Translate this page into:
Corticosteroid Responsive Sarcoidosis with Multisystemic Involvement Years after Initial Diagnosis: A Lymphoma Mimicker on 18-FDG PET/CT
Address for correspondence: Dr. Turker Acar, Department of Radiology, Abant İzzet Baysal University, Training and Research Hospital, Bolu Turkey. E-mail: drtacar@hotmail.com
-
Received: ,
Accepted: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Sarcoidosis is a chronic multisystemic inflammatory disease characterized by noncaseating epithelioid cell granulomas. 18-Fluorodeoxyglucose positron-emission tomography/computer tomography (FDG-PET/CT) is increasingly used in routine clinical practice to assess active sarcoidosis because it can detect active inflammatory granulomatous disease. However, active sarcoidosis lesions are observed to be hypermetabolic on FDG-PET/CT much like malignancies, which may lead to misinterpretation on imaging. In this case report, we present a rare case of sarcoidosis with multisystem involvement including lung, lymph nodes, bone, pleura, and soft tissue that mimicked lymphoma on FDG-PET/CT and responded to corticosteroid treatment.
Keywords
Computed tomography
lymphoma
positron-emission tomography
sarcoidosis

INTRODUCTION
Sarcoidosis is a chronic systemic inflammatory disease usually affecting middle-aged adults. Lymph nodes, pulmonary parenchyma, eyes, and skin are the tissues frequently affected by sarcoidosis. Noncaseating epithelioid cell granulomas are the key pathologic feature of sarcoidosis in the absence of granulomatous processes triggered by fungal infections, Crohn's disease, tuberculosis, autoimmune diseases, or foreign antigen induced delayed-type hypersensitivity reaction.[1] The lungs and lymph nodes are the most frequently involved organs with a frequency of 90% and 30%, respectively.[2] On the other hand, bone and pleural involvements are rarely seen in sarcoidosis with a frequency of 3–5% and 0.7%, respectively.[34] Herein, we aim to present a rare case of sarcoidosis with multisystem involvement identified on 18-fluorodeoxyglucose positron-emission tomography/computer tomography (FDG-PET/CT). The scan showed metabolic activity in the lungs, lymph nodes, bone, pleura, and soft tissue, mimicking lymphoma. The patient responded to corticosteroid treatment.
CASE REPORT
A 55-year-old woman who worked for 27 years in the cleaning sector presented to the Internal Medicine clinic of our institution with fatigue, swelling in the armpits, back pain, weight loss, and dry cough.
In her medical history, she described lymph node and gingival biopsies having been repeated three times during 2004 – 2009 at different institutions, due to lymph node enlargement and gingival hypertrophy. Pathology results were compatible with ‘non-necrotizing granulomatous lymphadenitis’. Because thoracoabdominal CT revealed enlarged mediastinal, hilar, para-aortic, and axillary lymph nodes, an excisional biopsy of axillary lymph nodes was performed with the preliminary diagnosis of lymphoma prior to the current submission. The pathology result was consistent with ‘non-necrotizing granulomatous lymphadenitis’ again and the patient was referred to the Department of Chest Diseases.
Physical examination revealed painless, but enlarged lymph nodes in the neck, axilla, and inguinal regions. A moderate hepatosplenomegaly was detected during abdominal palpation. Breath sounds were within normal limits. All hematological, biochemical results and pulmonary function tests (PFTs) were in normal limits, except for mildly elevated serum angiotensin converting enzyme (ACE) level which was found to be 60 U/l (reference value: 8–52 U/l). Sputum smear examinations repeated four times for acid-resistant bacilli were negative. After physical examination and laboratory tests, a FDG-PET/CT scan was performed to evaluate disease activity [Figure 1]. FDG-PET/CT examination showed hypermetabolic lymph nodes in all lymphatic regions (cervical, axillar, supraclavicular, bilateral hilar, mediastinal, retroperitoneal, iliac, and inguinal), disseminated bone, right pleural and right gluteal soft tissue involvement with standardized uptake values (SUVs) ranging from 5 to 13. There were also lung nodules in the upper zones, which were consistent with Stage 2 sarcoidosis [Figure 2]. Because FDG-PET/CT revealed accompanying disseminated bone lesions and diffuse lymph node involvement, a bone marrow biopsy was performed in an attempt to exclude metastasis or lymphoma infiltration. Bone marrow biopsy was found to be concordant with sarcoidosis and following biopsy, treatment with 0.5 mg/kg prednisolone was started. All complaints and physical findings totally regressed by the second month of corticosteroid treatment. FDG-PET/CT scan obtained in the 4th month of treatment showed normal physiological levels and the lung nodules regressed [Figure 3]. After treatment serum ACE level also decreased to 10 U/l, which was within normal limits. Currently, the patient has no complaints and is still on 8 mg/day corticosteroids.
