Translate this page into:
Breast MR Imaging: What the Radiologist Needs to Know
Address for correspondence: Dr. Gurpreet S. Dhillon, 601 Elmwood Ave. Box 648, Rochester, NY 14642, USA. E-mail: gurpreet_dhillon@urmc.rochester.edu
-
Received: ,
Accepted: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Magnetic resonance imaging (MRI) of the breast is being performed more frequently to improve primary and recurrent tumor detection, characterization, and response to therapy. Sensitivity of this test approaches 90% and the specificity ranges from 37% to 100%. We present a concise tutorial for the general radiologist with a pictorial review of common lesions identified with breast MRI.
Keywords
Benign
breast
MRI
malignant
INTRODUCTION

Magnetic resonance imaging (MRI) of the breast is being performed more frequently to improve primary and recurrent tumor detection, characterization, and patient's response to therapy. Sensitivity approaches 90%.[1] Specificity ranges from 37% to 100%.[1] MRI of the breast is indicated for the following: evaluation of the extent of spread of a suspected extensive high-grade carcinoma; evaluation of a suspected multifocal or bilateral neoplasm; monitoring of the response to neoadjuvant chemotherapy; screening of high-risk patients; characterization of an indeterminate lesion (after full assessment with other modalities); detection of occult breast carcinoma (in a patient with adenocarcinoma in an axillary lymph node, or in the presence of metastatic adenocarcinoma of unknown origin); detection of recurrent breast cancer after breast-conserving therapy; differentiation between scar tissue and recurrent tumor; and evaluation of implant rupture.
Contraindications to breast MRI include: contraindications to gadolinium-based contrast media due to allergy, pregnancy, or compromised renal function (eGFR < 30); inability to lie prone; marked kyphosis or kyphoscoliosis; marked obesity; extremely large breasts; implantable devices that are not MRI compatible; severe claustrophobia (which can be treated with sedatives, if necessary).
Equipment and patient positioning
The equipment necessary for the procedure include an MR device with a field strength of 1.5 Tesla or greater and a double breast surface coil [Figure 1]. The patient lies prone with both breasts lightly immobilized in the coil. Multichannel (4, 8, 16, 32) breast coils are commercially available and provide enhanced spatial and temporal resolution for improved visualization of small lesions. These coils are designed to enable breast intervention with both medial and lateral access to either breast.
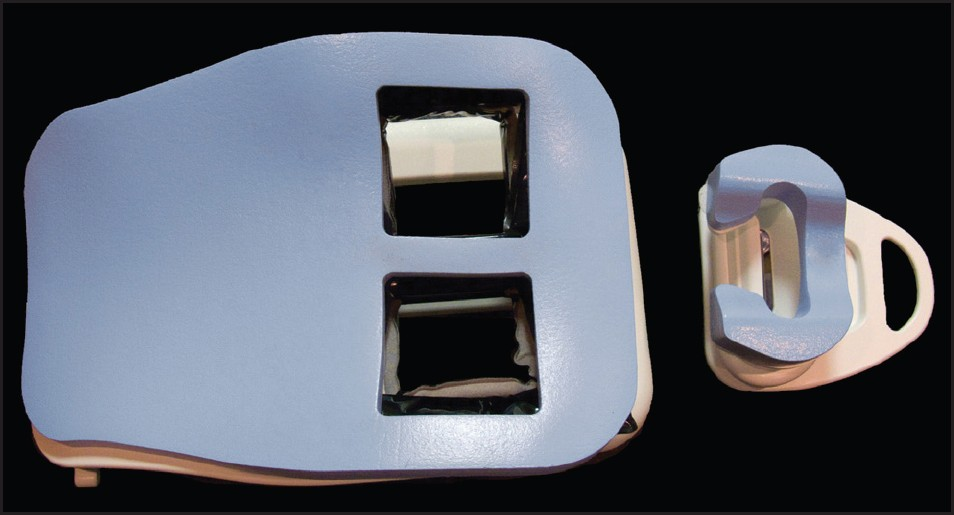
- Double breast MRI coil.
