Translate this page into:
Benign Sclerosing and Fibrosing Conditions of the Abdomen and Their Potential Mimics
Address for correspondence: Dr. James A Stephenson, Department of Radiology, Gastrointestinal Imaging Group, University Hospitals of Leicester, Leicester General Hospital, Leicester, LE5 4PW, UK. E-mail: james.stephenson@uhl-tr.nhs.uk
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
The process of abnormal reparative or reactive processes in the abdominal cavity, can lead to sclerosis and fibrous deposition. The relatively recent discovery of an IgG4 subgroup of immune mediated sclerosing disease 1,2 has thrown some light on the pathophysiology of these conditions. Firstly, our pictorial review aims to describe imaging findings to enhance the general radiologist's recognition and interpretation of this varied group of benign sclerotic and fibrotic abdominal processes. Secondly, along with the imaging findings, we bring into discussion the potential mimics of these pathologic processes to minimise interpretational errors. Moreover, some of the mimics of these processes are in the spectrum of malignant disease. Most importantly, to ensure a correct diagnosis thorough clinical and histopathological assessment are required to support the imaging findings presented in this review.
Keywords
Abdominal imaging
fibrosis
immunoglobulin G4
sclerosing disease

INTRODUCTION
The process of abnormal reparative or reactive processes can lead to sclerosis and fibrous deposition producing characteristic and shared imaging features [Table 1]. Benign sclerosing and fibrosing diseases of the abdomen are relatively rare and can be broadly categorized into two main groups idiopathic/immune-mediated and iatrogenic conditions. The group of immune-mediated fibrosing conditions can be further subdivided into those conditions that have been linked to immunoglobulin (Ig) G4 and those that do not have any current link to IgG4.

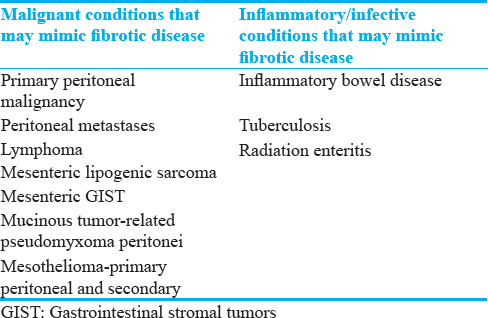
The relatively recent discovery of an IgG4 subgroup of immune-mediated sclerosing disease[12] has thrown some light on the pathophysiology of these conditions. Despite this, our understanding of this heterogeneous group of diseases is constantly evolving. There remains uncertainty and disparity in the imaging features and nomenclature used to describe and categorize these benign sclerosing and fibrosing abdominal conditions. As such, there is an understandable reliance on histopathology for a definitive diagnosis[3] as a number of malignant and infectious/inflammatory intra-abdominal processes can mimic the imaging features of benign sclerosing and fibrotic diseases within the abdomen [Table 2].
We present a pictorial review that aims to describe and clarify imaging findings to enhance the general radiologist's recognition and interpretation of this varied group of benign sclerotic and fibrotic abdominal processes. Some of these findings are shared with inflammatory and malignant processes and require further investigation. Other imaging features are more specific and recognition may help differentiate benign from malignant or infective processes [Table 3].
IGG4 LINKED IMMUNE-MEDIATED FIBROSING CONDITIONS OF THE ABDOMEN
IgG4-mediated disease is a systemic disorder first described within the abdomen as a cause for autoimmune pancreatitis (AIP).[4] Since this discovery, IgG4-mediated disease has been linked with a number of intra- and extra-abdominal sites.[25] The diagnosis is reliant on histological and biochemical analysis showing T lymphocytes and IgG4-positive plasma cells to make a definitive diagnosis. A number of the benign sclerosing and fibrosing abdominal diseases presented in this review have been linked with active or “burnt out” systemic IgG4 disease.
AIP can be divided into two subtypes. Type 1 is a manifestation of IgG4-related periductal fibrosis secondary to infiltration with IgG4-positive cells. It is a multisystem disease with synchronous or metachronous disease at numerous other organs sites.[5] Type 2 exclusively involves the pancreas with lack of IgG4-positive cells.[56] AIP is rare, accounting for 2%–11% of chronic pancreatitis with Type 1 being four times more common than Type 2.[6] The mean age for presentation of Type 1 is 60 years of age and Type 2 is 40 years. Type 2 is more common in men.[56]
Imaging findings
The imaging pattern in AIP can be diffuse, focal, or multifocal. Diffuse disease is overall the most common type[5] [Figure 1]. Type 2 AIP tends to be focal.[7]
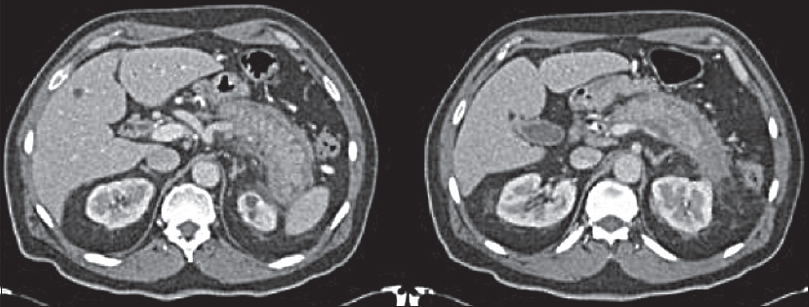
- Autoimmune pancreatitis: Axial contrast-enhanced computed tomography image showing diffuse pancreatic gland enlargement with loss of normal lobular architecture with a hypoattenuating rim. No pancreatic duct dilatation. No pancreatic necrosis.
