Translate this page into:
Current Opinion Regarding Multidisciplinary Cancer Clinic Utilization for the Management of Prostate Cancer

*Corresponding author: Daniel J. Lama, MD Division of Urology, Department of Surgery University of Cincinnati School of Medicine, Cincinnati, OH, United States lamadl@ucmail.uc.edu
-
Received: ,
Accepted: ,
How to cite this article: Lama DJ, Kasson M, Hoge C, Guan T, Rao M, Struve T, et al. Current opinion regarding multidisciplinary cancer clinic utilization for the management of prostate cancer. J Clin Imaging Sci 2021;11:29.
Abstract
Objectives:
Multidisciplinary cancer clinic (MDC) is an evaluation option for the management of prostate cancer (PCa). The purpose of MDC is to provide the patient with a comprehensive assessment and risk/benefit discussion of all pertinent treatment options. Our objective was to obtain a contemporary measure and analysis of urologists’ opinion regarding PCa MDC.
Material and Methods:
We created a 14-item questionnaire for respondent baseline characteristics, subjective and objective inquiries regarding MDC for PCa management. The survey was distributed through email to members of the Society of Urologic Oncology and the Endourological Society. Data were analyzed using R (R Core team, 2017).
Results:
One hundred and seven (51%) respondents reported participation in MDC; the majority of which were male (97.6%), academic (61.4%) urologists with urologic oncology fellowship training (50%), and >20 years in practice (40.3%). MDC patients were most commonly referrals (78.5%) and with high-risk disease (Gleason sum 8–10) (83.2%). A majority of the respondents felt that MDC was very or extremely beneficial for PCa research (45% and 19%, respectively) and treatment (35% and 20%, respectively). Responses dissuading the use of MDC included lack of infrastructure (41%) and time commitment (21%). On multivariate analysis, urologists with >10 years in practice were less likely to find MDC beneficial in the management of PCa (11–20 years, P = 0.028 and >20 years P = 0.009).
Conclusion:
A contemporary sampling of urologists’ opinion and practice patterns alludes to the benefits that advocate for and the resource demand that hinders routine use of MDC for PCa evaluation. Urologist training and practice environment can affect participation in PCa MDC.
Keywords
Multidisciplinary cancer clinic
Prostate cancer
Opinion
Survey
INTRODUCTION
Prostate cancer (PCa) remains the most common non-cutaneous malignancy and second most common cause of cancer-related mortality among American men in the 21st century.[1] As PCa management pathways continue to evolve, the process of treatment selection can become a challenging endeavor. Often, notwithstanding a shared decision-making approach, treatment is biased toward the consultant’s area of expertise.[2] Alternatively, PCa multidisciplinary cancer clinic (MDC) comprehensively addresses all applicable treatment options and can occur in the setting of separate risk/benefit discussions with PCa experts in the fields of urologic surgery, medical and radiation oncology following a review of imaging and histopathology by participating radiologists and genitourinary pathologists.[3-6] For some institutions, the use of MDC for PCa management has been a critical part of urologic practice for decades and a mandated modality for the evaluation of newly diagnosed cancer patients.[7,8]
Despite the advantages of MDC for PCa management, patient attendance and the decision to subsequently undergo treatment at the MDC center is variable.[3] However, a distinct benefit to the patient is the multidisciplinary expert review of preexisting pathologic and radiologic data that can result in reclassification of disease, alteration in the treatment algorithm,[4] as well as incur the possibility of a survival benefit.[7] Thus, as a consequence of vetting PCa treatment options within the context of MDC, the subsequent utilization of diagnostic and therapeutic resources is executed in an efficient and guideline adherent manner.
Given the inherent complexity, demand for time and physician resources the institutionalization of MDC is not ubiquitous. Herein, we present a query and analysis of physician characteristics and opinion regarding MDC, and discuss potential predictors of MDC utilization for the evaluation and treatment of PCa.
MATERIAL AND METHODS
Survey
A 14-item questionnaire was created to collect baseline characteristics and practice pattern data among the Society of Urologic Oncology (SUO) and the Endourological Society (ES) members regarding MDC for the management of PCa. We did not include a definition of MDC in the survey, thus the specific MDC setting was unknown. Urologist demographic data included age, sex, practice type, fellowship training, years in practice, and number of PCa patients seen in 1 month’s time. Response options included the selection of a single response, select all that apply, or freehand responses depending on the query. A complete version of the survey is included as a Supplementary Appendix.