Imaging parameters
Image acquisition is performed in an axial plane with 2 mm (or finer) sections. Sagittal and coronal reconstructions are made from this dataset. Sagittal image acquisition is usually preferred for biopsy procedures. The primary pulse sequences are fat-suppressed axial T1-weighted (T1W) without and with contrast and fat suppressed axial T2-weighted (T2W) or short TI inversion recovery (STIR). For the contrast portion of the exam, a paramagnetic gadolinium-based intravascular contrast (0.1 mmol/kg) is injected at a rate of 2 mL/s. A minimum of two postcontrast T1-weighted series should be obtained, with initial post-contrast images within 4 min and delayed post-contrast images within 8 min after contrast administration. Kinetic curves are generated from these T1W post-contrast images. Fat suppression is used because an enhancing cancer can be confused with nonsuppressed fat as they both have high signal intensity on T1W images. The most common way to reduce or remove fat signal and show enhancement more clearly is to use spectral fat saturation. Homogeneous fat suppression may not be possible with large breasts.
Image interpretation
ACR BI-RADS® Lexicon
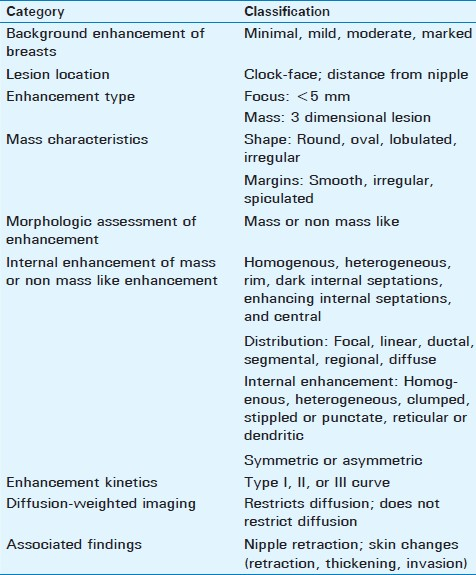
The American College of Radiology (ACR) has created a breast imaging and reporting data system (BI-RADS®) atlas[23] which contains terminology for describing lesion architecture and enhancement characteristics. Use of this terminology allows a comprehensive analysis of both morphological and kinetic features used in image interpretation and helps radiologists and other clinicians communicate more clearly and consistently. A radiological description should include lesion information including clock-face location and distance from nipple, morphologic assessment of enhancement, associated findings such as nipple retraction or inversion and skin changes (retraction, thickening, and invasion), and a kinetic curve assessment [Table 1].
Background enhancement
Assessment of background parenchymal enhancement pattern can be described with 4 M's: minimal, mild, moderate, and marked [Figure 2a and 2b]. This is analogous to mammographic breast tissue density in that breasts with greater background enhancement (or greater density in mammography) may limit accuracy of underlying lesion detection.[4] Background parenchymal enhancement fluctuates with the menstrual cycle, being highest during weeks 1 and 4 and lowest during week 2. Thus, imaging between days 7–14 of the menstrual cycle is recommended, unless precluded by clinical urgency. With background enhancement in mind, one must determine if there is a lesion that is conspicuous among its surroundings.
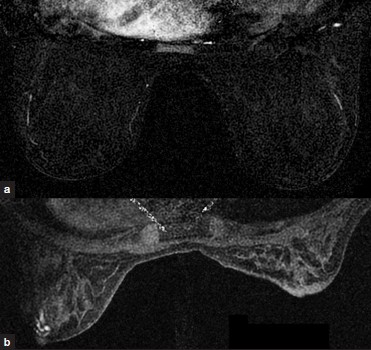
- (a) Minimal and (b) marked background enhancement.
Morphologic assessment of enhancement
A lesion of less than 5 mm should be described as a focus. A focus or multiple foci may result from hormonal changes (eg, fibrocystic changes) and are often stable on follow-up exams.
A 3-dimensional lesion (mass) should be characterized according to shape (round, oval, lobulated, or irregular), margins (smooth, irregular, or spiculated), and internal enhancement (homogenous, heterogeneous, rim, dark internal septations, enhancing internal septations, or central). More irregular and spiculated masses have a higher likelihood of malignancy. Specific internal enhancement patterns are often associated with certain entities: rim-enhancement is seen with high-grade invasive ductal carcinoma, cysts with inflammation, and fat necrosis; dark internal septations may be seen with fibroadenomas; enhancing internal septations are often seen with malignancy; central enhancement is seen with high-grade ductal carcinoma and vascular tumors.
