Translate this page into:
Computerized tomography-Guided Microwave Ablation of Patients with Stage I Non-small Cell Lung Cancers: A Single-Institution Retrospective Study

*Corresponding author: Michael Nance, School of Medicine, University of Missouri Columbia, One Hospital Drive, Columbia, Missouri-United States. mengxb@health.missouri.edu
-
Received: ,
Accepted: ,
How to cite this article: Nance M, Khazi Z, Kaifi J, Avella D, Alnijoumi M, Davis R, et al. Computerized tomography-guided microwave ablation of patients with Stage I non-small cell lung cancers: A single-institution retrospective study. J Clin Imaging Sci 2021;11:7.
Abstract
Objectives:
The objective of the study was to retrospectively investigate the safety and efficacy of computerized tomography-guided microwave ablation (MWA) in the treatment of Stage I non-small cell lung cancers (NSCLCs).
Material and Methods:
This retrospective, single-center study evaluated 21 patients (10 males and 11 females; mean age 73.8 ± 8.2 years) with Stage I peripheral NSCLCs treated with MWA between 2010 and 2020. All patients were surveyed for metastatic disease. Clinical success was defined as absence of FDG avidity on follow-up imaging. Tumor growth within 5 mm of the original ablated territory was defined as local recurrence. Welch t-test and Fisher’s exact test were used for univariate analysis. Hazard ratio (HR) and odds ratio (OR) were determined using Cox regression and Firth logistic regression. Significance was P < 0.05. Data are expressed as mean ± standard deviation.
Results:
Ablated tumors had longest dimension 17.4 ± 5.4 mm and depth 19.7 ± 15.1 mm from the pleural surface. Median follow-up was 20 months (range, 0.6–56 months). Mean overall survival (OS) following lung cancer diagnosis or MWA was 26.2 ± 15.4 months (range, 5–56 months) and 23.7 ± 15.1 months (range, 3–55 months). OS at 1, 2, and 5 years was 67.6%, 61.8%, and 45.7%, respectively. Progression-free survival (PFS) was 19.1 ± 16.2 months (range, 1–55 months). PFS at 1, 2, and 5 years was 44.5%, 32.9%, and 32.9%, respectively. Technical success was 100%, while clinical success was observed in 95.2% (20/21) of patients. One patient had local residual disease following MWA and was treated with chemotherapy. Local control was 90% with recurrence in two patients following ablation. Six patients (28.6%) experienced post-ablation complications, with pneumothorax being the most common event (23.8% of patients). Female gender was associated with 90% reduction in risk of death (HR 0.1, P = 0.014). Tumor longest dimension was associated with a 10% increase in risk of death (P = 0.197). Several comorbidities were associated with increased hazard. Univariate analysis revealed pre-ablation forced vital capacity trended higher among survivors (84.7 ± 15.2% vs. 73 ± 21.6%, P = 0.093). Adjusted for age and sex, adenocarcinoma, and neuroendocrine histology trended toward improved OS (OR: 0.13, 0.13) and PFS (OR: 0.88, 0.37) compared to squamous cell carcinoma.
Conclusion:
MWA provides a safe and effective alternative to stereotactic brachytherapy resulting in promising OS and PFS in patients with Stage I peripheral NSCLC. Larger sample sizes are needed to further define the effects of underlying comorbidities and tumor biology.
Keywords
Microwave ablation
Non-small cell lung cancer
Computerized tomography-guided
Early-stage lung cancer
INTRODUCTION
Lung cancer is the second most common cancer in both men and women with 228,150 new cases and 142,670 deaths in 2019.[1] Stage I (T1-T2aN0M0) lung cancer includes tumors up to size <4 cm diameter without evidence of nodal or metastatic disease.[2] According to National Comprehensive Cancer Network (NCCN) guidelines, surgical resection is the currently recommended first-line treatment for local control of Stage I non-small cell lung cancer (NSCLC).[3] However, a significant cohort of patients cannot tolerate (due to associated comorbidities, age, and poor lung function) or refuse major surgery.[4] Stereotactic ablative radiotherapy (SABR) or image-guided thermal ablation is less invasive options for local tumor control and has been increasingly offered in these patients. At present, the NCCN guidelines recommend SABR as the first-line therapy for Stage I NSCLC in patients who are not good surgical candidates.[3] Radiofrequency ablation (RFA) is a heat-based, safe, and effective ablation technique that has been used in the lung and other organs for local tumor control.[5-7] Due to some of its inherent limitations that include longer ablation times and susceptibility to “heat sink,” many operators now prefer microwave technology for heat-based ablations. In addition, microwave ablation (MWA) generates a larger heating radius within the poor conducting environment of the lung when compared to RFA.[8] Although there are many studies reporting the efficacy of MWA in the liver and kidney, there is a paucity of data evaluating MWA in Stage I NSCLC.[9-12] The relatively short follow-up periods, variable clinical efficacy, inclusivity, and methodology with the available data make meta-analysis challenging.[13-17] Therefore, further study is needed to bolster the available data on outcomes of MWA in NSCLC with the goal of strengthening the pool of evidence for patients and clinicians.