Study design
Between January and February 2018, the 14-item questionnaire was disseminated on two separate occasions through an email containing a hyperlink to the web-based survey platform (SurveyMonkey©, Palo Alto, CA, USA) to members of both the SUO and ES. Approximately 2–3 weeks’ time elapsed between survey disseminations. No incentive was offered for completion of the survey. We estimate approximately 6000–7000 total members of the SUO and ES collectively received the request to participate in the study. Survey responses were then collected and analyzed anonymously. For the purposes of discussion of our analysis, we utilized PCa disease risk categorization defined by the National Comprehensive Cancer Network (NCCN).[9] The study was determined to be exempt from review by Institutional Review Board.
Statistical analysis
Statistical analysis was performed using R (R Core team, 2017). Mann–Whitney U-test was used to compare distribution of continuous variables. Pearson Chi-square test and Fisher’s exact tests were used to compare proportions of categorical variables. Multivariate proportional odds models were fitted to identify predictors for urologists’ perception of benefit of MDC in treating PCa patients. The predictors studied were age, type of practice (academic vs. non-academic), years in practice, urologic oncology fellowship training, and number of new PCa patients seen per month. Statistical significance was defined as P < 0.05.
RESULTS
Respondents baseline characteristics are shown in Table 1. A total of 211 responses were obtained: 87 respondents from the SUO (41%) and 124 (59%) respondents from the ES. Thus, the estimated response rate was approximately 3%. Respondents were most commonly academic (61.4%), male urologists (97.6%) with median age of 48.5 years, fellowship training in urologic oncology (50%), and greater than 20 years of practice (40.2%). One hundred and seven respondents (51%) reported that they participated in MDC, of which 53% were fellowship trained. The majority of reported MDCs existed in an academic institution (65.3%).
| Median age (IQR) | 48.5 (18.5) |
|---|---|
| No. of male (%) | 203 (96.7) |
| Practice context (%) | |
| Academic | 129 (61.1) |
| Non-academic | 82 (38.8) |
| Years in practice | |
| 0–5 | 31 (14.7) |
| 6–10 | 35 (16.6) |
| 11–20 | 60 (28.4) |
| >20 | 85 (40.2) |
| Urologic oncology fellowship training (%) | |
| Yes | 105 (50.0) |
| No | 105 (50.0) |
| No. of patients seen with newly diagnosed prostate cancer monthly (%) | |
| None | 4 (1.9) |
| 1–5 | 68 (32.3) |
| 6–10 | 68 (32.3) |
| 11–20 | 46 (21.8) |
| >20 | 25 (11.8) |
IQR: İnterquartile range
Survey responses regarding patient characteristics and the logistics of MDC are depicted in Table 2. MDCs most commonly existed in an academic center (65.3%) and were managed by a similar distribution of urologists, radiation, and medical oncologists. Most respondents agreed that MDC was “very beneficial” for PCa treatment and research [Figure 1]. Criticisms against use of MDC included lack of infrastructure (65.4%) and time consumption (32.7%) [Figure 2].
| Query | Respondents* (%) |
|---|---|
| Where are most referrals to your PCa MDC directed from? | |
| Urology | 84 (78.5) |
| Radiation oncology | 14 (13.1) |
| Medical oncology | 3 (2.8) |
| Primary care provider | 2 (2) |
| Other provider | 4 (3.7) |
| How often do you schedule PCa MDC? | |
| Once per month | 23 (21.5) |
| Twice per month | 29 (27.1) |
| Four times per month | 42 (39.3) |
| Greater than 4 times per month | 11 (10.3) |
| Unknown | 2 (2) |
| What patient population comprises your PCa MDC?† | |
| Clinically localized low-risk disease (Gleason sum 6) | 73 (68.2) |
| Clinically localized intermediate-risk disease (Gleason sum 7) | 86 (80.4) |
| Clinically localized high-risk disease (Gleason sum 8–10) | 89 (83.1) |
| Active surveillance candidates | 61 (57.0) |
| Advanced/metastatic prostate cancer | 78 (72.9) |
| Recurrent prostate cancer | 40 (37.4) |
| What professionals participate in PCa MDC at your institution?† | |
| Urologist | 106 (99.1) |
| Radiation oncologist | 100 (93.5) |
| Medical oncologist | 91 (85) |
| Radiologist | 55 (51.4) |
| Pathologist | 54 (50.0) |
| Nurse practitioner | 50 (46.7) |
| Nutritionist | 13 (12.1) |
| Social worker | 23 (21.5) |
| Other | 7 (6.5) |

- Distribution of opinion regarding the benefits of multidisciplinary clinic for prostate cancer treatment or research. *For 211 responses.
