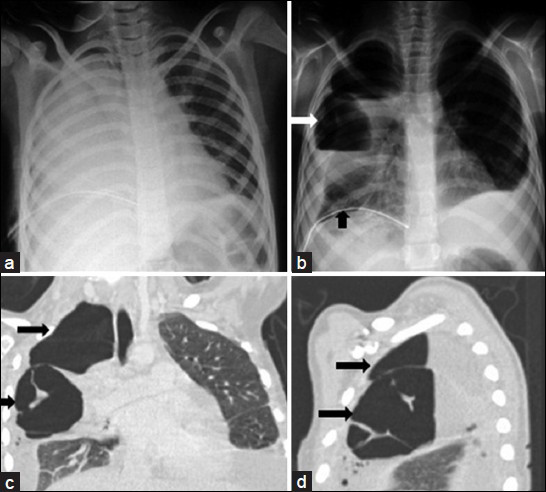
- Lung abscess due to streptococcal pneumonia in a 15-year-old girl. a) Admission chest radiograph shows a complete opacification of a right hemithorax. b) Chest radiograph obtained 5 days after initial radiograph shows a large cavitary lesion (white arrows) with an air-fluid level within the right upper lobe opacification. Note the right parapneumonic effusion and chest tube drainage catheter (black arrow). c-d) Coronal and sagittal reformatted CT images of the chest reveal two cavitary lesions (black arrows) with an air-fluid level, and a septate and outer margin obscured by the surrounding pneumonia.
Fungal Infections
Aspergillus species are typical opportunistic agents, with 80% of clinical infections caused by the Aspergillus fumigatus. The spectrum of Aspergillus infection ranges from saprophytic to invasive syndromes in the lungs, including allergic bronchopulmonary aspergillosis, aspergilloma, and invasive pulmonary aspergillosis. Saprophytic involvement of the lower respiratory tract is found with increased incidence in patients having underlying pulmonary diseases.[54]
Allergic bronchopulmonary aspergillosis is a recognized complication of asthma and cystic fibrosis. Radiologic manifestations include recurrent transient or permanent infiltrates in the middle or upper lobes. Bronchiectasis, involving the more central segmental bronchi, is a strong diagnostic criterion but not always present in patients during follow-up or at the time of diagnosis.[54]
Pulmonary aspergilloma (mycetoma) is a fungus ball that typically develops in the context of pre-existing cavitary disease. The fungus ball consists of a rounded conglomerate of hyphae, mucus, and cellular debris. The origin of pre-existing cavity is most commonly an old tuberculosis lesion.[54]
Invasive pulmonary aspergillosis (IPA) is a devastating infection that affects patients with neutropenia or neutrophil and/or macrophage dysfunction, cytotoxic chemotherapy, long-term corticosteroid therapy, bone marrow or organ transplantation, and congenital [Figure 16] or acquired immunodeficiency. Among pediatric patients, IPA is predominantly seen in those with hematological malignancies [Figure 17], chronic granulomatous disease, and individuals on corticosteroid and other immunosuppressive therapies.[5455] Radiologic manifestations of IPA include macronodules surrounded by a halo of ground glass opacification (halo sign) Figure 17 and central cavitation of small nodules with an air crescent formation within any area of consolidation. The characteristic halo of ground-glass opacification around the nodule corresponds to hemorrhagic infarcts.[55]
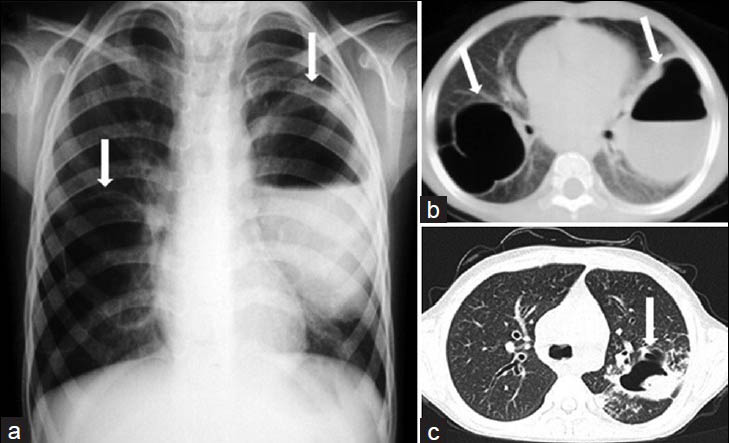
- Complicated staphylococcal pneumonia associated with hyperimmunoglobulin E syndrome (Job's syndrome) in a 5-year old girl. a) Chest radiograph and b) CT scan show bilateral pneumatoceles with thin walls (white arrows) on the right and with air-fluid level on the left (white arrow). c) Follow-up CT scan obtained 4 years after the initial CT scan shows a fungus ball or mycetoma within the pneumatocele (white arrow) on the left. Diagnosis was confirmed at post-mortem examination.