The primary aim of this study was to evaluate the technical success, survival outcomes, and complications in patients with Stage I peripheral NSCLC treated with MWA at a single, rural academic tertiary care hospital, in the Mid-West United States. A secondary aim was to identify factors contributing to survival in this cohort.
MATERIAL AND METHODS
Research ethics standards compliance
This original research was completed under an Institutional Review Board (IRB) approved protocol which waived the need for informed consent. The IRB number was 200,477. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Patient selection
A retrospective review of all patients who underwent MWA for biopsy proven Stage I NSCLC from 2010 to 2020 was performed. Patients that were deemed inoperable by a multidisciplinary team were included in the study. The maximum axial lesion diameter was 3 cm or less. All patients that were included had completed at least a 3-month follow-up after the ablation.
Tumor biopsy procedural details
Computerized tomography (CT)-guided lung biopsies were performed before the ablation. The Bard Mission (BD BARD, Tempe, AZ, USA) semi-automatic biopsy system was used for all the biopsies. At least three 20 G cores were obtained in each patient.
Computed tomography-guided MWA
Ablations were performed under general anesthesia using either the Covidien Evident System or Emprint Ablation System with Thermosphere Technology (Covidien, Boulder, CO, USA) with a frequency of 2.45 GHz and a power of 45 W or 100 W, respectively. The microwave antenna was placed percutaneously into the target lesion using CT guidance. The duration of ablation was selected as per the manufacturer’s recommendation to achieve an adequate ablation zone based on the tumor size. Immediate post-procedure CT and follow-up radiographs were obtained to identify early complications that may have needed additional treatment. If the patients had no complications, they were discharged home. If there was a symptomatic pneumothorax, a CT-guided chest tube was placed and the patient was admitted to the hospital for observation, till sequential chest radiographs demonstrated resolution of the pneumothorax and/or improvement of clinical status. The biopsy, ablations, and chest tube placements were performed by one of three fellowship trained interventional radiologists, two with 10 and one with 8 years of post-fellowship experience.
Post-procedural imaging and follow-up
An initial follow-up non-contrast CT was obtained in the 1st month after ablation, followed by a 3-month positron emission tomography (PET) scan, repeated every 6 months until either death/local tumor progression, or for at least 12 months post-procedure.
Statistical analysis
The statistical analysis was performed using the data analysis toolkit and statistical functions in Microsoft Excel 2020, Ver. 16.38, for MacOS (Microsoft Corp., Redmond, Washington, USA), IBM SPSS Statistics Ver. 26.0 (IBM, Armonk, New York, USA), and SAS Ver. 9.4 (SAS Institute Inc., Cary, North Carolina, USA). Intergroup comparisons were performed using an unpaired t-test assuming unequal variances (Welch’s t-test) or Fisher’s exact test. Kaplan–Meier and family life tables were used to model overall survival (OS) and progression-free survival (PFS) following microablation. A Cox regression analysis in SPSS was used to determine hazard ratio (HR) associated with OS. Odds ratios (ORs) with 95% confidence intervals and maximum likelihood estimates for OS or PFS were determined using PROC LOGISTIC in SAS with a Firth method penalized likelihood to adjust for small sample size bias in maximum likelihood estimation. A Hosmer–Lemeshow goodness-of-fit test was used to assess model fit for each estimate. Summary statistics are expressed as geometric mean ± standard deviation with statistical significance defined as P <0.05.
RESULTS
Patient demographics and tumor characteristics
In total, 21 patients (52.4% of female) who underwent MWA between 2010 and 2020 were identified and included in the study [Table 1]. Median follow-up was 20 months (range, 0.6–56 months). All patients had Stage I peripherally located NSCLC and were negative for metastatic disease as determined by PET imaging at the time of diagnosis. All patients that were deemed non-surgical candidates were offered MWA based on performance status (too poor for SABR) or personal preference. The average age of patients was 73.8 ± 8.2 years. Twenty patients (95.2%) had a history of smoking with an average of 52.1 ± 38.6 pack-years and 8 patients (38.1%) had a prior history of cancer. Information on tumor location, histology, and morphology were collected. Ablated tumors had longest dimension 17.4 ± 5.4 mm and depth 19.7 ± 15.1 mm from the pleural surface and were accessible to percutaneous MWA. Treated tumors were located in the right upper (33.3%), right lower (23.8%), left upper (28.6%), and left lower (14.3%) lobes. Majority of the tumors were either primary adenocarcinoma (33.3%) or squamous cell carcinoma (28.6%).