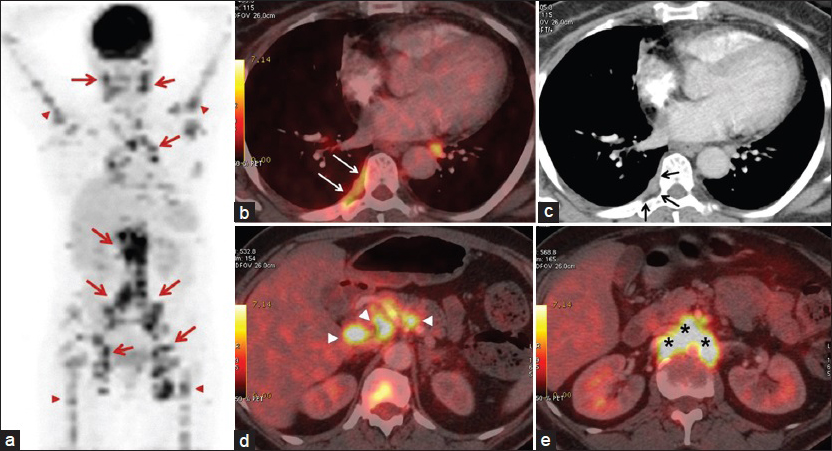
- 55-year-old woman presented with fatigue, swelling in the armpits, back pain, weight loss, and dry cough, later diagnosed with Stage 2 sacroidosis. (a) Positron emission tomography (PET) image shows involvement of lymph nodes (red arrows) and bones (red arrowheads). (b) Axial PET/CT and (c) contrast-enhanced CT images demonstrate right-sided hypermetabolic pleural thickening compatible with pleural sarcoidosis. Note that pleural involvement is indistinct on CT image (black arrows); however, the lesion is apparent on PET/CT (white arrows). Axial PET/CT image from (d) the level of porta hepatis shows discrete lymph nodes (arrowheads) and (e) the para-aortic region shows confluent lymph nodes (asterisk).
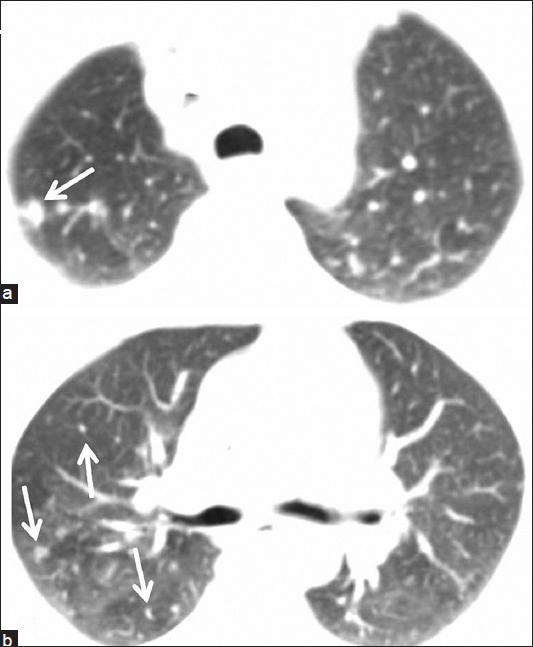
- 55-year-old woman presented with fatigue, swelling in the armpits, back pain, weight loss, and dry cough, later diagnosed with Stage 2 sacroidosis. (a and b) Axial thoracic CT images show right-sided lung nodules compatible with lung parenchyma involvement of sarcoidosis (arrows).
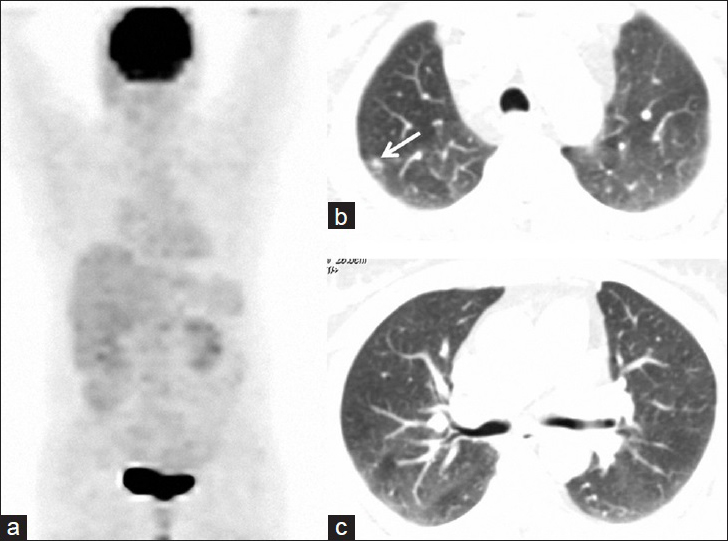
- 55-year-old woman presented with fatigue, swelling in the armpits, back pain, weight loss, and dry cough, later diagnosed with Stage 2 sacroidosis. (a) PET image obtained after corticosteroid treatment reveals total regression of hypermetabolic lesions. (b and c) Axial thoracic CT images from the same lung levels show regression in the parenchymal nodules. Note that right-sided nodules almost completely disappear and one prominent nodule of right apex significantly shrinks (arrow) after medical treatment.