Ultrasound (US) and endoscopic US provide fairly nonspecific findings including diffuse or focal hypoechoic pancreatic enlargement, bile duct wall thickening, and peripancreatic hypoechoic margins.
Dual-phase computed tomography (CT) with both an arterial and portal venous phase is the investigation of choice to assess the pancreas, particularly if pancreatic malignancy and pancreatitis is considered in the differential diagnosis. The most common finding is diffuse hypoattenuation and enlargement of the pancreas with loss of normal lobulation [Figure 1]. The loss of lobulation gives rise to the term “sausage-shaped pancreas.” About 30%–40% show focal areas of pancreatic enlargement, most commonly in the head[58] [Figure 1]. Interrogation of the pancreatic duct should reveal a nondilated duct.[8] The intrapancreatic common bile duct can be narrowed and strictured, especially when there is involvement of the pancreatic head.[9] Parenchymal calcification and pseudocyst formation are not common findings in AIP.[8] More commonly, there is a low attenuation rim/capsule that can show subtle enhancement on delayed images known as the “halo sign”[89] [Figure 2]. The parenchymal enhancement pattern in AIP is variable. In the arterial phase, affected parenchyma is hypoenhancing, and in the venous phase, it may remain hypoenhancing or be isoenhancing compared to normal parenchyma.[8]
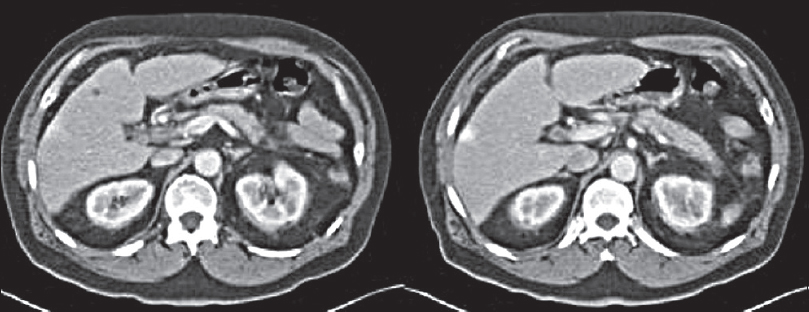
- Autoimmune pancreatitis poststeroid treatment: Axial contrast-enhanced computed tomography images of the same patient in Figure 1 after 3 months of steroid treatment shows resolution of the pancreatic gland changes.
On magnetic resonance imaging (MRI), the pancreas is hypointense on T1-weighted (W) sequences. The capsule is low signal on T2W images and shows delayed enhancement postgadolinium. Magnetic resonance cholangiopancreatography (MRCP) sequences should be used to assess the pancreatic duct and biliary tree. The hallmark of AIP is diffuse narrowing of the pancreatic duct without dilatation.[10] A key feature to support a diagnosis of AIP is a marked response to steroid treatment on interval imaging[7] [Figure 2].
Potential mimics of autoimmune pancreatitis
Peripancreatic fat stranding in the early AIP may mimic a mild acute pancreatitis. Imaging features to help differentiate AIP from acute pancreatitis include the described “halo sign” and lack of local fat necrosis.[8] Focal areas of inflammation in the pancreatic head can mimic a mass lesion; however, upstream main pancreatic duct dilatation is rare in AIP, and if present, suggests a pancreatic ductal adenocarcinoma.
Potential mimics of immunoglobulin G4-related sclerosing disease of the abdomen
Periportal soft-tissue thickening and strictures secondary to IgG4-related sclerosing disease is known to mimic hilar cholangiocarcinoma or metastatic disease. When compared to IgG4-related sclerosing disease, hilar cholangiocarcinoma tends to be more focal than IgG4-related disease of the biliary tree.[2] Parenchymal cholangiocarcinoma often has characteristic liver capsule retraction which is not seen in IgG4, however, lack of retraction does not reliably exclude a cholangiocarcinoma.[2] In practice, histology is required for a definitive diagnosis and differentiation. IgG4-related biliary disease and primary sclerosing cholangitis (PSC) can also mimic each other and are discussed later in this review.[5]
Primary retroperitoneal fibrosis (RPF) encompasses a range of disease processes resulting in fibroinflammatory proliferation in the retroperitoneum with encasement of the ureters, infrarenal abdominal aorta, inferior vena cava (IVC), and iliac vessels.[11] RPF can be categorized into the primary or secondary disease. The primary form of RPF accounts for two-thirds of cases.[11] The pathophysiology of primary RPF is poorly understood. Atherosclerotic disease is common in the patient group, and conventionally, an excessive local inflammatory response to atherosclerotic plaque was thought to play a key role. However, it is now recognized to be a manifestation of IgG4-related sclerosing disease.[2]
Imaging findings
Abdominal radiography can be normal. Nonspecific loss of the normal psoas shadow can be seen in the late stages of RPF due to the presence of a central soft-tissue mass, however, this is rare.
Abdominal US scan can demonstrate bilateral hydronephrosis. In the later stages, RPF can typically be seen as a well-defined hypoechoic or isoechoic retroperitoneal mass. It often has an irregular contour and sits anterior to the lower lumbar spine.[12]
Due to the superior sensitivity and specificity of CT urography, intravenous (IV) urography is rarely used today. It classically showed the triad of medial deviation of the middle-third of the ureters, tapering of both ureteral lumens, and hydronephrosis with delayed excretion of contrast,[13] and these features can be seen on CT urographic coronal reformats.
CT is the initial investigation of choice in the most cases to assess for RPF due its accessibility and ability to evaluate the location and extent of organ involvement.[13] It is also useful to assess for secondary causes of RPF and secondary effects such as hydronephrosis.