If enhancement is located in an area that is not associated with a mass (nonmasslike enhancement), the description should give details of the distribution (focal, linear, ductal, segmental, regional, or diffusive), internal enhancement (homogenous, heterogeneous, clumped, stippled, punctate, reticular or dendritic), and whether it is symmetric or asymmetric. Ductal and segmental distribution of enhancement can be seen with ductal carcinoma in situ (DCIS) or invasive ductal cancer, sclerosing adenosis, atypical ductal hyperplasia, or papillary neoplasms. Diffuse enhancement is seen with benign processes and normal fibroglandular tissues. Reticular or dendritic internal enhancement is seen with lymphatic involvement such as that seen with inflammatory breast cancer.
Enhancement kinetics
Three basic curve shapes have been described [Figure 3].[5] Type-I curves are slowly enhancing, in which gradual, steady enhancement occurs over about 5 min. Malignancy is seen in approximately 6% of lesions with a Type-I curve.[6] Type-II curves show early strong enhancement (increase over a 1–2 min period) with a subsequent plateau phase. Malignancy is seen in approximately 6–29% of lesions with a Type-II curve.[6] Type III or “washout” curves show early strong enhancement (over 1–2 min), with subsequent decline in enhancement. This produces a characteristic peak dubbed the “the cancer corner,” and is strongly associated with malignancy. Malignancy is seen in approximately 29-77% of lesions with a Type-III curve.[6] Both Type-II and Type-III curves should be considered suggestive of malignancy.
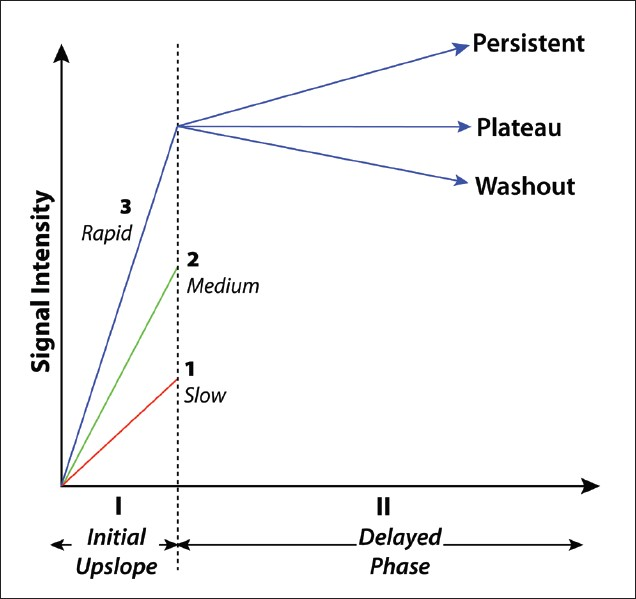
- Three types of enhancement kinetics curves seen with breast MRI.
Diffusion-weighted Imaging
Diffusion-weighted imaging (DWI) is a technique that takes into account the differences in diffusion rate of water molecules in normal and pathologic tissue. This technique, although not commonly used, has a higher specificity to differentiate between benign and malignant breast lesions compared to that of contrast-enhanced MRI (84% compared to 37%).[7] It relies on differences in cellularity to distinguish between benign and malignant lesions. Malignant lesions, which frequently have a higher degree of cellularity compared with benign lesions, often demonstrate restricted diffusion.
Computer-aided detection in breast MRI
Computer-aided detection (CAD) can be performed using a software adjunct package for enhancement kinetics. A maximal intensity projection (MIP), kinetics curve, and color map overlay can be generated [Figure 4]. CAD does not evaluate anatomy or pathology. Advantages of CAD include the ability to quickly analyze large numbers of images, aid in visual subtraction, and facilitate reconstructions and future comparisons.