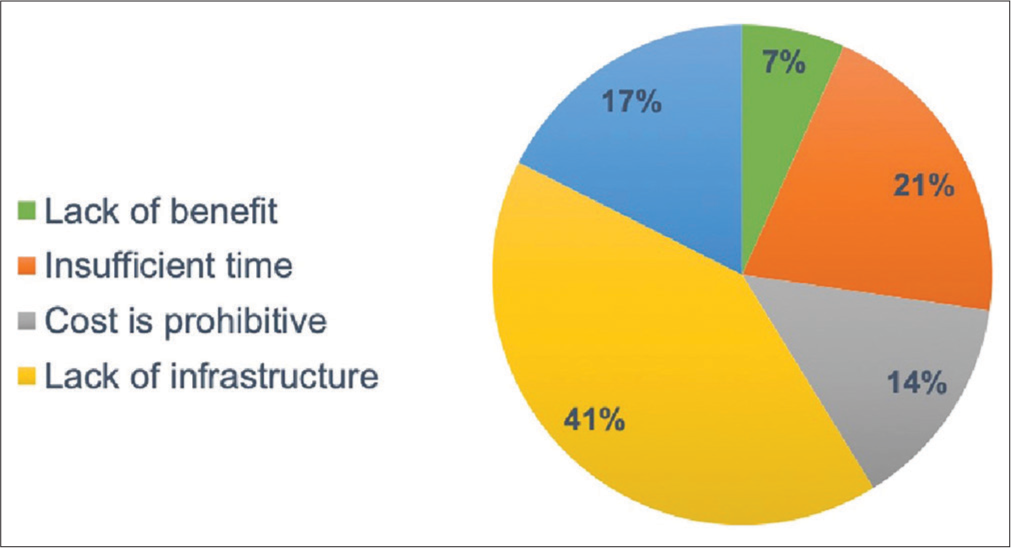
- Survey responses in opposition to the use of prostate cancer multidisciplinary clinic.
Table 3 shows both univariate and multivariate analyses to identify predictors for respondents to agree that MDC benefits management of PCa. On multivariate analysis years in practice impacted, the likelihood of positively viewing MDC, that is, the longer a respondent was in clinical practice, the less beneficial MDCs were reported (11–20 years in practice OR 0.32, 95% CI 0.113–0.882, P < 0.028; >20 years in practice OR 0.14, 95% CI 0.031–0.616, P < 0.009). Specifically, respondents with 11–20 years and >20 years in practice were 68% and 86% less likely to find MDC beneficial for the management of PCa patients. Although respondent age approached statistical significance, no other variables on multivariate analysis were predictive of reporting benefit of MDC.