| Parameter | Cases (%) |
|---|---|
| Gender | n=21 |
| Male | n=10 (47.6) |
| Female | n=11 (52.4) |
| Age (y) | 73.8±8.2 (range:56–87) |
| Male | 72.5±7.5 |
| Female | 74.9±9.0 |
| BMI (kg/m2) | 30.1±11.0 (range:19–65) |
| Male | 26.6±7.6 |
| Female | 33.4±12.9 |
| Race | |
| Caucasian | n=19 (90.5) |
| African American | n=1 (4.8) |
| Asian | n=1 (4.8) |
| Prior history of cancer | n=8 (38.1) |
| NSCLC | n=1 (4.8) |
| PSCC | n=2 (9.5) |
| PAC | n=4 (19.0) |
| HNSCC | n=1 (4.8) |
| Smoking history | n=20 (95.2) |
| Avg. PY | 52.1±38.6 (range:1–130) |
| Comorbidities | |
| Diabetes | n=5 (23.8) |
| CHF | n=1 (4.8) |
| HTN | n=15 (71.4) |
| CKD | n=2 (9.5) |
| HLD | n=13 (61.9) |
| A.Fib | n=4 (19.0) |
| Obesity | n=12 (57.1) |
| Malnutrition | n=2 (9.5) |
| CAD | n=6 (28.6) |
| OSA | n=6 (28.6) |
y: year, BMI: Body mass index, NSCLC: Non-small cell lung cancer, PSCC: Pulmonary squamous cell carcinoma, PAC: Pulmonary adenocarcinoma, HNSCC: Head-and-neck squamous cell carcinoma, PY: Pack-year, CHF: Congestive heart failure, HTN: Hypertension, CKD: Chronic kidney disease, HLD: Hyperlipidemia, A. Fib: Atrial fibrillation, CAD: Coronary artery disease, OSA: Obstructive sleep apnea
Technical, clinical success, local control, and complications
Pre-ablation tumors demonstrated avid FDG activity [Figure 1a]. Technical success was defined as the ability to successfully place the probe within the tumor and perform MWA as per the manufacturer’s recommendation to achieve the intended ablation zone [Figure 1b and c]. This was achieved in 100% of the cases. Clinical success, defined as the absence of FDG activity within the mass on initial follow-up PET imaging [Figure 1d], was achieved in 95.2% of the cases following percutaneous MWA (20/21 cases). The one patient who had residual PET activity was subsequently treated with chemotherapy. Local recurrence, defined as absence of FDG activity on initial follow-up but eventual return of activity within 5 mm of the ablation on subsequent scans, was observed in 10% (2/20) of cases. Of these patients, one opted for chemotherapy while the other opted for observation. Complications were minor as defined by Society of Interventional Radiology guidelines (requiring therapy, hospital admission <48 h) and occurred in 28.6% (6/21) of cases. Complications included pneumothorax requiring a chest tube (n = 5) and small pleural effusion (n = 1). There was no difference in the occurrence of a complication and OS following MWA (P = 0.439).
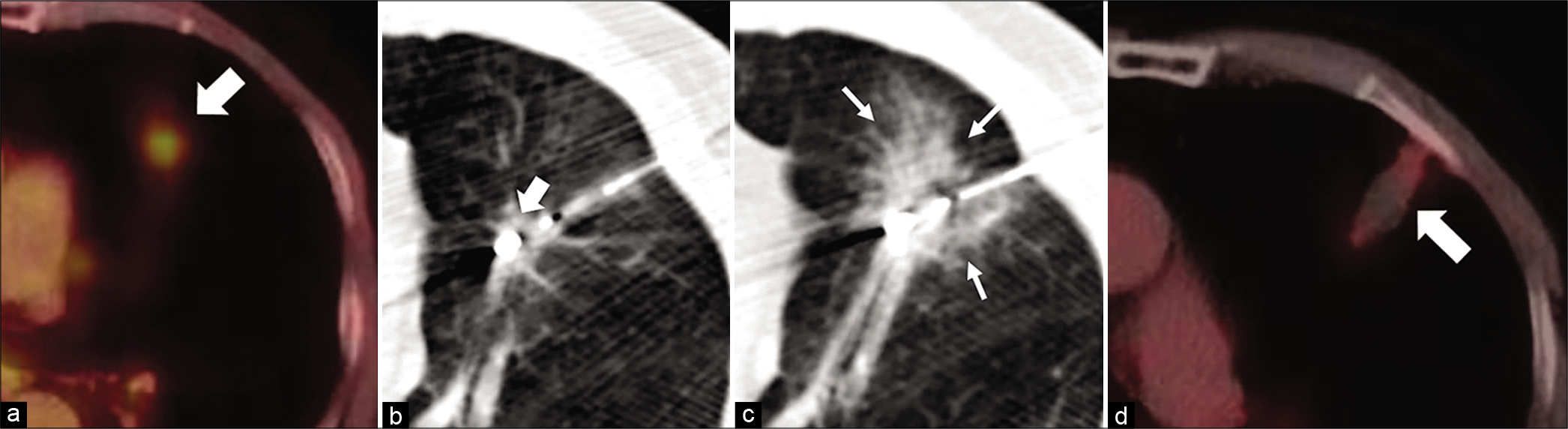
- A 65 year-old female patient presenting with dyspnea diagnosed with biopsy proven Stage I adenocarcinoma of the left lung, who was deemed a non-surgical candidate. Axial fused pre ablation PET (a) showing a FDG active lesion in the lingula (white arrow). Axial procedural CT in a (b) showing the microwave probe in the lingular lesion (white block arrows). Post procedure axial CT (c) showing a ground glass opacity around the ablated zone (white line arrows) indicating a technical success. The axial fused PET 3 months after ablation (d) shows no activity in the lesion (white arrow), indicating clinical success.