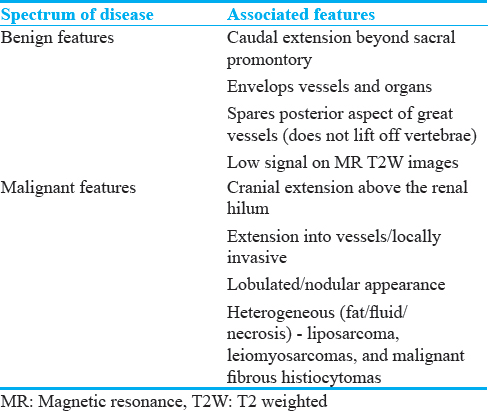
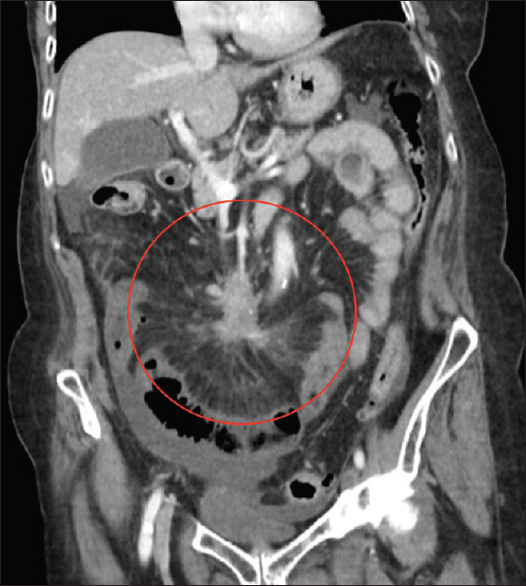
Typical CT appearances of idiopathic RPF are a lobulated soft-tissue density mass extending from the level of the renal to the iliac vessels.[1113] Classically, RPF produces nonstenotic vessel wall thickening with irregular margins.[2] The soft-tissue incorporates retroperitoneal structures often including the IVC and ureters [Figure 3]. Involvement of the duodenum is less common. While most involved retroperitoneal structures are displaced, the aorta is not lifted from the spine [Figure 3]. Less typical locations of RPF include extension into the pelvis, and superiorly, to include the renal hila. A quarter of cases have local reactive lymphadenopathy.[11] Administration of IV contrast is often contraindicated by poor renal function, but if administrated, the retroperitoneal mass may enhance avidly in the early stages of the disease, enhancing less avidly as the disease progresses, and fibrosis predominates.[1113] On positron-emission tomography CT, there is avid fluorodeoxyglucose uptake early in the disease,[2] thus RPF is often thought to be malignant in the early phase of the disease.
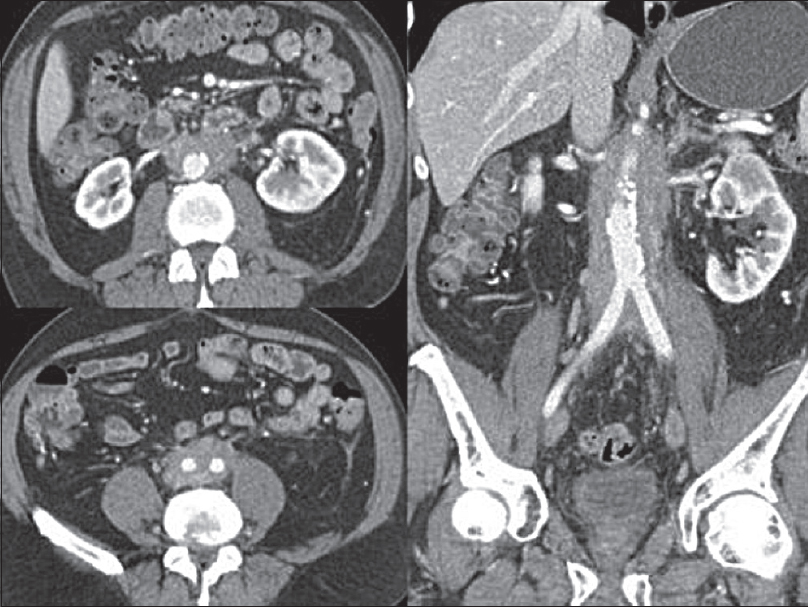
- Selected axial and coronal contrast-enhanced computed tomography images of the retroperitoneal fibrosis which is isoattenuating to muscle showing some subtle postcontrast enhancement. It is surrounding but not lifting the aorta. Computed tomography is the initial investigation of choice in the most cases to assess for retroperitoneal fibrosis due its accessibility and ability to evaluate the location and extent of the organ involvement.
In patients with established renal failure, MRI gives superior contrast resolution than unenhanced CT.[1113] The urinary tract can also be assessed without the need for IV contrast by utilizing heavily T2W sequences. RPF itself is low signal on T1W sequences with variable signal intensity on T2W sequences, which increases with degree of active inflammation. In the early stages of disease, there is often high T2-signal intensity, and this becomes predominantly low as fibrosis progresses.
Potential mimics of retroperitoneal fibrosis
Imaging lacks specificity for the definitive differentiation between benign and malignant causes of RPF, thus histological analysis is thought to be mandatory in some centers.[13] Lymphoma can mimic the appearance of primary RPF [Figures 4 and 5] and tends to also envelop vessels but is more likely to lift the aorta from the spine than RPF. Other mimics include metastatic disease and primary retroperitoneal sarcoma [Figure 6]. These tend to be more infiltrative and invade vessels with heterogeneous contrast enhancement and can have central necrosis.