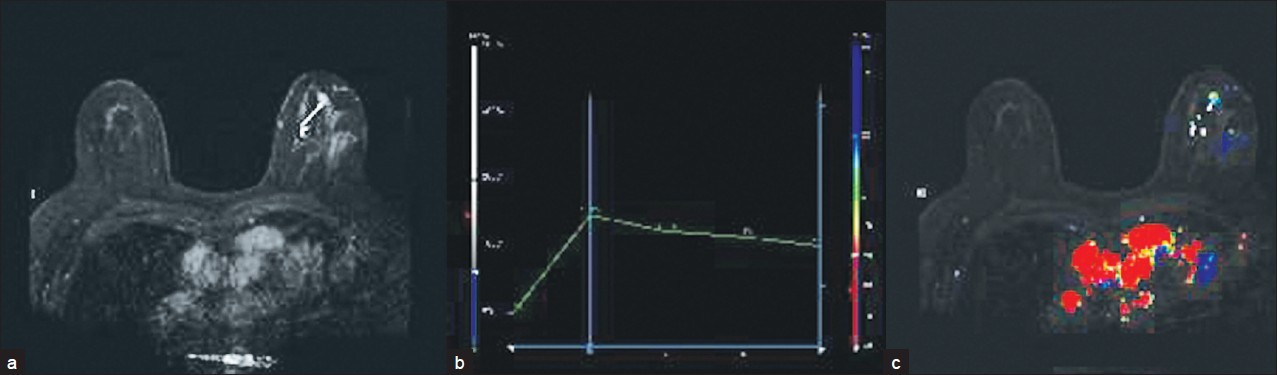
- (a) MIP, (b) kinetics curve, and (c) color map overlay obtained using CAD software.
Artifacts
Breast MRI is susceptible to artifacts common in MRI of all organ systems: ghosting, motion, wrap-around, magnetic susceptibility, signal void, field inhomogeneity, and chemical shift. Artifacts specific to breast imaging include background parenchymal enhancement (discussed above), which can be avoided by imaging between days 7–14 of the menstrual cycle, and artifact due to breast tissue (usually large breasts) abutting the radiofrequency coil, leading to signal voids and magnetic susceptibility. Poor fat saturation, which can be due to incorrect identification of the fat peak or field inhomogeneity, is often seen with breasts composed of larger amounts of fat. Care must be taken that the proper fat peak is selected, shimming is used to improve field uniformity, and appropriately sized breast coils are used to ensure adequate fat suppression.
Common benign lesions
Benign breast lesions can have a variable appearance on MRI. However, a few important principles regarding benign lesions have been described. Lesions with high signal on T1W imaging often contain fat and are thus most often benign, unless they are rapidly growing. Lesions that show intensely high signal on T2W imaging often contain water and are also generally benign. One important exception is colloid carcinoma, which also exhibits high signal on T2W images. Benign lesions often do not show enhancement. However, as described above, variable enhancement kinetics can be seen with benign lesions. Benign lesions often do not show restricted diffusion.
A simple cyst [Figure 5a–e] is the most common benign breast lesion. It is best seen with ultrasound as a well circumscribed, anechoic mass, with an imperceptible wall and posterior acoustic enhancement. On MRI, simple cysts show low signal on T1W images, high signal on T2W images, and do not enhance. Mammography cannot distinguish between cyst and solid mass.
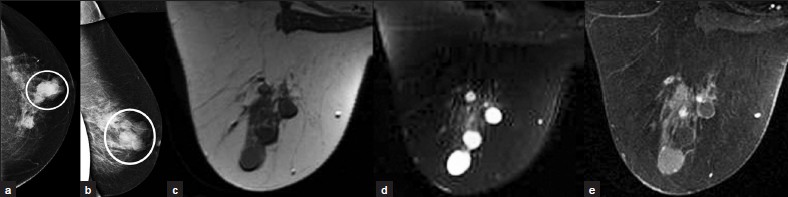
- Simple cyst. (a) CC and (b) ML views of the left breast demonstrate several well-circumscribed round/oval masses nearly isodense to the parenchyma (circles). MR images demonstrate these masses to be (c) hypointense on T1WI, (d) hyperintense on T2WI, and (e) nonenhancing.
A fibroadenoma [Figure 6] is the second-most common benign breast lesion behind the simple cyst. On MRI, it is a focus or mass of enhancement with benign morphologic characteristics (without spiculations or microlobulations), nonenhancing dark internal septations, and follows a Type-I kinetics curve.