Overall and progression-free survival outcomes
Kaplan–Meier analysis was performed to evaluate overall (OS) and progression-free (PFS) survival following lung cancer MWA [Figure 2a and b]. Mean OS following diagnosis or MWA was 26.2 ± 15.4 months (range, 5–56 months) and 23.7 ± 15.1 months (range, 3-55 months). OS at 1, 2, and 5 years was 67.6%, 61.8%, and 45.7%, respectively. Mean PFS was 19.1 ± 16.2 months (range, 1–55 months). PFS at 1, 2, and 5 years was 44.5%, 32.9%, and 32.9%, respectively.

- Kaplan-Meier curves for overall (a) and progression free (b) survival. PFS, progression free survival.
Factors impacting survival outcomes
To investigate factors associated with increased risk of death following MWA of peripheral NSCLC, we performed a Cox regression analysis [Table 2]. Female gender was the only factor associated with a statistically significant reduction in the risk of death (HR: 0.10, 95% CI: 0.03, 0.7, P = 0.014). Increasing age trended toward an increased risk but was not statistically significant. Comorbidities like hyperlipidemia (HR: 4.0, 95% CI: 0.8, 20.1, P = 0.091), among others, trended toward increased hazard, but were not statistically significant. To specifically evaluate differences in patient characteristics before ablation between surviving and non-surviving groups, a univariate analysis was performed using either Welch’s t-test or Fisher’s exact test [Table 3]. There was no statistical difference between any of the factors tested including age, gender, BMI, smoking history, tumor histology, location, or morphology. However, better lung function before ablation demonstrated a strong trend toward higher values in surviving patients (FVC, P = 0.093, FEV1, P = 0.132).
| Parameter | HR (95% CI) | P-value |
|---|---|---|
| Age (y) | ||
| 55–65 | Ref value 1 | 0.114 |
| 66–75 | 6.8 (0.7, 70.4) | 0.108 |
| >75 | 1.6 (0.2, 16.2) | 0.707 |
| Female gender | 0.1 (0.03, 0.7) | 0.014 |
| Smoking history (PY) | 1.0 (0.95, 1.01) | 0.121 |
| Time to ablation | 1.0 (0.99, 1.01) | 0.950 |
| Tumor longest dim. | 1.1 (1.0, 1.3) | 0.197 |
| Pred. FVC % | 1.0 (0.95, 1.04) | 0.843 |
| Pred. FEV1 % | 1.0 (0.91, 1.03) | 0.295 |
| FEV1:FVC ratio | 0.94 (0.85, 1.03) | 0.178 |
| Diabetes | 1.0 (0.16, 6.5) | 0.997 |
| CAD | 0.7 (0.11, 0.4.2) | 0.663 |
| CHF | 1.8 (0.2, 19.1) | 0.623 |
| HLD | 4.0 (0.8, 20.1) | 0.091 |
| A. Fib | 1.1 (0.18, 6.6) | 0.937 |
| Obesity | 1.1 (0.17, 6.6) | 0.945 |
| OSA | 2.2 (0.5, 10.5) | 0.327 |
| HTN | 1.5 (0.25, 9.1) | 0.648 |
| CKD | 2.5 (0.18, 34.8) | 0.503 |
y: Year, PY: Pack-year, dim.: Dimension, pred. FVC%: Predicted forced vital capacity, FEV1%: Forced expiration volume in 1 s, CHF: Congestive heart failure, HTN: Hypertension, CKD: Chronic kidney disease, HLD: Hyperlipidemia, A. Fib: Atrial fibrillation, CAD: Coronary artery disease, OSA: Obstructive sleep apnea
| Parameter | Surviving | Dead | P-value |
|---|---|---|---|
| Age (y) | 73.8±9.6 | 73.8±6.5 | 0.994 |
| Female gender | n=7 (63.3%) | n=4 (36.4%) | 0.670 |
| BMI (kg/m2) | 29.9±13.7 | 30.5±6.7 | 0.903 |
| Smoking history (PY) | 47.5±39.2 | 52.4±41.3 | 0.785 |
| Tumor location | 0.083 | ||
| RUL | n=4 (19.0%) | n=3 (14.3%) | |
| RML | n=0 (0%) | n=0 (0%) | |
| RLL | n=2 (9.5%) | n=3 (14.3%) | |
| LUL | n=3 (14.3%) | n=3 (14.3%) | |
| LLL | n=3 (14.3%) | n=0 (0%) | |
| Tumor pathology | 0.115 | ||
| PAC | n=6 (28.6%) | n=1 (4.8%) | |
| PSCC | n=3 (14.3%) | n=3 (14.3%) | |
| CG | n=0 (0%) | n=1 (4.8%) | |
| NET | n=1 (4.8%) | n=0 (0%) | |
| NSC-US | n=1 (4.8%) | n=1 (4.8%) | |
| Tumor longest dimen. (mm) | 17.0±3.4 | 18.0±7.5 | 0.716 |
| Tumor shortest dimen. (mm) | 12.7±3.6 | 13.7±4.6 | 0.596 |
| Depth from pleura (mm) | 21.1±15.3 | 17.5±15.7 | 0.620 |
| Pred. FVC % | 84.7±15.2 | 73±21.6 | 0.093 |
| Pred. FEV1 % | 57.1±19.9 | 47.3±13.7 | 0.132 |