| Variable | Benefit of MDC for treatment | |||
|---|---|---|---|---|
| Univariate OR (CI) | P-value | Multivariate OR (CI) | P-value | |
| Age | 0.99 (0.97–1.01) | 0.574 | 1.04 (0.99–1.09) | 0.063 |
| Practice type | ||||
| 0=Non-academic | ||||
| 1=Academic | 0.87 (0.50–1.52) | 0.630 | 0.85 (0.45–1.6) | 0.54 |
| Fellowship training* | ||||
| 0=No | ||||
| 1=Yes | 0.89 (0.54–1.44) | 0.632 | 0.77 (0.43–1.36) | 0.37 |
| Years in practice | ||||
| 1=0–5 | ||||
| 2=6–10 | 0.98 (0.41–2.35) | 0.969 | 0.74 (0.26–2.04) | 0.561 |
| 3=11–20 | 0.71 (0.32–1.55) | 0.391 | 0.31 (0.11–0.88) | 0.028 |
| 4≥20 | 0.59 (0.28–1.24) | 0.168 | 0.13 (0.03–0.61) | 0.001 |
| No. of newly diagnosed prostate cancer patients seen per month | ||||
| 0=None | ||||
| 1=1–5 | 0.68 (0.11–4.25) | 0.687 | 0.07 (0.10–4.79) | 0.721 |
| 2=6–10 | 0.55 (0.09–3.39) | 0.519 | 0.70 (0.10–4.75) | 0.716 |
| 3=11–20 | 1.07 (0.17–6.79) | 0.941 | 1.24 (0.18–8.65) | 0.826 |
| 4≥20 | 1.56 (0.23–10.54) | 0.648 | 2.03 (0.27–14.92) | 0.486 |
DISCUSSION
MDC evaluation for men with newly diagnosed PCa represents a modality of management unlike that achievable within a single area of expertise. The premise of MDC lies within the comprehensive review of clinicopathologic and radiologic data such that patient management is deliberated and the most appropriate treatment options, albeit on the basis of national guideline recommendations, are presented to the patient. We sought contemporary opinion of MDC for PCa management from the urologist’s perspective. Our results suggest a positive view of MDC and notable barriers for implementation. We found that the modern PCa MDC was reported to occur within the context of an academic center and is staffed by experienced urologists with fellowship training. The majority of respondents supported MDC with respect to the benefits for patient care and research, whereas criticisms of MDC adoption included complex logistics and the resource demand that is required. Importantly, on multivariate analysis years in clinical practice tended to decrease the probability, a provider would find MDC beneficial for the treatment of PCa.
A tenant of MDC is adherence to current guideline-directed practice. To this end, several centers have explored their MDC experience, compared treatment pathways, and outcomes to a matched cohort of men from the Surveillance, Epidemiology, and End Results (SEER) database. The National Cancer Institute retrospectively compared treatment outcome of their MDC patients with SEER patients and reported overall survival approaching 100% at 5 and 10 years for localized PC, as well as significantly improved survival for locally advanced PCa.[7] Tang et al.[6] found PCa treatment selection differed than that occurring on a national basis; MDC patients with low-risk disease were more often treated with active surveillance and those with high-risk disease were treated with definitive treatment (e.g., prostatectomy or radiation therapy) compared to matched SEER controls over the period of study. Importantly, specific discrepancies were identified in the treatment of intermediate- and high-risk African-American men compared to Caucasian men, which are well-known phenomena.[10] Since MDC provides unbiased PCa counseling, it can help mitigate racial disparities seen in treatment pathways.[11] Similar guideline adherence in appropriately risk stratified PCa treatment selection in the setting of MDC was also identified by Aizer et al. who demonstrated that evaluation at MDC was a significant independent predictor for choosing active surveillance in the setting of low-risk disease.[12]
Our survey responses found most patients presenting to MDC had at least intermediate-risk or advanced PCa. As such, the impact of MDC evaluation for patients with complex disease is seemingly relevant. Reichard et al.’s[13] comparison of their MDC patients with NCCN high-risk and very high-risk PCa to a matched SEER cohort who underwent primary surgical or radiation-based treatment found a mean 17-month survival advantage for all patient’s treated at their MDC. The authors reported that in addition to their radiation treatment protocols, use of neoadjuvant and multimodal treatment algorithms was felt to be the result of appropriately managing the “volume of information processed in a clinical visit” and mitigating “cognitive biases that can affect decision-making.”