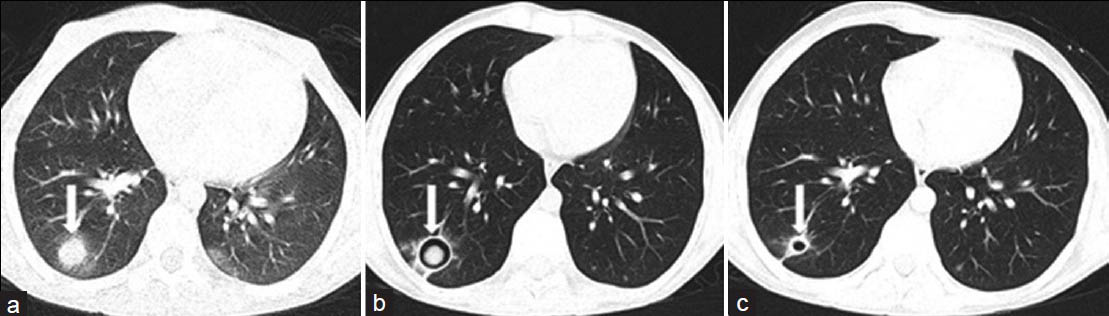
- Invasive pulmonary aspergillosis in a 10-year-old boy with acute lymphoblastic leukemia. a) Active early lesion of invasive pulmonary aspergillosis with CT halo sign (white arrow). b) One year later, follow-up CT scan shows an intracavitary aspergilloma (white arrow) in the right lower lobe. c) Six months later, the lesion is healed. CT scan shows residual thin-walled cyst (white arrow).
Pulmonary blastomycosis, histoplasmosis, and coccidioidomycosis include diffuse infiltrates, fibrosis associated with thick- or thin-walled cavitary lesions. These are endemic in different parts of the world, particularly in the south-central and midwest regions of the United States.[56]
MISCELLANEOUS
Destroid Lung
Destroid lung is an uncommon condition in children, causing irreversible changes in the lung parenchyma, which necessitate surgical intervention. Inflammatory lung diseases, such as pulmonary tuberculosis, whole lung bronchiectasis, necrotizing pneumonia, multiple or extensive lung abscesses, fungal infections, lung gangrene, and mycobacteria other than tuberculosis are considered major causes for this lung disorder.[57] Chronic progressive pulmonary tuberculosis is observed in 5-10% of patients with primary tuberculosis because the lowered cellular immune response of patients. Complete destruction of the whole or a major part of a lung may result from a progressive primary infection. The radiologic features are similar to those of post-primary tuberculosis [Figure 18].[58] Chest radiographic criteria for destroid lung in patients with severe pulmonary infection include loss of normal pulmonary parenchymal architecture and the presence of areas of liquefaction that are progressively replaced by multiple small or large air [Figure 19] or fluid-filled cavities.[5059] Chronic infectious complications in patients with destroid lung include cavitary necrosis, pleural thickening with fibrosis, and marked herniation of the remaining lung with a mediastinal shift to the opposite side on follow-up chest radiograph or CT [Figure 19].[57]
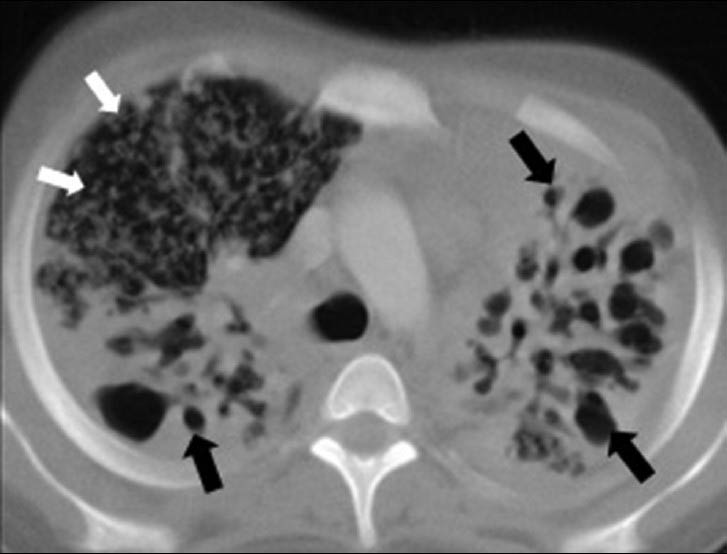
- Destroid lung due to progressive pulmonary tuberculosis in a 16-year-old boy. CT scan (lung window) shows diffuse bilateral small and large air-filled cystic lesions (black arrows) associated with ground-glass attenuation and disseminated miliary micronodular lesions on the right (white arrows).