| FEV1/FVC ratio | 53.8±19.3 | 50.9±14.7 | 0.726 |
| Comorbidities | 0.921 | ||
| Diabetes | n=1 (4.8%) | n=3 (14.3%) | |
| CHF | n=0 (0%) | n=1 (4.8%) | |
| HTN | n=7 (33.3%) | n=6 (28.6%) | |
| CKD | n=1 (4.8%) | n=1 (4.8%) | |
| HLD | n=5 (23.8%) | n=7 (33.3%) | |
| A.Fib | n=1 (4.8%) | n=3 (14.3%) | |
| Obesity | n=4 (19.0%) | n=5 (23.8%) | |
| Malnutrition | n=0 (0%) | n=1 (4.8%) | |
| CAD | n=3 (14.3%) | n=2 (9.5%) | |
| OSA | n=2 (9.5%) | n=3 (14.3%) |
y: Year, BMI: Body mass index, NSC-US: Non-small cell unspecified, PSCC: Pulmonary squamous cell carcinoma, PAC: Pulmonary adenocarcinoma, NET: Neuroendocrine tumor, CG: Caseating granuloma, pred. FVC%: Predicted forced vital capacity, FEV1%: Forced expiration volume in 1 s, PY: Pack-year, CHF: Congestive heart failure, HTN: Hypertension, CKD: Chronic kidney disease, HLD: Hyperlipidemia, A. Fib: Atrial fibrillation, CAD: Coronary artery disease, OSA: Obstructive sleep apnea
Age- and sex-adjusted parameter effects on survival outcomes
To evaluate age- and sex-adjusted parameter effect on OS and PFS, we performed a multivariate analysis with Firth penalized maximum likelihood to compensate for the small sample size [Table 4]. Similar to univariate analysis, there were no statistically significant factor effects identified.
| Parameter | OR (95% CI) | P-value |
|---|---|---|
| OS | ||
| Tumor histology | ||
| Squamous cell carcinoma | Ref value 1 | |
| Adenocarcinoma | 0.13 (0.005, 3.6) | 0.175 |
| Non-small cell unspecified | 0.51 (0.013, 19.3) | 0.936 |
| Neuroendocrine | 0.13 (0.001, 32) | 0.482 |
| Tumor location | ||
| Left upper lobe | ref value 1 | |
| Left lower lobe | 0.18 (0.01, 7.0) | 0.253 |
| Right upper lobe | 0.52 (0.05, 5.7) | 0.543 |
| Right lower lobe | 1.6 (0.13, 18.6) | 0.607 |
| Pre-ablation lung function | ||
| Pred. FVC % | 0.95 (0.89, 1.02) | 0.155 |
| Pred. FEV1 % | 0.96 (0.89, 1.03) | 0.288 |
| FEV1/FVC ratio | 0.99 (0.93, 1.07) | 0.859 |
| Comorbidities | ||
| Diabetes | 3.8 (0.36, 40) | 0.268 |
| Coronary artery disease | 1.9 (0.20, 18) | 0.578 |
| Hyperlipidemia | 4.3 (0.60, 31) | 0.145 |
| Atrial fibrillation | 3.5 (0.31, 38) | 0.309 |
| Obstructive sleep apnea | 2.3 (0.28, 18) | 0.443 |
| Obesity | 3.1 (0.40, 24) | 0.279 |
| PFS | ||
| Tumor histology | ||
| Squamous cell carcinoma | ref value 1 | |
| Adenocarcinoma | 0.88 (0.06, 12.8) | 0.419 |
| Non-small cell unspecified | 1.2 (0.03, 41.2) | 0.902 |
| Neuroendocrine | 0.37 (0.002, 90) | 0.484 |
| Tumor morphology | ||
| Longest dimension | 0.99 (0.82, 1.2) | 0.915 |
| Depth from the pleura | 0.98 (0.93, 1.04) | 0.522 |
| Smoking pack years | 1 (0.98, 1.03) | 0.631 |
OS: Overall survival, PFS: Progression-free survival, pred. FVC%: Predicted forced vital capacity, FEV1%: Forced expiration volume in 1 s
DISCUSSION
NCCN guidelines recommend surgical resection for Stage I NSCLC.[3] Patients who are not surgical candidates should be offered SABR or thermal ablation that can be performed with significantly less morbidity, yet a curative intent. The advantages of SABR are that it is non-invasive and does not require placement of a chest tube or overnight stay in hospital; however, dose fractionation with SABR means it does require multiple hospital visits. SABR is also associated with complications such as radiation-induced pneumonitis, chronic chest wall pain, and rib fractures.[18-20] Furthermore, when the radiation dose is reduced to protect central mediastinal, hilar structures, or the chest wall, it appears to be less effective.[21] A few studies suggest that SABR may be more toxic and less effective in elderly population.