Implementation of MDC demands financial resources and a significant time commitment from respective personnel. Our survey respondents reported that most MDCs were at least staffed with a urologist and radiation oncologist and occurred at least more than once per month, which is consistent with the literature.[5,12,14,15] Importantly, nearly 40% of respondents declared a lack of infrastructure as limiting initiation of MDC followed by “insufficient time” and “cost is prohibitive.” Cost and efficiency measures of MDC were studied by De Leso et al.[8] at 52 MDCs across various types of malignancies in the United Kingdom over a 1-month period. Cost analysis accounted for the hourly cost of physicians and ancillary personnel at the MDC meeting, overhead facility cost, and the cost of time spent preparing radiologic and pathologic patient data for review. The authors reported cost estimates between £14,000 and £38,000 ($21,000 to $57,000 based on 2011/2012 years figures) per month depending on the MDC. Interestingly, 42% of patients required greater than 1 MDC meeting, which was most often attributed to lack of acquisition and/or preparation of radiologic and pathologic studies before MDC. Indeed, lack of preparation implicating increased cost of MDC was echoed through interviews regarding protocolization and content of a urologic MDC with member physicians.[15] Criticisms most commonly reported from the study included the lack of dedicated time for MDC preparation, especially for participating radiologists and pathologists, haphazard preparation on the part of the urologist, the lack of a nominated consultant in charge to make executive decisions, a referral pattern rendering patients possibly not meeting MDC inclusion criteria, and the overall time constraint to conduct the MDC. As demonstrated in the abovementioned study, our survey respondents also considered the cost and time commitments as impediments to wider adoption of MDC for PCa.
Although the use of MDC exists outside of PCa management, it is not ubiquitous among all other oncologic specialties in the United States. In the United Kingdom, however, MDCs are a mandated practice for the management of newly diagnosed malignancy.[14] In an effort to improve MDC, Lamb et al.[16] solicited for consensus among the United Kingdom MDC participants across a number of visceral and hematologic malignancies by querying opinions for various aspects of MDC. Their study included >1000 participants and identified consensus for ~85% of queries (113/136) with respect to infrastructure, governance, meeting logistics, and the shared decision-making process of MDC. Therefore, it may be prudent to perform a similar consensus study in the United States across various MDCs to create solutions to our respondents’ concerns that limit MDC adoption.
The results of our multivariate analysis suggest that urologist respondents in practice for longer than 10 years are less likely to find MDC beneficial for PCa management. We found this to be an interesting finding, as fellowship training can impact years in practice and the practice setting but did not significantly affect our queries of MDC. Notwithstanding the limits of our survey to further dissect this finding, we speculate that perhaps urologists, who one can conjecture that after a decade have established themselves in practice, may have created a referral pattern of consultants that would supplement MDC. Moreover, it can be inferred that the decision to establish or participate in an MDC has been already occurred. As previously discussed, the implementation of MDC for cancer care in the United States is not as ubiquitous or mandatory as elsewhere, and therefore, the urologist who is unfamiliar with the logistics and commitments of MDC may in itself be a reason to not participate.
The role of the radiologist in MDC will continue to grow as diagnostic and therapeutic interventions for PCa evolve. The advent of multiparametric magnetic resonance imaging (mpMRI) and the prostate imaging reporting and data system[17] has led to increasing adoption of and advocation for mpMRI-targeted prostate biopsy. A recent consensus of the literature estimates a negative predictive value close to 90% with over a third of men who undergo pelvic mpMRI avoiding the cost, morbidity, and potential overtreatment associated with prostate biopsy.[18] With respect to treatment, partial or focal gland ablation has become a formidable therapeutic modality highly reliant on the interpretation of pelvic mpMRI. In 2020, a European consensus survey of urologic oncologists (72%) and radiologists (28%) was performed to standardize partial gland ablation management of focal PCa, thus highlighting the importance of a continued multidisciplinary approach to PCa. Moreover, as evident in recent survey of radiologists who participate in oncologic MDCs, and echoing our survey results from urologists, time constraints and lack of incentive limit the preparation for and participation in MDC.[19] Ultimately, multidisciplinary efforts will be required to resolve these multidisciplinary issues.
