Translate this page into:
Computed Tomography Findings of an Unusual Maxillary Sinus Mass: Brown Tumor Due to Tertiary Hyperparathyroidism
Address for correspondence: Dr. Canan Altay, Department of Radiology, Dokuz Eylul University, School of Medicine, Mithatpaşa Street Inciralti 35340, Izmir, Turkey. E-mail: cananaltay@yahoo.com
-
Received: ,
Accepted: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Brown tumor is a non-neoplastic bone lesion that develops secondary to hyperparathyroidism and it is very rare in the maxillofacial region. We report the case of a 59-year-old man who presented with pain and a swelling in the left cheek. Computed tomography (CT) demonstrated an expansile and radioluscent lesion in the left maxillary sinus. Incisional biopsy was performed, and the diagnosis was Brown tumor. Brown tumor must be considered in the differential diagnosis of expansile lesions of maxillary sinus.
Keywords
Brown tumor
computed tomography
hyperprathroidism
maxillary sinus
INTRODUCTION

Brown tumors are non-neoplastic lesions caused by complications of hyperparathyroidism (HPT). It arises from areas of intense bone resorption and fibroblastic tissue fills in this cavity. Brown tumor is characterized by locally expansile behavior. The ribs, pelvic flat bones, and extremities are most commonly affected sides.[1] This lesion associated with high parathyroid hormone levels due to HPT result in elevated osteoclastic resorption of bone. Brown tumor of the maxillary sinus has rarely been reported.[2]
CASE REPORT
A 59-year-old man presented with a 10-month history of progressively increasing swelling in the left maxillary sinus and associated pain. He also had signs and symptoms of chronic renal failure developing following long-standing pyelonephritis with urolithiasis. He was maintained on hemodialysis 4 h, three times per week. Physical examination revealed a large mass located in the left maxillary sinus. The mucosa-covered lesion was discernible at the left maxillary vestibule. Routine laboratory exams revealed the following: Blood urea nitrogen 33 mg/dL, serum creatinine 7.8 mg/dL, serum calcium 11.7 mg/dL (normal range: 8.6-10.2 mg/dL), alkaline phosphatase 304 IU/L (normal range: 40-150 IU/L), and parathormone 1942 pg/mL (normal range: 12-65 pg/mL). Due to the high serum calcium level, the patient was diagnosed with tertiary HPT associated with long-standing chronic renal failure. Maxillofacial computed tomography (CT) scan revealed a 38 × 46 × 68 mm, well-defined, lobular, expansile radiolucent mass in the left maxillary sinus extending through the left nasal cavity, floor of the orbit, left infratemporal fossa, and premaxillary soft tissue. The anterior, medial, and posterior walls of the maxillary sinus were eroded. The mass was slightly hyperdense with adjacent soft tissue on contrast-enhanced maxillofacial CT scan [Figure 1]. The differantial diagnosis of the lesion included benign and malignant tumors of jaw, odontogenic tumors, infectious diseases, metastasis, simple bone cyst, and Brown tumor.
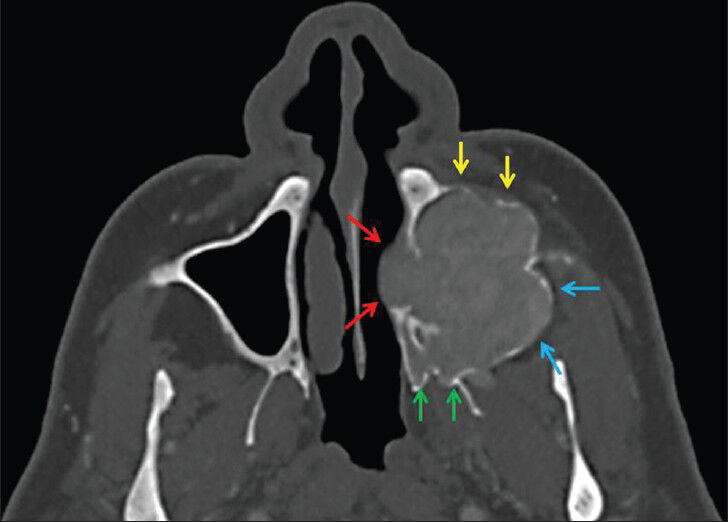
- 59-year-old man presented with a 10-month history of pain and swelling inthe left maxillary sinus diagnosed with Brown tumor associated with tertiary parathyroidism. (October 14, 2010) Axial maxillofacial computed tomography scan shows a lesion on the floor of the left maxillary sinus extending through the nasal cavity (red arrows), infratemporal fossa (blue arrows), pterygopalatine fossa (green arrows), and premaxillary region (yellow arrows). The expansion of the left maxillary sinus is visible.