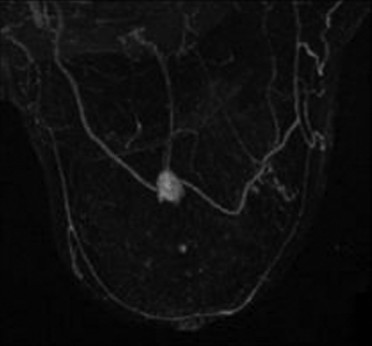
- Fibroadenoma. MIP image demonstrates an enhancing mass without spiculations or microlobulations.
An intramammary lymph node [Figure 7a–c] appears as an intraparenchymal breast mass with an eccentric fatty hilum. It is often small, oval, and smoothly marginated. Though it may be located anywhere in the breast, it is more commonly located in the upper-outer quadrant. MRI characteristics include a high-signal fatty hilum on T1W images, high signal on T2W images, and rapid, intense enhancement with contrast. On mammography, an intramammary lymph node may have a reniform or lobulated mass with a fatty hilum or notch. On ultrasound, it is a hypoechoic reniform mass with an echogenic fatty hilum.
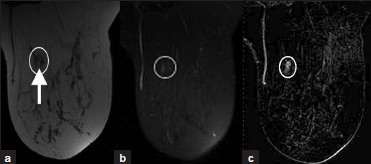
- Intramammary lymph node. (a) T1W MR image without fatsuppression demonstrates a hypointense oval mass (oval) with a hyperintense fatty hilum (arrow). (b) The mass demonstrates high signal with T2W (oval) and (c) intense enhancement with contrast (oval).
Intraductal papilloma [Figure 8a–d] is a benign often periareolar ductal neoplasm that is the most common source of bloody nipple discharge. On ultrasound, dilated ducts are seen around a solid lesion. On MRI, intraductal papilloma appears as a focus or mass of variable enhancement, with a hyperintense duct and hypointense mass on T2W imaging.
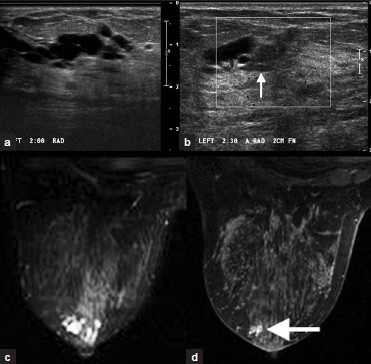
- Intraductal papilloma. (a) Grayscale and (b) color Doppler images of the left breast demonstrate anechoic dilated ducts with a hypoechoic mass proximal to the dilatation (arrow, b). (c) T2W and (d) post-contrast images of the left breast demonstrate hyperintense dilated ducts (c) due to an enhancing periareolar mass (arrow, d).
Common malignant lesions
Malignant breast lesions can also have a variable appearance on MRI. These lesions often show low-signal intensity on T1W imaging, and low or moderate signal intensity on T2W imaging. Malignant lesions enhance with variable enhancement kinetics, as above. They often show restricted diffusion.
Ductal Carcinoma in situ (DCIS) [Figure 9a–c] is a neoplasm of variable grade and may not be visualized on MRI. It may have non-mass-like enhancement that can be clumped, ductal, linear, or segmental in shape. Enhancement kinetics are not useful because this lesion shows slow initial enhancement without washout (Type-I curve).
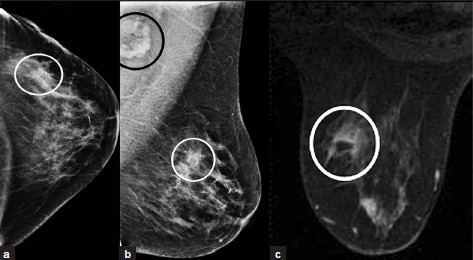
- DCIS. (a) CC and (b) MLO views of left breast with microcalcifications (not well projected), soft tissue density, and biopsy clip in the outer aspect (white ovals). Axillary lymphadenopathy (black oval) is noted on the MLO view. (c) Post-contrast MR image of left breast showing clumped non-masslike enhancement in the outer aspect (oval). Note central low-signal artifact from a biopsy tract.