[22] Thermal ablation is a minimally invasive option for local tumor control in patients with early-stage NSCLC who are not suitable for major surgery. RFA is the most established thermal ablation technique in terms of safety and efficacy. Although there are no randomized control prospective studies comparing SABR to RFA, recent systematic review and pooled analysis have shown better local control rates with SABR compared to RFA.[23] This may be in part due to the poor performance of RFA in the poor conducting environment of the lung parenchyma.[7] MWA is a newer technique that has been used as an alternative to RFA. The purported advantages of MWA over RFA include achieving higher temperatures, larger ablation zones, shorter ablation times, and reduced susceptibility to the heat-sink effect (inefficient ablation from the cooling effect of blood in adjacent vessels). These properties of MWA may be especially beneficial in the lung which has a high heat-sink effect and low thermal conductivity due to its large perfusion volume and ventilated air-filled spaces, respectively. However, studies evaluating MWA in Stage I peripheral NSCLC are sparse, and the available studies are limited in the reporting of long-term outcomes.[13,16,24-29] There are no studies directly comparing MWA to SABR and the available data are limited to retrospective meta-analyses.[30,31] The local control and OS in these studies are comparable to SABR.[30,32] This suggests that MWA may provide an alternative treatment for patients with early-stage, inoperable NSCLC who cannot receive or would like to avoid SABR. In addition, MWA ablation can be repeated in the same location (in case of residual disease or local recurrence), which is generally not recommended following SABR due to dosimetric constraints.
In the present study, we achieved a very promising clinical success of 95.2% following MWA of early-stage NSCLC. OS in these patients at 1, 2, and 5 years post-ablation was 67.6%, 61.8%, and 45.7%, respectively. These results are consistent with the literature for MWA in early-stage NSCLC.[24,25,29,32-35] Our study also observed PFS at 1, 2, and 5 years of 44.5%, 32.9%, and 32.9%, respectively, which is much lower than previously reported by Healey et al.[16] However, most of these were from distant progression rather than local recurrence. Our local control following MWA was 90.5%. Symptomatic pneumothorax requiring a chest tube was the most common complication, and these were all recognized early and treated successfully. We did not observe any effect of complications on survival.
To identify potential prognostic factors following MWA, we performed univariate and multivariate analyses. Consistent with these prior studies, our results demonstrated a significant protective effect of female gender following MWA of NSCLC (HR: 0.10).[36,37] Lung function before MWA was also identified as a potential prognostic factor in the present study. On average, we observed that long-term survivors had FEV1/FVC ratio and FEV1 values consistent with Global Initiative for Chronic Obstructive Lung Disease criteria Class II (GOLD) while non-survivors had a relative decrease in mean FEV1 (GOLD III), as reported in prior studies.[38,39]
The negative effects of comorbidities on lung cancer mortality have been well established.[40-42] Lung cancer patients are also more likely to have comorbidities such as COPD, diabetes, and hypertension.[42,43] These associations are likely due to the strong connection between smoking and lung cancer.
In line with the previous studies, we also observed a strong trend toward negative effects on survival with several comorbidities. This included hyperlipidemia, diabetes, atrial fibrillation, obesity, and obstructive sleep apnea among others. Our results as well as others support the importance of managing and aggressively treating comorbidities in lung cancer regardless if surgery, SABR, or MWA is used to treat the primary tumor.