Limitations of this study include the inherent subjectivity in a questionnaire-based investigation and the low survey response rate. Importantly, the majority of respondents were academic urologists participating in an academic institution MDC, which introduces sampling bias to our results. Future sampling of contemporary opinion for MDC should target urologists in the private setting, as private urologists comprise the majority of practicing urologists and also include the perspective of participating and non-participating medical and radiation oncologists, radiologists, and pathologists to address this bias. Our decision to survey only urologists was in part to better understand their perspective as it pertains to initiating mechanisms of change in support of MDC; the contrary could prove valuable should future surveys include radiologists or pathologists exclusively. In addition, a majority of respondents identified men with high-risk PCa as the greatest population of MDC patients, which suggests selection bias. The greater proportion of high-risk PCa patients is likely because men in this group are less often candidates for unimodal treatment or active surveillance and more likely to require a multimodal multidisciplinary approach. Furthermore, the management of low- and intermediate-risk patients can also benefit from a multidisciplinary approach, especially as the role of prostate mpMRI in excluding men from prostate biopsy.[18] In the past decade, the role of ablative focal and partial gland therapy is becoming part of the treatment armamentarium of PCa, which cannot be pursued without obtaining a pelvic mpMRI and suspicious lesions identified by a dedicated radiologist. Finally, depending on the urologists’ practice environment, MDC may be defined differently. For example, patients may encounter a specialist from each respective discipline (surgery, medical, and radiation oncology) in a single visit, require more than a single visit through referral to a consultant following an initial encounter, or “MDC” may refer to a tumor board-like meeting of providers who discuss treatment options before patient interaction, or any combination thereof. Thus, the interpretation of the objective findings and opinions in the present study is likely distributed among different MDC settings and cannot be generalized. Ultimately, providing a definition of MDC would benefit a more targeted approach to determining what works and what does not with respect to creating and maintaining MDC.
CONCLUSION
Most urologists consider MDC to be beneficial for PCa management with respect to the enhanced evaluation and collaboration among physicians, improved patient communication, and quality of care. Endorsement of and participation in MDC can be dependent on the setting in which the urologist initiates practice; interestingly, urologists in practice for longer than 10 years were less likely to find MDC beneficial for the management of PCa. The challenge in incentivizing providers, the logistic, and financial barriers to partake in MDC are shared among urologists, radiologists, and likely other MDC personnel. With a shift toward a targeted tissue diagnosis of PCa, the benefits of a centralized multidisciplinary evaluation and involvement of diagnosticians will become more necessary.
ACKNOWLEDGMENT
Daniel J. Lama: The definition of intellectual content, literature search, manuscript preparation, editing and review Matt Kasson: Data acquisition, manuscript preparation Connor Hoge: Data acquisition, manuscript preparation Tianyuan Guan: Data analysis, statistical analysis Marepalli B. Rao: Data analysis, statistical analysis Timothy Struve: Design, manuscript editing and review Sadhna Verma: Concept, design, manuscript editing and review Abhinav Sidana: Concept, design, manuscript editing and review.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
SUPPLEMENTARY APPENDIX
What is your age? What is your gender (choose one of the following)? Male Female. What is the context of your practice (choose one of the following)? Academic Non-academic. How many years have you been practicing urology (choose one of the following)? 0–5 years 6–10 years 11–20 years Greater than 20 years. Are you fellowship trained in urologic oncology (choose one of the following)? Yes No. On average, how many patients do you see per month with newly diagnosed prostate cancer (choose one of the following)? None Between 1 and 5 patients Between 6 and 10 patients Between 11 and 20 patients Greater than 20 patients. How beneficial do you feel prostate cancer multidisciplinary clinics are in the management of prostate cancer (choose one of the following)? No benefit at all Slightly beneficial Moderately beneficial Very beneficial Extremely beneficial. How beneficial do you feel prostate cancer multidisciplinary clinics are in promoting prostate cancer research and patient enrollments in prostate cancer clinical trials (choose one of the following)? No benefit at all Slightly beneficial Moderately beneficial Very beneficial Extremely beneficial. Does your center/practice use prostate cancer multidisciplinary clinic to manage prostate cancer (choose one of the following)? Yes No. If your practice does not utilize prostate cancer multidisciplinary clinic what is the reason (select all that apply)? Lack of benefit Insufficient time Cost is prohibitive Lack of infrastructure Other ______. If your practice does utilize prostate cancer multidisciplinary clinic at your institution what professional(s) participate in patient evaluation (select all that apply)? Urologist Radiation oncologist Medical oncologist Radiologist Pathologist Advanced practice registered nurse Social worker Other ______. On average, how often do you schedule prostate cancer multidisciplinary clinics (choose one of the following)? Once per month Twice per month Four times per month Greater than 4 times per month. What patient population comprises your prostate cancer multidisciplinary clinic (select all that apply)? Clinically localized low-risk patients (Gleason Grade 3+3) Clinically localized intermediate-risk patients (Gleason Grade 3+4 or 4+3) Clinically localized high-risk patients (>Gleason 4+4) Active surveillance candidates Advanced/metastatic prostate cancer Recurrent prostate cancer. Where are most referrals to your prostate cancer multidisciplinary clinic directed from (choose one of the following)? Urology clinic Primary care clinic Radiation oncology clinic Medical oncology clinic Other provider ________.