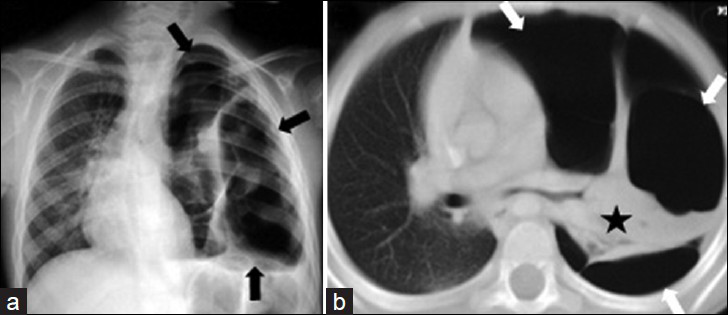
- Destroid lung due to staphylococcal pneumonia in a 13-year-old girl with severe dyspnea and exertional syncope. a) Chest radiograph shows unilateral hyperlucency affecting the entire left lung (black arrows). b) CT scan (lung window) of the same children shows the loss of normal lung architecture and vascularity, necrosis areas that is replaced by multiple small and large air-filled pneumatoceles or thin-walled cavities on the left lung (white arrows), and shrinkage of the left lower lobe (asterisk). Note marked mediastinal shift to the right lung.
Traumatic Pulmonary Pseudocyst
Blunt chest trauma frequently results in pulmonary contusion, hematomas, or effusions, but rarely leads to the appearance of a cystic lesion. Traumatic pulmonary pseudocyst as a result of a chest trauma is a rare event seen in children and young adults in whom the thorax is elastic, the visceral pleura intact, and the parenchyma easily injured. These lesions are either a direct result of the injury itself or develop after resolution of a pulmonary hematoma. On the chest radiograph, an air-fluid level is usually seen and the surrounding lung often shows consolidation due to pulmonary contusion. On CT, post-traumatic pseudocyst appears as round, well-circumscribed single or multiple cavitary lesions with an air-fluid level and a thin wall [Figure 20]. In children, the lesion may be confused with a postpneumonic pneumatocele, bronchogenic cyst, lung abscess, or pulmonary sequestration. Radiologically, pseudocysts usually diminish in size and resolve within 2 or 3 months.[60]
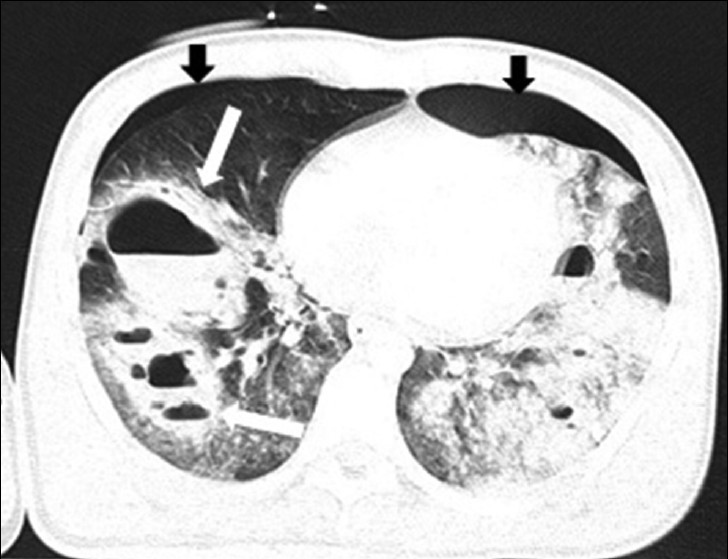
- Traumatic pulmonary pseudocyst in a 15-year-old boy. CT scan shows multiple bilateral cystic lesions with air-fluid level (white arrows) on the right lung and patchy areas of ground-glass opacification. There are bilateral pneumothorax (black arrows).