The incisional biopsy followed by histopathologic investigation of the excised mass revealed a lesion with regions of discrete bone resorption with osteoclast activity, fibroblasts with areas of hemorrahage, and multinucleated giant cells [Figure 2]. Finally, together with the medical history, imaging and pathological findings, and laboratory results, our case was diagnosed as tertiary HPT with Brown tumor of maxillary sinus. Ultrasound examination of the neck showed enlargement of all four parathyroid glands. A subtotal parathroidectomy was performed. Postoperatively, there was distinct improvement as shown in the blood profile with serum phosphate, calcium, and parathormone levels dropping to 5.2 mg/dL, 9.8 mg/dL, and 3 pg/mL, respectively. There have been no signs of enlargement or destruction of adjacent bone tissue during the 27-month follow-up period. CT scan (in January, 2013) 27-months post-treatment showed thickened cortical bone of the tumor walls, decreased tumor size, and increased density of the lesion matrix [Figure 3].
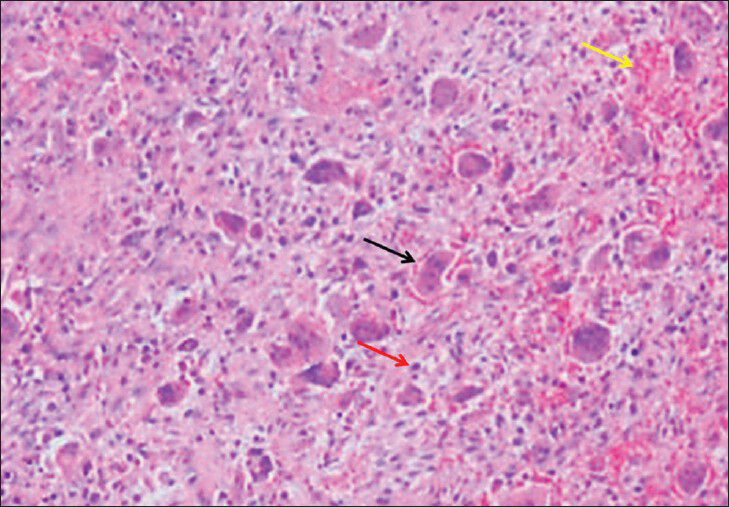
- 59-year-old man presented with a 10-month history of pain and swelling inthe left maxillary sinus diagnosed with Brown tumor associated with tertiary parathyroidism. Histopathologic slide shows multiple giant cells (black arrow) with spindle-shaped stromal cells, fibrous connective tissue (red arrow) proliferation, and areas of hemorrhage (yellow arrow) (H and E, × 40).