DISCUSSION
Sarcoidosis is a multisystemic inflammatory disease of unknown etiology. The prevalence is reported to be 2–60 per 100,000 people and women are more frequently affected.[5] Pulmonary system and lymph nodes are the most common sites involved in sarcoidosis. Persistent dry cough, shortness of breath, wheezing, and chest pain are the symptoms of pulmonary involvement. Pulmonary sarcoidosis may be classified via chest radiograph into five stages.[6] The current case had faint complaints such as nonproductive cough, but PFTs were preserved. According to FDG-PET/CT and CT scan, the patient had Stage 2 sarcoidosis.
Lymph node enlargement is an expected finding of sarcoidosis. Hilar or paratracheal lymph node enlargement is seen in more than 90% of the cases.[7] Peripheral lymph nodes are enlarged in about 30% of patients and they present a good site for biopsy.[8] Axillary lymph node enlargement was a presentation in our case and that provided a good access point for biopsy.
The abdomen is a common extrathoracic involvement site in sarcoidosis, with a frequency of 50–70%. Liver (50–80%), spleen (40–80%), and abdominal lymph nodes (30%) are the frequently involved abdominal regions.[9] Enlarged abdominal lymph nodes are frequently detected in celiac axis, para-aortic region, and porta hepatis. Contrary to lymphoma, the lymph nodes are more discrete rather than confluent in sarcoidosis.[10] We found discrete lymph nodes in porta hepatis; however, para-aortic lymph nodes were seen in confluent form suggesting lymphoma infiltration.
Bone and bone marrow involvement is rarely seen in sarcoidosis and has a frequency of 3–5%.[3] Primary skeletal involvement without other organ involvement is extremely rare. According to Sparks et al., axial skeleton (primarily the pelvis and the lumbar spine) is the most commonly involved bone.[11] In the present case, the patient presented with back pain on initial submission. FDG-PET/CT examination revealed multiple hypermetabolic bone lesions in the lumbar spine, pelvis, proximal femur, and humerus. Interestingly, these aforementioned osseous lesions were only visible on PET and hybrid images. Based on the extensive bone lesions and involvement of lymph nodes, metastasis or lymphoma could not be ruled out; therefore, a bone marrow biopsy was required to confirm the diagnosis.
Pleural cavity is a rare site of involvement in sarcoidosis. Its incidence ranges between 0.7% and 10% and it can present as nodular pleural thickening, pneumothorax, or pleural effusion.[4] We found faint focal pleural thickening in the right inferomedial pleura, which could have been easily overlooked if only CT scan had been performed. However, the same region demonstrated avid enhancement on FDG-PET/CT facilitating detection of pleural involvement.
Brincker first described an association between sarcoidosis and the development of a lymphoproliferative disease in a group of 46 cases which is called sarcoidosis-lymphoma syndrome (SLS) in 1986.[12] Lymphoma can develop either years after or before the diagnosis of sarcoidosis.[13] It is postulated that an alteration of the immune system in the form of a cell reaction against tumor antigens leads to an increase in T-helper cells in the granulomatous tissues and reduction in circulation of immune system cells. Eventually, this condition can reduce the immune response against oncogenic viruses.[14] Moreover, increased mitotic activity of lymphocytes, particularly B cells, enhances the risk of mutation and malignant transformation. In addition to lymphoma, the risk of developing solid tumor in organs such as the skin, testis, uterus, cervix, lung, and liver is also increased in sarcoidosis.[14] There were strong findings suggestive of SLS in the current case. Firstly, pathology investigations from different clinics proved the lesions to be non-necrotizing granulomatous lesions. Secondly, FDG-PET/CT scan revealed multiple lymph node involvement including retroperitoneal confluent lymph nodes, resembling lymphoma. Thirdly, multiple axial and appendicular osseous lesions were detected, also suggesting lymphoma involvement. However; response of the lesions to corticosteroid and several repeated biopsies ruled out a concurrent lymphoma. Another reason of lymph node, lung, and bone–bone marrow involvement is tuberculosis, which in the active stage is seen as avid lesions on FDG-PET scan. But tuberculosis was excluded through repeated sputum analysis in the current case.