CONCLUSIONS
There are recognizable radiologic and pathologic patterns of lung parenchymal abnormalities that characterize the various causes of cystic and cavitary lung lesions in the pediatric population. The chest radiograph is a useful, inexpensive examination that is always a part of the initial examination of children with cystic and cavitary lung diseases. However, chest radiograph is less sensitive than CT. Although CT has an ionizing radiation exposure, it is an excellent tool for the evaluation of children with a number of very common clinical complaints, including chronic cough and progressive shortness of breath or exertional dyspnea. Because HRCT has a greater risk of radiation-induced damage in children than adults, low-dose HRCT of the chest in children may be used to obtain further reduction in radiation exposure. MDCT with 2D and 3D reconstructions provides optimal evaluation of the thoracic and abdominal aorta. In the same study, with a single-bolus contrast injection, pulmonary sequestration and arterio-venous malformations can be excellently depicted. However, diagnostic difficulties occur because of the diverse manifestations in children of different ages. There may be some overlap in characteristics among various cystic and cavitary lung lesions in most cases. Thus, needle biopsy or open-lung biopsy may still be required, although in proper clinical settings, results can be suggestive.
Available FREE in open access from: http://www.clinicalimagingscience.org/text.asp?2013/3/1/60/124087
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- Multiple, thin-walled cystic lesions of the lung. AJR Am J Roentgenol. 1980;135:593-604.
- [Google Scholar]
- Cystic and cavitary lung diseases: Focal and diffuse. Mayo Clin Proc. 2003;78:744-52.
- [Google Scholar]
- Imaging evaluation of congenital lung abnormalities in infants and children. Radiol Clin North Am. 2005;43:303-23.
- [Google Scholar]
- CT appearances of solitary and multiple cystic and cavitary lung lesions. Eur Radiol. 2001;11:612-22.
- [Google Scholar]
- High resolution computed tomography of pediatric pulmonary parenchymal disorders. Radiol Clin North Am. 1993;31:533-51.
- [Google Scholar]
- Low-dose high resolution CT of the chest in children and young adults: Dose, cooperation, artifact incidance, and image quality. AJR Am J Roentgenol. 2000;175:985-92.
- [Google Scholar]
- Congenital pulmonary malformations in pediatric patients: Review and update on etiology, classification, and imaging findings. Radiol Clin North Am. 2011;49:921-48.
- [Google Scholar]
- Congenital lobar emphysema: Evaluation and long term follow-up of thirty cases at a single center. Paediatr Pulmonol. 2003;35:384-91.
- [Google Scholar]
- Developmental lung anomalies in the adult: Radiologic-pathologic correlation. RadioGraphics. 2002;22:525-43.
- [Google Scholar]
- The clinical and imaging spectrum of findings in patients with congenital lobar emphysema. Pediatr Pulmonol. 1992;14:160-70.
- [Google Scholar]
- Congenital cystic adenomatoid malformation of the lung: CT-pathologic correlation. AJR Am J Roentgenol. 1997;168:47-53.
- [Google Scholar]
- Late presentation of congenital cystic adenomatoid malformation of the lung. Radiology. 1984;151:569-73.
- [Google Scholar]
- Congenital cystic adenomatoid malformation of the lung. Classification and morphologic spectrum. Hum Pathol. 1977;8:155-71.
- [Google Scholar]
- Congenital pulmonary airway malformation: A new name for an expanded classification of congenital cystic adenomatoid malformation of the lung. Histopathology. 2002;41(Suppl 2):S424-31.
- [Google Scholar]
- CT imaging of mass-like nonvascular pulmonary lesions in children. Pediatr Radiol. 2007;37:1253-63.
- [Google Scholar]
- Pleuropulmonary blastoma. The so-called pulmonary blastoma of childhood. Cancer. 1988;62:1516-26.
- [Google Scholar]
- Pleuropulmonary blastoma is the pulmonary blastoma of the childhood. Semin Diagn Pathol. 1994;11:144-51.
- [Google Scholar]
- Pulmonary cysts in early childhood and the risk of malignancy. Pediatr Pulmonol. 2009;44:14-30.
- [Google Scholar]
- Bronchogenic cyst: Imaging features with clinical and histopathologic correlation. Radiology. 2000;217:441-6.