In addition, tumor histology may have important impact on the success of MWA. In this study, we observed a diagnosis of adenocarcinoma or neuroendocrine pathology had improved survival (87% increased odds of survival) following MWA compared to squamous cell carcinoma. This supports the findings of Gao et al. who observed survival following MWA with a diagnosis of adenocarcinoma was 14.7 months compared to 10.3 months non-adenocarcinoma.[44] Interestingly, the histological subtype of adenocarcinoma has been identified as a prognostic factor for time to local recurrence following thermal ablation. Specifically, solid and micropapillary subtypes may demonstrate increased cumulative incidence of recurrence.[44]
Limitations
The present study has several limitations which should be considered when interpreting the conclusions. The retrospective nature of the study has inherent limitations including a lack of available medical information for all patients and limited enrollment. Furthermore, retrospective analysis limits causal conclusions and only associations can be ascertained. Medical records containing the exact pathological diagnosis were not available for all patients, these were classified as NSCLC unspecified. The ability to include the exact type of NSCLC for these patients may have altered the results of statistical sub-analyses. This was further compounded by the number of patients lost to follow-up and variability in follow-up duration. Furthermore, this study was a case series design without a control group. Future prospective studies are needed to directly compare MWA to SABR and/or surgical resection. In addition, the study was conducted at a rural, single tertiary care hospital in Midwestern United States, which may influence the generalizability of the results as there are certain factors unique to our population (e.g., higher smoking rates and pulmonary histoplasmosis). As a corollary, the sample size in this study was small. Even though statistical tests designed for small sample sizes were used, the results should still be interpreted with caution. However, the OS and PFS are mostly consistent with the prior literature on MWA at other institutions. Finally, the present study was unable to assess the effect of large tumor size as the average size of tumors included in this study was 1.74 cm. The effectiveness of MWA, compared to surgery and other ablative techniques, is an important consideration when treating larger tumors. For instance, Healey et al. observed significant decrease in effectiveness with tumors >3.0 cm with 7% increase in success with every mm decrease in size.[16]
CONCLUSION
Our results are consistent with previous MWA studies with good clinical success and local control of Stage I peripheral NSCLC, thus serving as a positive endorsement for MWA as a safe and effective alternative to SABR. In addition, our long-term follow-up showed promising OS in patients with Stage I NSCLC following MWA. Larger sample sizes are needed to further define the effects of pre-ablation lung functions, comorbidities, and tumor biology on MWA outcomes and to discern the most optimal indications and guidelines for MWA in NSCLC.
Declaration of patient consent
Institutional Review Board permission obtained for the study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- AJCC Cancer Staging Manual. 2020. Berlin: Springer; Available from: https://www.springer.com/gp/book/9783319406176. [Last accessed on 2020 Oct 23]
- [Google Scholar]
- Factors associated with decisions to undergo surgery among patients with newly diagnosed early-stage lung cancer. JAMA. 2010;303:2368-76.
- [CrossRef] [PubMed] [Google Scholar]
- Radiofrequency ablation for lung tumors: Outcomes, effects on survival, and prognostic factors. Diagn Interv Radiol. 2016;22:65-71.
- [CrossRef] [PubMed] [Google Scholar]
- Radiofrequency ablation of cancer. Cardiovasc Intervent Radiol. 2004;27:427-34.
- [CrossRef] [PubMed] [Google Scholar]
- Computed tomography guided radio-frequency ablation of osteoid osteomas in atypical locations. Indian J Radiol Imaging. 2019;29:253-7.
- [CrossRef] [PubMed] [Google Scholar]
- Radiofrequency and microwave ablation of the liver, lung, kidney, and bone: What are the differences? Curr Probl Diagn Radiol. 2009;38:135-43.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy and safety of microwave ablation for malignant renal tumors: An updated systematic review and meta-analysis of the literature since 2012. Korean J Radiol. 2018;19:938-49.
- [CrossRef] [PubMed] [Google Scholar]
- Image-guided percutaneous microwave ablation of small renal tumours: Short-and mid-term outcomes. Quant Imaging Med Surg. 2015;5:649-55.
- [Google Scholar]
- Microwave ablation compared with hepatic resection for the treatment of hepatocellular carcinoma and liver metastases: A systematic review and meta-analysis. World J Surg Oncol. 2019;17:98.
- [CrossRef] [PubMed] [Google Scholar]
- Malignant liver tumors: Treatment with percutaneous microwave ablation-complications among cohort of 1136 patients. Radiology. 2009;251:933-40.
- [CrossRef] [PubMed] [Google Scholar]
- Microwave ablation for lung cancer patients with a single lung: Clinical evaluation of 11 cases. Thorac Cancer. 2018;9:548-54.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical analysis on 113 patients with lung cancer treated by percutaneous CT-guided microwave ablation. J Thorac Dis. 2017;9:590-7.
- [CrossRef] [PubMed] [Google Scholar]
- Percutaneous thermal ablation for stage IA non-small cell lung cancer: Long-term follow-up. J Thorac Dis. 2017;9:4039-45.
- [CrossRef] [PubMed] [Google Scholar]
- Microwave ablation for lung neoplasms: A retrospective analysis of long-term results. J Vasc Interv Radiol. 2017;28:206-11.
- [CrossRef] [PubMed] [Google Scholar]
- CT-guided percutaneous microwave ablation of pulmonary malignancies: Results in 69 cases. World J Surg Oncol. 2012;10:80.
- [CrossRef] [PubMed] [Google Scholar]
- Organizing pneumonia after stereotactic ablative radiotherapy of the lung. Radiat Oncol. 2012;7:123.
- [CrossRef] [PubMed] [Google Scholar]
- Low incidence of chest wall pain with a risk-adapted lung stereotactic body radiation therapy approach using three or five fractions based on chest wall dosimetry. PLoS One. 2014;9:e94859.
- [CrossRef] [PubMed] [Google Scholar]
- Patterns of disease recurrence after stereotactic ablative radiotherapy for early stage non-small-cell lung cancer: A retrospective analysis. Lancet Oncol. 2012;13:802-9.