Conventional imaging techniques used in sarcoidosis are chest radiography and CT. Magnetic resonance imaging (MRI) is the preferred imaging method for neurosarcoidosis and in the evaluation of cardiac involvement. However, these aforementioned methods are unable to reveal active inflammation. FDG-PET/CT has a great advantage in the detection of reversible, inflammatory, active granulomatous disease in patients with thoracic sarcoidosis. Teirstein et al., reviewed 188 FDG-PET scans and according to their study, mediastinal lymph nodes and lungs were the most frequent sites of positive PET findings. Positive pulmonary findings were present in 65% of patients in Stage 2 and Stage 3 sarcoidosis.[15] In the current case, hypermetabolic mediastinal, axillar, and hilar lymph nodes were successfully demonstrated on FDG-PET/CT, but we were unable to reveal avid FDG accumulation in lung nodules. We believe the small dimension of pulmonary nodules can be an explanation.
In a recent review published by Keijsers et al., the authors showed that FDG-PET/CT is recommended for identifying extrathoracic involvement of sarcoidosis.[16] In the present case, right pleural involvement was successfully revealed by FDG-PET/CT despite indistinct CT findings. There is also a likelihood of bone–bone marrow involvement in sarcoidosis, as reported by Mostard et al.[3] In the current case, we found significantly increased FDG uptake in lumbar spine, pelvis, humerus, and proximal femur, indicating bone–bone marrow involvement despite no pronounced features being visible on CT.
The cornerstone therapy in sarcoidosis is corticosteroids. But corticosteroid-sparing drugs such as methotrexate, azathioprine, leflunomide, tumor necrosis factor alpha (TNF-α) antagonists, and several monoclonal antibodies (e.g., rituximab) should be considered for management, particularly in patients with the history of corticosteroid side effects and in whom long-term corticosteroid therapy will be required. However, medical therapy should be individualized based on the patient's specific clinical features or side effects.[17] Interestingly, in the current case the patient responded well to corticosteroid treatment despite multisystem involvement.
CONCLUSION
Sarcoidosis may present with multisystem involvement even years after initial diagnosis. FDG-PET/CT is excellent in the assessment of active inflammatory lesions as well as in assessing the response to treatment. Disseminated lesions should alert clinician to consider SLS or tuberculosis in the differential diagnosis. Undoubtedly, histological confirmation will be required in such complex cases.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Available FREE in open access from: http://www.clinicalimagingscience.org/text.asp?2015/5/1/40/161850
REFERENCES
- Pulmonary disorders. In: McPhee SJ, Papadakis MA, Tierney LM, eds. Current Medical Diagnosis and Treatment (49th ed). New York: McGraw Hill; 2010.
- [Google Scholar]
- F-18 FDG PET/CT for detecting bone and bone marrow involvement in sarcoidosis patients. Clin Nucl Med. 2012;37:21-5.
- [Google Scholar]
- Sarcoidosis. In: Fauci A, Braunwald E, Kasper DL, Hauser SL, Longo DL, Jameson JL, Loscalzo J, eds. Harrison's Principles of Internal Medicine (17th ed). New York: McGraw Hill; 2008.
- [Google Scholar]
- Diagnostic value of peripheral lymph node biopsy in sarcoidosis: A report of 67 cases. Can Respir J. 2007;14:209-11.
- [Google Scholar]
- Abdominal sarcoidosis: Cross-sectional imaging findings. Diagn Interv Radiol (Ank). 2015;21:111-7.
- [Google Scholar]
- Imaging manifestations of abdominal sarcoidosis. AJR Am J Roentgenol. 2004;182:15-28.
- [Google Scholar]
- Osseous sarcoidosis: Clinical characteristics, treatment, and outcomes–experience from a large, academic hospital. Semin Arthritis Rheum. 2014;44:371-9.
- [Google Scholar]
- Splenic and lymph nodal involvement in sarcoidosis mimicking lymphoma on fluorine-18 fluorodeoxyglucose positron emission tomography/computed tomography. Indian J Nucl Med. 2015;30:135-8.
- [Google Scholar]
- Results of 188 whole-body fluorodeoxyglucose positron emission tomography scans in 137 patients with sarcoidosis. Chest. 2007;132:1949-53.
- [Google Scholar]
- Advances in the diagnosis and treatment of sarcoidosis. F1000 Prime Rep. 2014;6:89.
- [Google Scholar]