Invasive ductal carcinoma [Figure 10a–f] is the most common primary malignant tumor of the breast. MRI demonstrates an irregularly shaped, spiculated mass, with rim or heterogeneous enhancement. These lesions often display Type-II or Type-III washout curves. However, morphology is always more significant a tool for diagnosis than kinetic curve assessment.
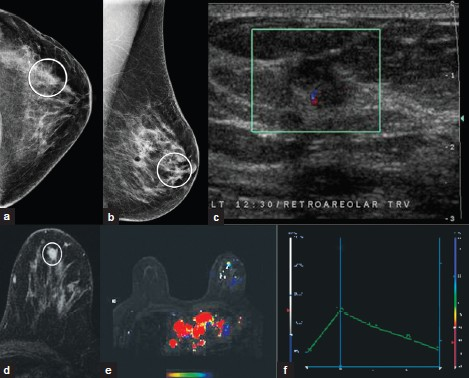
- Invasive ductal carcinoma. (a) CC and (b) MLO views of the left breast demonstrate an irregular mass with indistinct spiculated margins (white ovals) (c) Ultrasound image demonstrates a taller-than-wide hypoechoic irregular mass with indistinct margins. (d) Postcontrast MR images demonstrate heterogeneous enhancement of the mass (oval). Note (e) the color map overlay and (f) type III enhancement curve of the mass.
Invasive lobular carcinoma [Figure 11a–d] comprises about 10% of all breast carcinomas. It is very difficult to detect mammographically due to an insidious growth pattern and a density equal or less than that of normal breast tissue. Both mammography and ultrasound often underestimate the lesion size, which has implications for staging and treatment. MRI has a higher sensitivity for lobular carcinoma and can more accurately assess the lesion size.[8] It commonly appears as multicentric/multifocal, spiculated focus or mass with architectural distortion. Enhancement can be asymmetric and nonmasslike in a ductal, segmental, regional, or diffuse pattern.
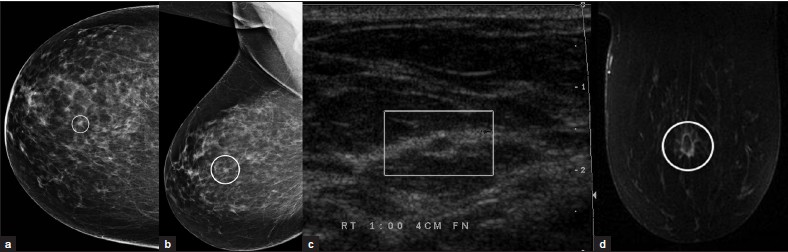
- Invasive lobular carcinoma. (a) CC and (b) MLO views of the right breast demonstrate a spiculated focus (ovals). (c) Ultrasound image demonstrates a hypoechoic lesion with echogenic rim. (d) Postcontrast MR image demonstrates rim-enhancement of the mass with extension of enhancement within adjacent tissue (oval).
Post-treatment evaluation
MRI can be used to help distinguish scar from tumor recurrence in post-treatment evaluation. A treated tumor generally exhibits decreased enhancement, with false negative rates of 67% and false positive rates up to 56%.[9] Hematoma is hyperintense on T1W imaging, hypointense on T2W imaging, and shows no enhancement. Seroma [Figure 12a–e] is hypointense on T1W imaging, hyperintense on T2W imaging, and displays smooth peripheral enhancement (< 4 mm thickness) with contrast. A scar generally shows no enhancement, though an early scar may enhance. Enhancement can be seen with recurrence [Figure 13a–d], though non-tumor related contrast enhancement can be seen with fibrosis, necrosis, and inflammation.
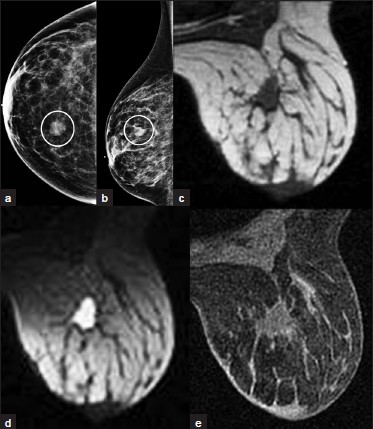
- Posttreatment seroma. (a) CC and (b) ML views of the right breast from initial screening mammogram demonstrates a mass in the inner aspect (oval, a). This was found to be invasive lobular carcinoma and the patient underwent lumpectomy. MRI exam 6 months after lumpectomy demonstrates a lesion in the surgical bed that is (c) hypointense on T1WI, hyperintense on (d) T2WI, and shows (e) mild contrast enhancement. This lesion is consistent with a postoperative seroma.