- [CrossRef] [Google Scholar]
- Prediction of chest wall toxicity from lung stereotactic body radiotherapy (SBRT) Int J Radiat Oncol Biol Phys. 2012;82:974-80.
- [CrossRef] [PubMed] [Google Scholar]
- Stereotactic ablative body radiation therapy for octogenarians with non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2013;86:257-63.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of the effectiveness of radiofrequency ablation with stereotactic body radiation therapy in inoperable stage I Non-small cell lung cancer: A systemic review and pooled analysis. Int J Radiat Oncol Biol Phys. 2016;95:1378-90.
- [CrossRef] [PubMed] [Google Scholar]
- Percutaneous high-energy microwave ablation for the treatment of pulmonary tumors: A retrospective single-center experience. J Vasc Interv Radiol. 2016;27:474-9.
- [CrossRef] [PubMed] [Google Scholar]
- Microwave ablation versus radiofrequency ablation for the treatment of pulmonary tumors. Oncotarget. 2017;8:109791-8.
- [CrossRef] [PubMed] [Google Scholar]
- Radiotherapy, lobectomy or sublobar resection? A meta-analysis of the choices for treating stage I non-small-cell lung cancer. Eur J Cardiothorac Surg. 2017;51:203-13.
- [CrossRef] [PubMed] [Google Scholar]
- Microwave ablation therapy: Clinical utility in treatment of pulmonary metastases. Radiology. 2011;261:643-51.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy and safety of microwave ablation in the treatment of patients with oligometastatic non-small-cell lung cancer: A retrospective study. Int J Hyperthermia. 2019;36:827-34.
- [CrossRef] [PubMed] [Google Scholar]
- Microwave ablation of lung malignancies: Effectiveness, CT findings, and safety in 50 patients. Radiology. 2008;247:871-9.
- [CrossRef] [PubMed] [Google Scholar]
- Is microwave ablation an alternative to stereotactic ablative body radiotherapy in patients with inoperable early-stage primary lung cancer? Interact Cardiovasc Thorac Surg. 2019;29:539-43.
- [CrossRef] [PubMed] [Google Scholar]
- Stereotactic body radiotherapy versus percutaneous local tumor ablation for early-stage non-small cell lung cancer. Lung Cancer. 2019;138:6-12.
- [CrossRef] [PubMed] [Google Scholar]
- Percutaneous microwave ablation of stage I medically inoperable non-small cell lung cancer: Clinical evaluation of 47 cases. J Surg Oncol. 2014;110:758-63.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison between microwave ablation and lobectomy for stage I non-small cell lung cancer: A propensity score analysis. Int J Hyperthermia. 2018;34:1329-36.
- [CrossRef] [PubMed] [Google Scholar]
- A meta-analysis of clinical outcomes after radiofrequency ablation and microwave ablation for lung cancer and pulmonary metastases. J Am Coll Radiol. 2019;16:302-14.
- [CrossRef] [PubMed] [Google Scholar]
- Microwave ablation (MWA) of pulmonary neoplasms: Clinical performance of high-frequency MWA with spatial energy control versus conventional low-frequency MWA. AJR Am J Roentgenol. 2019;213:1388-96.
- [CrossRef] [PubMed] [Google Scholar]
- Gender and outcomes in non-small cell lung cancer: An old prognostic variable comes back for targeted therapy and immunotherapy? ESMO Open. 2018;3:e000344.
- [CrossRef] [PubMed] [Google Scholar]
- Sex differences in presentation, management, and prognosis of patients with non-small cell lung carcinoma. J Thorac Cardiovasc Surg. 2000;119:21-6.
- [CrossRef] [Google Scholar]
- Strategy Independent Professional Body Guideline Guidelines. 2020. Available from: https://www.guidelines.co.uk/respiratory/gold-copd-2020-strategy/455088 article. [Last accessed on 2020 Oct 21]
- [Google Scholar]
- Impact of COPD on prognosis of lung cancer: From a perspective on disease heterogeneity. Int J Chron Obstruct Pulmon Dis. 2018;13:3767-76.
- [CrossRef] [PubMed] [Google Scholar]
- Comorbidity and survival in lung cancer patients. Cancer Epidemiol Biomarkers Prev. 2015;24:1079-85.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of comorbidity on lung cancer diagnosis timing and mortality: A nationwide population-based cohort study in Taiwan. Biomed Res Int. 2018;2018:1252897.
- [CrossRef] [PubMed] [Google Scholar]
- Impact of comorbidity on lung cancer survival. Int J Cancer. 2003;103:792-802.
- [CrossRef] [PubMed] [Google Scholar]
- Lung cancer survival and comorbidities in lung cancer screening participants of the Gdańsk screening cohort. Eur J Public Health. 2019;29:1114-7.
- [CrossRef] [PubMed] [Google Scholar]
- Micropapillary and/or solid histologic subtype based on pre-treatment biopsy predicts local recurrence after thermal ablation of lung adenocarcinoma. Cardiovasc Intervent Radiol. 2018;41:253-9.
- [CrossRef] [PubMed] [Google Scholar]