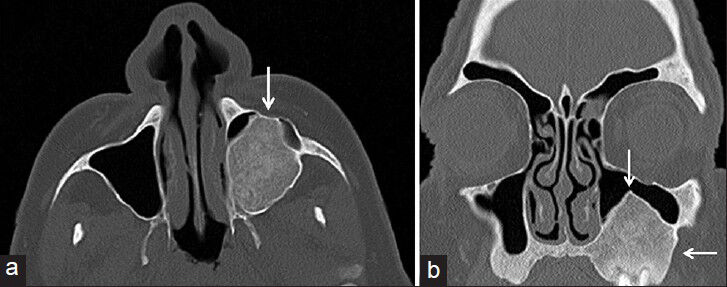
- 59-year-old man presented with a 10-month history of pain and swelling in the left maxillary sinus diagnosed with Brown tumor associated with tertiary parathyroidism. (January 30, 2013) 27-month post-treatment (a) axial and (b) coronal maxillofacial computed tomography images reveal thickening of bone walls of the lesion with a reduction of the tumor volume (arrow) and increase in the density of the lesion.
DISCUSSION
Within the group of HPT three subtypes are recognized: Primary, secondary, and tertiary.[3] Primary HPT occurs due to increased parathyroid hormone (PTH) secretion caused by abnormality in one or more of the parathyroid glands. Adenomas and glandular hyperplasia are the main causes of primary HPT. Secondary HPT is one of the most important complications of chronic renal failure. Secondary HPT occurs due to compensatory hypersecretion of parathormone and this condition may cause a decrease in blood calcium level.[2] In rare cases, longstanding secondary HPT leads to hyperplasia of one or more parathyroid glands, and the system for normal inhibition of PTH secretion by calcium is ruined.[4] This event is called as tertiary HPT.
HPT leads to cortical bone resorption, development of microfracture, and subsequent bleeding in bone tissue. After this stage, multinucleated macrophage migrate to this area and the ingrowth of granulation tissue occurs. This lesion is known as Brown tumor. Brown tumor is a member of bone-expanding giant cell lesions, these lesions are giant cell reparative granuloma, cherubism, and brown tumor.[1] Histologically, brown tumor is composed of numerous multinucleated giant cells, dense fibroblasts and fibrous tissue, oseoid, hemosiderin deposits within macrophages, small microhemorrhages, and area of cystic degeneration of necrosis.[5] Identifying these lesions on the basis of histology or radiology examination is not easy. The presence of chronic renal failure findings is a guide for the diagnosis of Brown tumor in cases with secondary or tertiary HPT.[6]
The osseous lesion resulting from HPT is Brown tumor and is found in approximately 10% of cases.[1] Brown tumors may occur as solitary or multiple lesions. This lesion is painless and is usually found incidentally. These lesions can cause destruction to adjacent structures and lead to symptoms related to compression of surrounding organs. However, they do not undergo malignant transformation. The sinonasal cavity is an unusual site for Brown tumors. Usually, this lesion orginates from the pelvis, ribs, clavicles, mandible, and extremities; however, Brown tumors can be detected in any osseous structure, including chondral tissue.[5] In the head and neck region, the mandible is a common site for Brown tumor.[7] This lesion is related with a high incidence of fractures if located in weight-bearing bones.[6] Symptoms of brown tumors of the sinonasal tract include epistaxis, facial swelling, pain, and bulging of the adjacent tissues. Our case presented mainly with facial swelling and pain.
The most useful imaging studies for evaluation of Brown tumor are CT and magnetic resonance imaging (MRI). On multislice CT, the most common manifestation of Brown tumor is expansive and radiolucent bone lesion. CT may also show erosion of adjacent bone tissue. On MRI, Brown tumor shows expansile behavior with low to iso- signal intensity on the T1- and T2-weighted images. After intravenous gadolinium chelates, lesions display strong and homogeneous enhancement.[8] CT is the first-step imaging modality in defining and evaluating effect of maxillofacial Brown tumors, while MRI is the problem solving imaging modality.
The differential diagnosis of an expansive and erosive maxillary sinus mass includes paranasal sinus malignancies, odontogenic tumors, metastatic carcinoma, fibrous dysplasia, giant cell reparative granuloma, aneurismal bone cysts, cherubism, or plasma cell tumor.[139] When a tumor of the maxillary sinus has been diagnosed as a giant cell tumor, HPT should be ruled out to exclude the possibility that it is a Brown tumor. Surgical resection of Brown tumor is usually not necessary, because dimensional regression and increased mineralization may take place once the PTH level of blood is corrected.