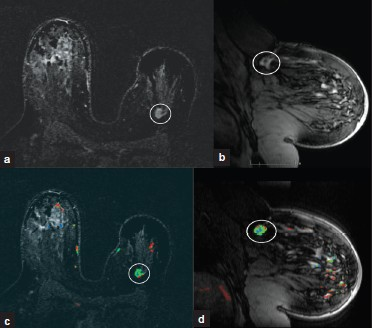
- Cancer recurrence. (a) MIP axial and (b) sagittal and (c) color map overlay axial and (d) sagittal MR images demonstrate an asymmetrically smaller left breast related to lumpectomy for invasive ductal carcinoma. In the deep 12:00 position of the left breast there is an irregular, enhancing mass with spiculations (white oval) consistent with biopsy-proven recurrence of invasive ductal carcinoma.
Silicone implant evaluation
MRI can be used to evaluate for rupture of silicone implants.[10] A noncontrast exam with a fluid-sensitive sequence (water-suppressed STIR) is used, as only silicone is hyperintense on this pulse sequence. With extracapsular rupture, silicone can be seen outside the capsule of the implant. MRI is useful to determine the extent of damage of the implant. On ultrasound, there is a “snowstorm” appearance. When intracapsular rupture occurs, silicone is seen external to the implant shell. Silicone that is seen within folds of the implant gives the “keyhole” and “teardrop” sign [Figure 14]. When intracapsular rupture results in a fully collapsed implant, the “linguine” sign can be visible on MRI, analogous to the “stepladder” sign on ultrasound.
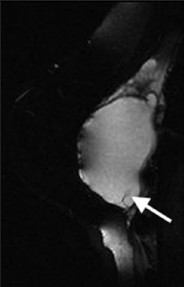
- Intra- and extracapsular silicone implant rupture. STIR MR image demonstrates hyperintense silicone within the extracapsular soft tissues and within implant shell folds (arrow).
CONCLUSIONS
Breast MRI is increasingly being performed. It is important for the general radiologist to be familiar with indications and contraindications, equipment and patient positioning, imaging basics, the ACR BI-RADS® lexicon, common artifacts, common lesions, post-treatment evaluation, and silicone implant evaluation. MRI is a versatile modality for evaluating breast conditions with which general radiologists should be familiar.
Source of Support: Nil
Conflict of Interest: None declared.
Available FREE in open access from: http://www.clinicalimagingscience.org/text.asp?2011/1/1/48/85655
REFERENCES
- American College of Radiology. In: Breast imaging reporting and data system atlas (BI-RADS atlas). Reston, VA: American College of Radiology; 2003.
- [Google Scholar]
- Does the degree of background enhancement in breast MRI affect the detection and staging of breast cancer? Eur Radiol 2011 in press
- [Google Scholar]
- Do T2-weighted pulse sequences help with the differential diagnosis of enhancing lesions in dynamic breast MRI? J Magn Reson Imaging. 1999;9:187-96.
- [Google Scholar]
- Diagnostic architectural and dynamic features at breast MR imaging: Multicenter study. Radiology. 2006;238:42-53.
- [Google Scholar]
- Meta-analysis of quantitative diffusion-weighted MR imaging in the differential diagnosis of breast lesions. BMC Cancer. 2010;10:693.
- [Google Scholar]
- MRI compared to conventional diagnostic work-up in the detection and evaluation of invasive lobular carcinoma of the breast: A review of existing literature. Breast Cancer Res Treat. 2008;107:1-14.
- [Google Scholar]
- Accuracy of MRI in the detection of residual breast cancer after neoadjuvant chemotherapy. AJR Am J Roentgenol. 2003;181:1275-82.
- [Google Scholar]
- Evaluation of the rupture of silicone breast implants by mammography, ultrasonography and magnetic resonance imaging in asymptomatic patients: Correlation with surgical findings. Sao Paulo Med J. 2004;122:41-7.
- [Google Scholar]