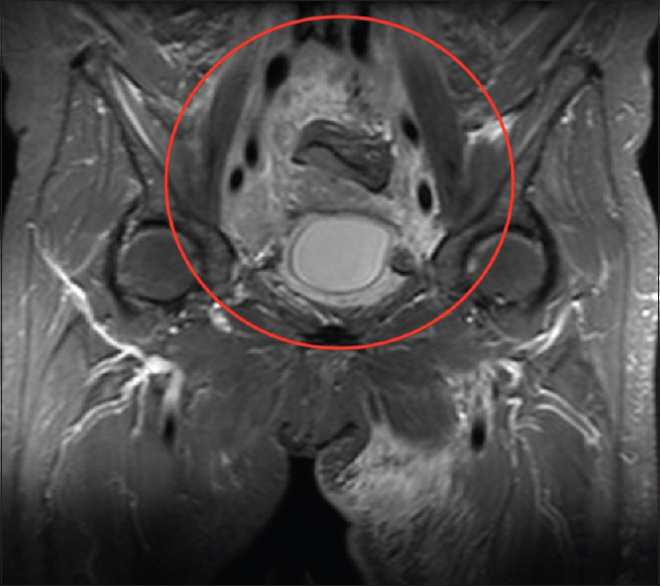
- Coronal T2-weighted fat-saturated sequence magnetic resonance image showing heterogeneous high T2-signal (compared to muscle) enveloping the iliac vessels (red circle). Histopathology confirmed lymphoma.
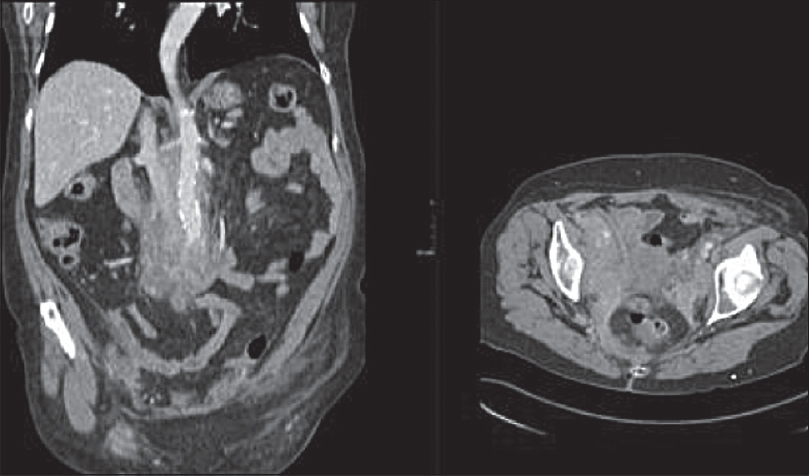
- Coronal and axial contrast-enhanced computed tomography images showing heterogeneous enhancing soft tissue in the retroperitoneum extending into the pelvis. Histopathology confirmed lymphoma.
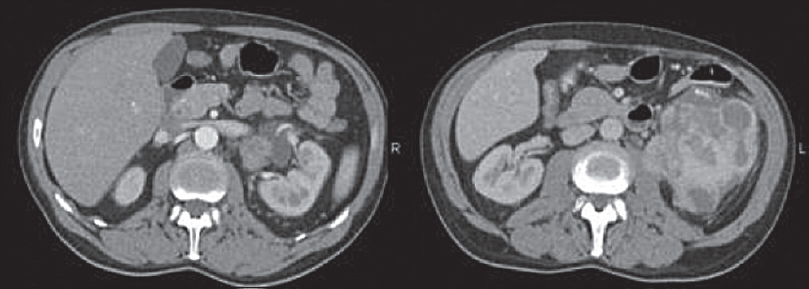
- Axial contrast-enhanced computed tomography images showing a heterogeneously enhancing retroperitoneal mass with associated left-sided hydronephrosis. Histology confirmed retroperitoneal liposarcoma.
Inflammatory pseudotumors (IPTs) of the abdomen are rare, poorly understood, and can affect any part of the body.[14] They are well described at various sites within the abdomen [Table 4]. Histopathological analysis is often required for diagnosis, which shows acute and chronic inflammatory cells with a mixture of both T- and B-lymphocytes.[141516] A number of IPTs are proven to be part of IgG4-related sclerosing disease but are also associated with trauma, surgery, and infections such as Epstein–Barr virus, Mycobacterium, and actinomycosis.[16]
Imaging findings
The imaging findings for IPTs are variable as they can affect many different organs. A detailed description of the imaging findings for each organ is beyond the scope of this review, but the common imaging findings and potential mimics for selected abdominal organs are summarized in Table 4. In general, the gastrointestinal and biliary tract lesions are ill-defined whereas the liver, spleen, and retroperitoneal lesions are well circumscribed[16] although as our examples show this is not always the case [Figures 7-9].
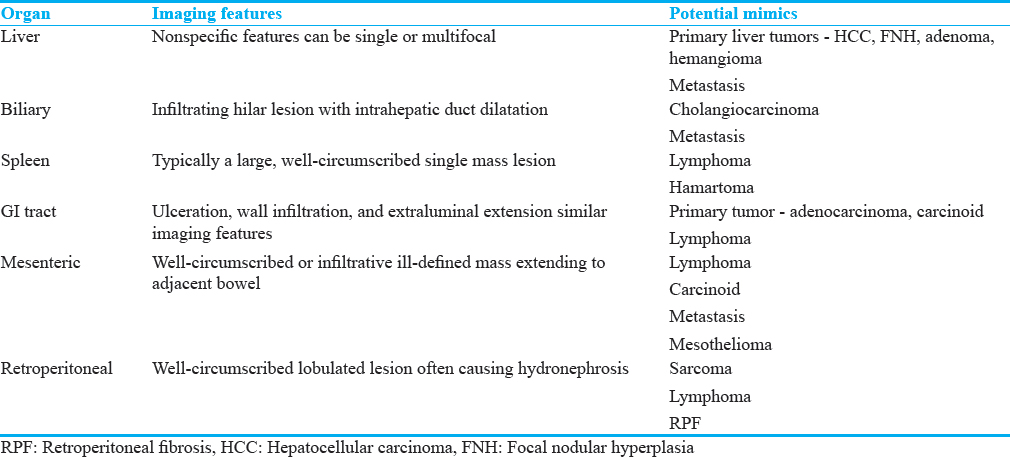
- Image from axial contrast-enhanced computed tomography showing a heterogeneous mass within the right lobe of the liver with irregular peripheral and internal enhancement.