Radiological differential diagnosis between a Brown tumor and other histological giant cell tumors is not simple. True giant cell tumors are more locally destructive than Brown tumors. Craniofacial fibrous dysplasia generally invades the bones of the face or the skull base, and is observed most commonly in young women.[1] Reparative granulomas often arise in the alveolar arch of mandible or maxilla and affect children and young adults, generally females. Reparative granulomas are a reactive process, that is caused by trauma or inflammation.[8] A reparative granuloma can be similar histologically and radiologically in appearance with a Brown tumor. The presence of HPT and hypercalcemia may be useful to differentiate these granulomas from Brown tumors. In the craniofacial region, aneurismal bone cyst is found more frequently in the mandible contains multiple septations and fluid-fluid level within the lesion. This lesion generally occurs in first 2 decades of life and mostly in females.[4] Cherubism is a rare benign and familial disease of early childhood that is limited to the mandible and the maxilla. The radiologic appearance of this lesion is similar to a fibrous dysplasia.[10] Paranasal sinus malignancies, odontogenic tumors, metastatic carcinoma, and plasma cell tumor have nonspecific radiological findings and generally occur in adulthood with equal frequency among males and females.
The treatment of Brown tumor is to control the HPT. Normalization of the PTH level is essential and can be done with medications or by parathyroidectomy. In patients with a Brown tumor in primary HPT, the parathyroid glands should be removed surgically. Calcium carbonate, vitamin D, aluminium hydroxide antacids, lantum carbonate, sevelamer, and calcimimetics can be used for medical treatment.[3] Clinical studies on calcimimetics have shown that these drugs ensures the increased calcium receptor sensitivity, causing a rapid decrease in PTH levels. Nevertheless, surgical approach is preferred to the use of medications. Parathyroidectomy should be the first choice of treatment in tertiary HPT when the disease is refractory to medical therapy. Tumor regression or complete remission following parathyroidectomy has been shown in patients with HPT. The treatment of choice for the patient in the present case was total parathyroidectomy.
CONCLUSION
Intramaxillary sinus Brown tumor is a rare neoplasm that is difficult to diagnose. Cross-sectional imaging techniques are available for tumor localization and behavior. Although rare, the possibility of Brown tumor should be kept in mind when assessing well-defined expansile maxillary sinus lesions.
Available FREE in open access from: http://www.clinicalimagingscience.org/text.asp?2013/3/1/55/122325
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- Brown tumor of the maxillary sinus in a patient with hyperparathyroidism: A case report. J Med Case Rep. 2009;3:7495.
- [Google Scholar]
- Brown tumors of the jaws associated with primary or secondary hyperparathyroidism. A clinical study and review of the literature. Am J Otolarygol. 2006;27:281-6.
- [Google Scholar]
- Brown tumour of the maxilla and mandible: A rare complication of tertiary hyperparathyroidism. Dentomaxillofac Radiol. 2009;38:53-8.
- [Google Scholar]
- Peripheral brown tumor of hyperparathyroidism in the oral cavity. Oral Oncol Extra. 2006;42:91-3.
- [Google Scholar]
- Brown tumor – a rare manifestation of renal osteodistrophy and severe secondary hyperparatyhroidism: Case report. Acta Clin Croat. 2010;49:299-304.
- [Google Scholar]
- Brown tumor of the maxilla and mandible: Progressive mandibular brown tumor after removal of parathyroid adenoma. J Oral Maxillofac Surg. 2003;61:719-22.
- [Google Scholar]
- Emphasis on the MR imaging findings of brown tumor: A report of five cases. Skeletal Radiol. 2011;40:205-13.
- [Google Scholar]
- Aneurysmal bone cyst of the mandible: A case report. Int J Oral Maxillofac Surg. 2003;32:419-22.
- [Google Scholar]