- [Google Scholar]
- Surgical management and radiological characteristics of bronchogenic cysts. Ann Thorac Surg. 1993;55:476-81.
- [Google Scholar]
- Bronchogenic cysts of the lung. Report of 29 cases. Heart Lung Circ. 2009;18:214-8.
- [Google Scholar]
- Intrapulmonary bronchogenic cyst: CT and pathologic findings in five adult patients. AJR Am J Roentgenol. 2002;179:167-70.
- [Google Scholar]
- Imaging of bronchopulmonary sequestration. J Med Imaging Radiat Oncol. 2009;53:22-31.
- [Google Scholar]
- Severe isolated pulmonary langerhans cell histiocytosis in a 6-year-old girl. Eur J Pediatr. 2004;163:320-2.
- [Google Scholar]
- Langerhans cell histiocytosis, diagnosis, natural history, management, and outcome. Cancer. 1999;85:2278-90.
- [Google Scholar]
- Pulmonary diseases of unknown origin and miscellaneous lung disorders. In: Armstrong P, Wilson AG, Dee P, Hansell DM, eds. Imaging of diseases of the chest (2nd ed). St. Louis: Mosby; 1995. p. :585-8.
- [Google Scholar]
- Radiographic manifestations of anomalies of the lung. Radiol Clin North Am. 1991;29:258-9.
- [Google Scholar]
- Bronchial atresia: Reports of a case and review of the literature. Surg Today. 1993;23:449-54.
- [Google Scholar]
- Diseases of the airways. In: Armstrong P, Wilson AG, Dee P, Hansell DM, eds. Imaging of diseases of the chest (2nd ed). St. Louis: Mosby; 1995. p. :856.
- [Google Scholar]
- Giant pulmonary bulla with mediastinal shift in a 12 1/2 -year-old girl. J Pak Med Assoc. 2012;62:503-4.
- [Google Scholar]
- A diagnostic approach to chest diseases. (2nd ed). Baltimore: Williams and Wilkins; 1987. p. :245-6.
- [Google Scholar]
- Chest computed tomography scans should be considered as a routine investigation in cystic fibrosis. Paediatr Respir Rev. 2006;7:202-8.
- [Google Scholar]
- High-resolution CT in the acute exacerbation of cystic fibrosis: evaluation of acute findings, reversibility of those findings, and clinical correlation. AJR Am J Roentgenol. 1997;169:375-80.
- [Google Scholar]
- Congenital bronchiectasis due to deficiency of bronchial cartilage (Williams-Campbell Syndrome): A case report. J Pediatr. 1975;87:230-4.
- [Google Scholar]
- Pleuropulmonary paragonimiasis: Radiologic findings in 471 patients. AJR Am J Roentgenol. 1992;159:39-43.
- [Google Scholar]
- Radiographic, CT and MRI spectrum of hydatid disease of the chest: A pictorial essay. Eur Radiol. 1993;3:62-70.
- [Google Scholar]
- Single stage transthoracic approach for the right lung and liver hydatid disease. J Thorac Cardiovasc Surg. 2003;126:769-73.
- [Google Scholar]
- Spectrum of imaging findings in pediatric hydatid disease. AJR Am J Roentgenol. 1997;169:1627-31.
- [Google Scholar]
- Traumatic rupture of hydatid cysts: A 12-year experience from an endemic region. J Trauma. 1999;46:164-7.
- [Google Scholar]
- Imaging of cavitary necrosis in complicated childhood pneumonia. Eur Radiol. 2002;12:391-6.
- [Google Scholar]
- Pneumonia in children: Decreased parenchymal contrast enhancement: CT sign of intense illness and impending cavitary necrosis. Pediatr Radiol. 1997;205:817-20.
- [Google Scholar]
- Clinical manifestations and diagnosis of invasive aspergillosis in immunocompromised children. Eur J Pediatr. 2002;161:563-74.
- [Google Scholar]
- Pneumonectomy in children for destroyed lung and the long term consequences. J Thorac Cardiovasc Surg. 2003;126:571-81.
- [Google Scholar]
- Necrotising pneumococcal pneumonia in children. The role of pulmonary gangrene. Pediatr Pulmonol. 2006;41:623-9.
- [Google Scholar]
- Primary traumatic pulmonary pseudocysts: A rare entity. Eur J Cardiothorac Surg. 2003;23:43-5.
- [Google Scholar]