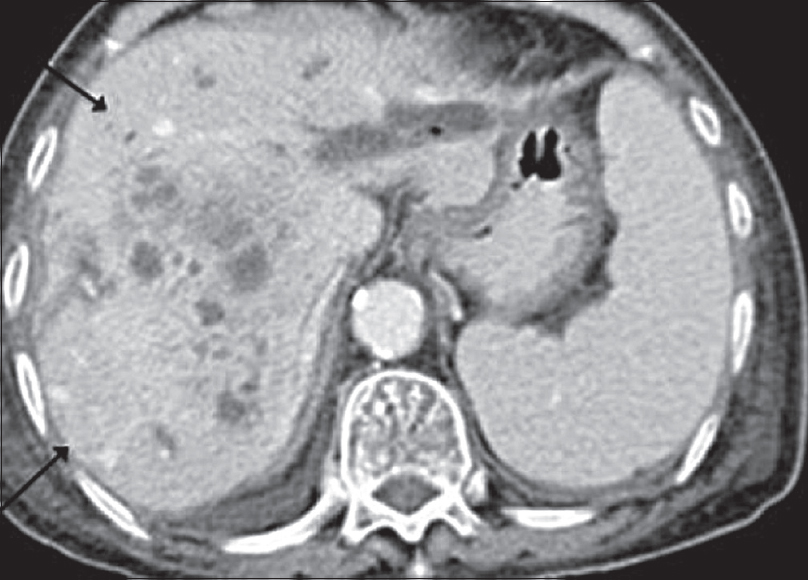
- Image from axial contrast-enhanced computed tomography with positive oral contrast showing a heterogeneously enhancing intramural small-bowel mass. Histopathology confirmed pseudotumor.
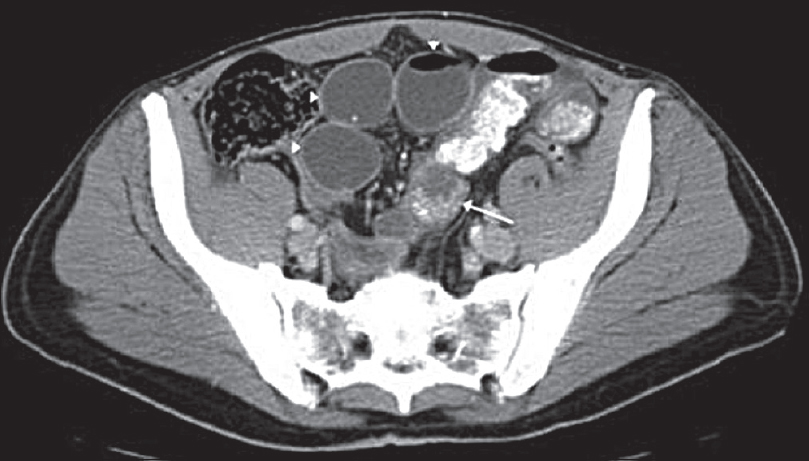
- (a) Axial contrast-enhanced computed tomography demonstrating a well-defined splenic mass (arrows) with an irregular central region of high attenuation (arrowhead). (b) Contrast-enhanced axial T1-weighted fat-saturated magnetic resonance imaging demonstrating predominately a low-attenuation splenic lesion (arrows) with focal peripheral enhancement (arrowhead). (c) In-phase axial gradient-echo magnetic resonance imaging showing marked susceptibility artifact (secondary to iron). (d) Axial T2-weighted fat-saturated magnetic resonance imaging demonstrating a hypointense splenic lesion (arrow). Histopathology confirmed inflammatory pseudotumor.
On CT imaging, IPTs are, generally, low-density mass lesions with varying enhancement pattern that can calcify. They are low signal on T1W and T2W MRI and do not restrict on MRI diffusion-weighted imaging. The MRI enhancement pattern is variable, but IPTs rarely avidly enhance.[16] On US, IPTs are usually well-defined, predominantly hypoechoic, heterogeneous solid lesions with color flow on Doppler assessment.[1416]
Potential mimics of inflammatory pseudotumors
IPTs have a wide differential and they often mimic primary malignancy or metastasis due to their mass such as appearance, multifocality, and locally aggressive features [Table 4]. There are few specific imaging features; therefore, histological analysis is required for definitive diagnosis.
MESENTERIC PANNICULITIS
There is uncertainty and disparity in the literature regarding the understanding and nomenclature of mesenteric lipodystrophy, mesenteric panniculitis (MP), and sclerosing mesenteritis (SM). In the past, the authors have described SM as the end-point of a single disease process progressing from mesenteric lipodystrophy to panniculitis and then mesenteric sclerosis. There is little or no evidence to support this theory, and there is a growing consensus that these processes are separate entities that share some histopathological and imaging features. However, there are imaging features specific to MP and SM enabling confident distinction on imaging.[17] This is an important distinction to make as MP, and “misty mesentery” is a common finding that requires limited or no further imaging or follow-up.[171819]
Imaging features
The US findings are nonspecific, but echogenic mesenteric fat or lymphadenopathy with the root of the mesentery and with or without mass effect can be seen.