References
- Predictors of patient preferences and treatment choices for localized prostate cancer. Cancer. 2008;113:2058-67.
- [CrossRef] [PubMed] [Google Scholar]
- Utilization trends at a multidisciplinary prostate cancer clinic: Initial 5-year experience from the duke prostate center. J Urol. 2012;187:103-8.
- [CrossRef] [PubMed] [Google Scholar]
- Establishment of a new prostate cancer multidisciplinary clinic: Format and initial experience. Prostate. 2015;75:191-9.
- [CrossRef] [PubMed] [Google Scholar]
- Longitudinal regret after treatment for lowand intermediate-risk prostate cancer. Cancer. 2017;123:4252-8.
- [CrossRef] [PubMed] [Google Scholar]
- Contemporary prostate cancer treatment choices in multidisciplinary clinics referenced to national trends. Cancer. 2020;126:506-14.
- [CrossRef] [PubMed] [Google Scholar]
- Enhancing prostate cancer care through the multidisciplinary clinic approach: A 15-year experience. J Oncol Pract. 2010;6:e5-10.
- [CrossRef] [PubMed] [Google Scholar]
- A study of the decision outcomes and financial costs of multidisciplinary team meetings (MDMs) in oncology. Br J Cancer. 2013;109:2295-300.
- [CrossRef] [PubMed] [Google Scholar]
- NCCN guidelines updates: Management of prostate cancer. J Natl Compr Canc Netw. 2019;17:583-6.
- [Google Scholar]
- Racial differences in the surgical care of medicare beneficiaries with localized prostate cancer. JAMA Oncol. 2016;2:85-93.
- [CrossRef] [PubMed] [Google Scholar]
- Multidisciplinary clinics: A possible means to help to eliminate racial disparities in prostate cancer. Cancer. 2020;126:2938-39.
- [CrossRef] [PubMed] [Google Scholar]
- Multidisciplinary care and pursuit of active surveillance in low-risk prostate cancer. J Clin Oncol. 2012;30:3071-76.
- [CrossRef] [PubMed] [Google Scholar]
- Radical prostatectomy or radiotherapy for high-and very high-risk prostate cancer: A multidisciplinary prostate cancer clinic experience of patients eligible for either treatment. BJU Int. 2019;124:811-9.
- [CrossRef] [PubMed] [Google Scholar]
- The impact of multidisciplinary team meetings on patient assessment, management and outcomes in oncology settings: A systematic review of the literature. Cancer Treat Rev. 2016;42:56-72.
- [CrossRef] [PubMed] [Google Scholar]
- Case review in urology multidisciplinary team meetings: What members think of its functioning. J Clin Urol. 2014;7:394-402.
- [CrossRef] [Google Scholar]
- Multidisciplinary team working across different tumour types: Analysis of a national survey. Ann Oncol. 2012;23:1293-300.
- [CrossRef] [PubMed] [Google Scholar]
- Prostate imaging reporting and data system version 2.1: 2019 update of prostate imaging reporting and data system version 2. Eur Urol. 2019;76:340-51.
- [CrossRef] [PubMed] [Google Scholar]
- A multifaceted approach to quality in MRI-directed biopsy pathway for prostate cancer diagnosis. Eur Radiol. 2021;31:4386-89.
- [CrossRef] [PubMed] [Google Scholar]
- Standardized nomenclature and surveillance methodologies after focal therapy and partial gland ablation for localized prostate cancer: An international multidisciplinary consensus. Eur Urol. 2020;78:371-8.
- [CrossRef] [PubMed] [Google Scholar]