Mesenteric panniculitis has been suggested to be present in up to 3% of the abdominal CTs by some authors[19] and is, therefore, often discovered as an incidental finding.
Appearances on CT are predominantly inflammatory and vary in severity from ill-defined hyperattenuating mesenteric fat? (-60 to -40 Hounsfield Units) similar to ground-glass opacification seen in the chest imaging, also known as “misty mesentery” to a well-demarcated lesion causing local mass effect[2021] [Figures 10 and 11]. The soft tissue or mixed fat and soft-tissue mesenteric root mass may or may not have surrounding fluid density secondary to lymphatic or venous obstruction. The presence of a capsule-like soft-tissue attenuation surrounding the lesion called a “pseudocapsule” has been described in 60% of cases.[1922] Surrounding fluid, a soft-tissue rim, or apparent mass increase the likelihood of need for biopsy.
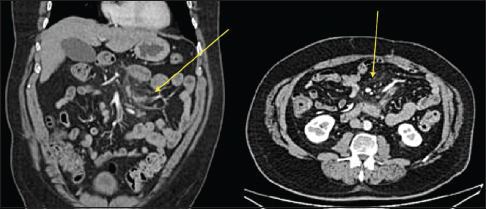
- Axial and coronal contrast-enhanced computed tomography image of early mesenteric panniculitis. There is an increased attenuation (fat stranding) within the bowel mesentery with sparing of the fat surrounding mesenteric vessels and small lymph nodes (yellow arrows). The fat stranding is relatively well circumscribed and limited to the mesentery.
- Axial contrast-enhanced computed tomography image of mesenteric panniculitis of indeterminate cause. The features resolved on follow-up computed tomography. The process is enveloping mesenteric vessels and local lymph nodes with preservation of a ring of fat around the vessels and nodes called the “fat halo” or “fat ring” sign which is fairly specific for mesenteric panniculitis and can help differentiate from sclerosing mesenteritis and other mimics.
The process envelops mesenteric vessels and local lymph nodes with preservation of a ring of fat around the vessels and nodes called the “fat halo” or “fat ring” sign which is fairly specific for MP and can help differentiate from SM and other mimics[15192021] [Figure 11]. There are often minimally enlarged lymph nodes related to inflammatory MP, which have been described as an additional supportive finding in MP.[17] These nodes are homogeneous in appearance and connected along a chain.
There is sparse literature of the MR findings of in MP. Many of the findings on CT such as the “fat halo” sign translate to MR. Other findings such as diffuse-increased T2-signal intensity of the affected mesenteric fat and postcontrast enhancement have been recently described.[17]
Potential mimics of mesenteric panniculitis
Misty mesentery is nonspecific and is also seen in mesenteric hemorrhage, edema, lymphoma, and metastatic disease.[1415] A mesenteric root soft-tissue mass commonly seen in MP can be mimicked by a number of malignant conditions including lymphoma and RPF. However, if the fairly specific imaging features of ground-glass changes in the mesenteric fat, with preservation of the perivascular/perilymphatic soft tissue and connected homogenous minimally enlarged lymph nodes, are present, a confident diagnosis can be made on imaging alone.
SM is an idiopathic disease process characterized by chronic inflammation, fat necrosis and fibrotic thickening of the small bowel mesentery and mesocolon, shortening and kinking the mesentery.[15] It is often associated with other idiopathic inflammatory disorders including RPF, Riedel's thyroiditis, and pseudotumor and have been linked with prior abdominal surgery. The disorder occurs more frequently in men with an average age of presentation of 60 years.[1520] The condition is now considered separate to MP by a number of authors and has more severe inflammation, imaging findings, and clinical symptoms.[17]
Imaging findings
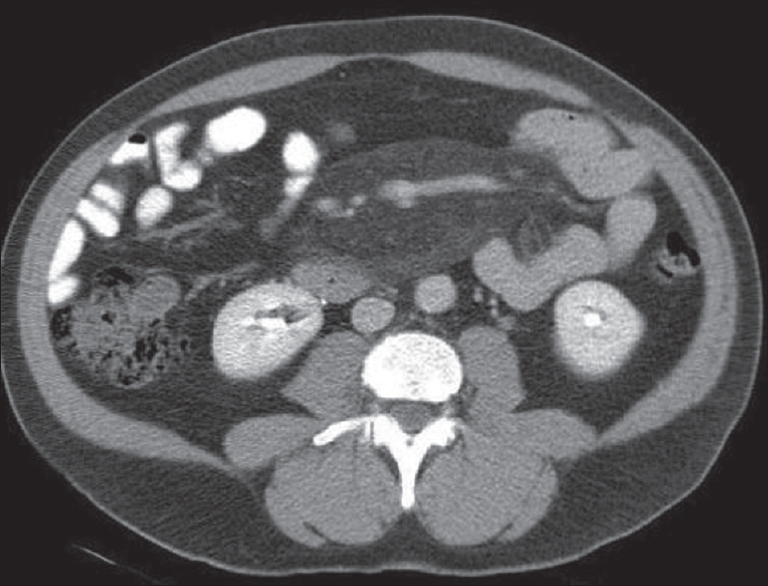
CT is the investigation of choice in the assessment of SM and shows ill-defined soft tissue extending within and into the mesentery [Figure 12]. Infiltration of the porta hepatis and retroperitoneal extension has also been described.
- Axial contrast-enhanced computed tomography image showing a mesenteric root, ill-defined soft-tissue mass extending within and into the mesentery.
Unlike MP, the soft tissue will encircle and encase the mesenteric vessels without preservation of fat. It may also involve bowel loops [Figure 12]. Severe cases of retractile SM have been shown to cause bowel obstruction and ischemia.[21] Calcification of the mass lesion is a rare but recognized finding affecting areas of fat necrosis[20] [Figure 12].
MRI features of SM predominantly fit the pattern expected for fibrosis low T1- and T2-signal intensity mesenteric root soft tissue, but high T2-signal intensity has been described.[15]
Potential mimics of sclerosing mesenteritis
Histopathological analysis is necessary for definitive diagnosis of SM due to the number of important mimics.
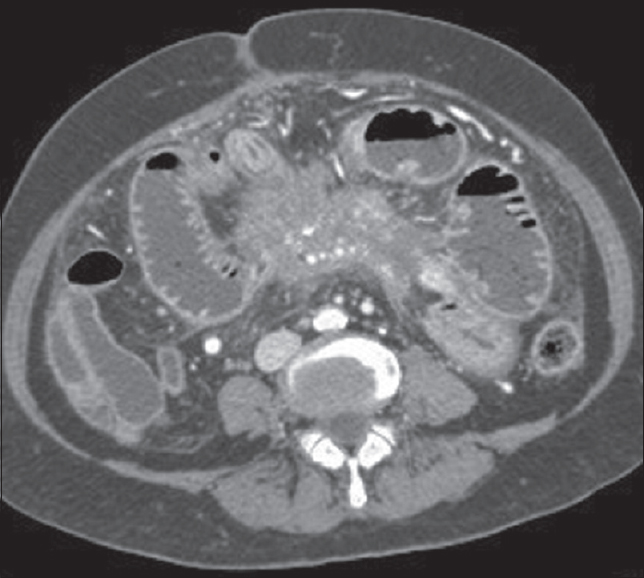
Neuroendocrine tumors, specifically carcinoid of the ileum and jejunum, often have mesenteric lymph node involvement. The desmoplastic reaction is an important mimic of the retractile type of SM. Infiltration rather than encasement of local vessels is more consistent with carcinoid [Figure 13]. Differentiation of these conditions is often impossible on CT alone.[20] Tumor markers and a positive somatostatin receptor scintigraphy scan (indium-111 pentetreotide) are specific for carcinoid tumor and a helpful imaging tool to differentiate the two,[2] but biopsy is often still required.
- Coronal contrast-enhanced computed tomography image showing a heterogeneously enhancing mass within the root of the mesentery with surrounding satellite nodules, in-drawing bowel loops, and obliterating the superior mesenteric vein (red circle). Histopathology confirmed carcinoid.
Other mimics of SM include treated lymphoma, mesenteric carcinomatosis, primary mesenteric mesothelioma, and lipogenic liposarcoma.[20] The presence of large discrete nodes will point to a malignant cause[20] and calcification suggests carcinoid tumor.[23] Although this is not always the case as both SM and carcinoid metastases can show calcification [Figure 12].
AUTOIMMUNE-MEDIATED FIBROSING DISEASES OF THE ABDOMEN NOT LINKED TO IMMUNOGLOBULIN G4
PSC is a chronic, idiopathic, fibrosing, and stricturing disease of the biliary tree. There is a strong association with other autoimmune-mediated conditions such as inflammatory bowel disease, particularly ulcerative colitis, RPF, mediastinal fibrosis, and Sjogren's syndrome.[24] The mean age at presentation is 40 and is twice as common in men.[24]
Imaging finding
US will often show intra- and extra-hepatic duct dilatation with a varied duct caliber [Figure 14]. In advanced disease, the liver will appear cirrhotic often with caudate lobe hypertrophy. CT will also show intrahepatic duct dilation and evidence of cirrhosis.
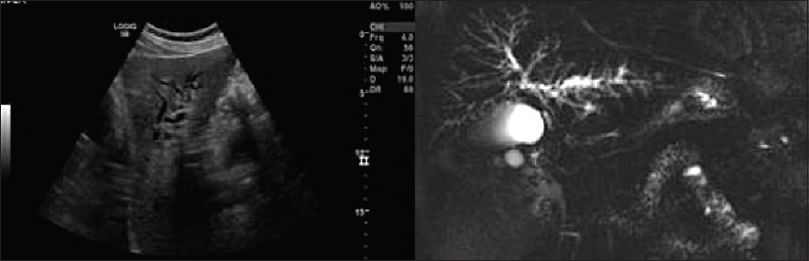
- Selected ultrasound image and thick slab maximum intensity projection magnetic resonance cholangiopancreatography image showing the dilated left-sided intra-hepatic ducts with evidence of beading in patient with primary sclerosing cholangitis.
Cholangiography is the gold standard for diagnosis of PSC.[24] Both endoscopic retrograde cholangiopancreatography (ERCP) and MRCP will show multifocal intrahepatic bile duct strictures interspersed with normal caliber or slightly dilated bile ducts resulting in a “beaded” appearance[24] [Figure 14]. Other reported findings include biliary webs and diverticula. Later, in the disease process, bile duct diseases become obliterated in the periphery resulting in a “pruned-tree” appearance.[24] ERCP has the advantage of interventional capabilities including biopsy, stent placement, and stricture dilatation but also carries the significant risks of cholangitis, pancreatitis, and perforation.[24] MRCP, on the other hand, is noninvasive and allows the assessment of other organs such as the pancreas and thus is the first-line cholangiographic technique.
Potential mimics of primary sclerosing cholangitis
Especially, in the early disease, PSC can resemble cholangiocarcinoma.[25] Cholangiocarcinoma also occurs in 10%–15% of PSC patients.[25] It should be considered clinically if there is a rapid decline in LFTs and/or weight loss. On MRI, periductal high signal on T2-weighted imaging (periportal edema) and periductal soft tissue (>1 cm) with delayed and persistent enhancement suggest cholangiocarcinoma.[24] Rapid progression of focal strictures on follow-up imaging in PSC should also raise the suspicion of transformation to cholangiocarcinoma. Other imaging features more suggestive of cholangiocarcinoma include shouldered, high-grade strictures, liver capsule retraction, and marked bile duct dilatation.[25]
IgG4-related sclerosing disease can result in multiple extrahepatic strictures mimicking PSC and cholangiocarcinoma,[35] thus careful histological analysis is recommended. There are a number of other causes of secondary sclerosing cholangitis, which share many imaging features and should be excluded before a diagnosis of PSC is made [Table 5].

Mesenteric fibromatoses are a group of fibroproliferative processes that are benign but can be locally aggressive. They are also known as desmoid tumors due to their tendon-like appearance histologically. In this review, we will focus on mesenteric fibromatosis, which can be superficial or deep. Intra-abdominal fibromatosis can infiltrate local organs and reoccur after resection, but it does not metastasize.[1526] The majority of cases occur sporadically and have no gender predilection with a wide age range at presentation. Thirteen percent of patients have the Gardner syndrome variant of familial adenomatous polyposis.[15] An additional risk factor in these patients is previous surgery usually developing within 4 years of the first surgical procedure.[15]
Imaging findings
Abdominal fibromas contain a mix of collagenous tissue and myxoid stroma, thus may have varied image characteristics depending on the predominant tissue type.[15]
US performed for nonspecific abdominal pain may reveal homogeneously anechoic or hypoechoic masses.
Cross-sectional imaging is preferred for the preoperative assessment of mesenteric masses, but imaging characteristics are variable due to varied histological components [Figure 15]. The mass is often homogenous and isoattenuating to muscle. If mixed then a striated or “whorled” texture is seen. In postintravenous contrast administration, there is mild-to-moderate enhancement depending on vascularity. Lesions with a predominant myxoid histology are classically hypoattenuating and do not enhance postcontrast.[15]
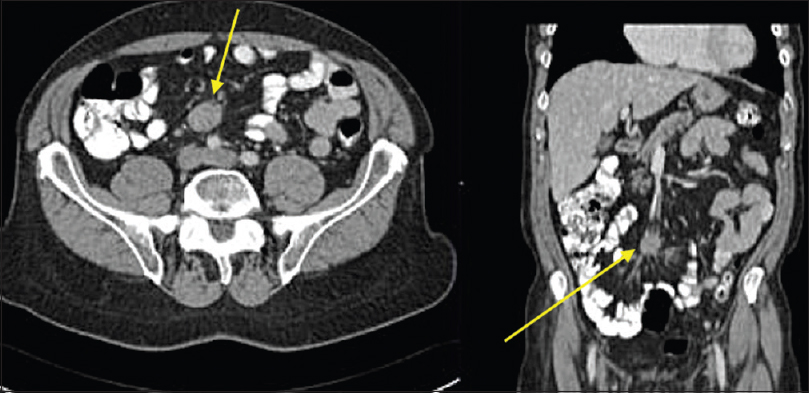
- Axial and coronal contrast enhanced computed tomography showing homogenous mass in the root of mesentery which is isoattenuating to muscle and shows minimal contrast enhancement (yellow arrow). Surgical biopsy confirmed desmoid tumor.
At MRI, most lesions are low or intermediate signal intensity on T1. T2 characteristics are more variable with heterogeneous intermediate/high signal with bands of low signal. If there is predominantly high T2 signal, the lesion is likely to be predominantly made of myxoid stroma. On postcontrast MRI, most lesions will show variable enhancement much like CT.[15]
Potential mimics of mesenteric fibromatosis
In general, malignant disease of the mesentery can mimic mesenteric fibromatosis. More specifically, lymphoma can present with a well-marginated lesion of homogenous attenuation involving the small bowel. Gastrointestinal stromal tumors of the small bowel can have extensive mesenteric involvement and occur as a primary mesenteric mass mimicking benign fibromatosis both radiologically and histopathologically.[1527]
Benign mimics include mesenteric hemangioma, which may have a similar appearance to myxoid mesenteric fibromatoses. They are predominantly hypoattenuating to muscle. Hemangiomas often have prominent peripheral vessels that help in differentiating from other benign fibromatoses [Figure 16].
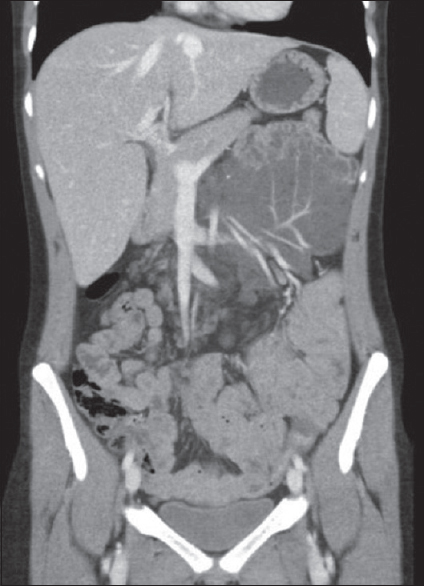
- Coronal contrast-enhanced computed tomography image showing a low-attenuation lesion with multiple prominent peripheral veins in keeping with a mesenteric hemangioma.
Other differentials for mesenteric deposits include endometriosis, soft-tissue sarcomas, RPF, and ITPs.
IATROGENIC FIBROSING CONDITIONS OF THE ABDOMEN
Sclerosing peritonitis (SP) is a chronic fibrotic thickening of the peritoneum. When severe, it can progress to envelop and encapsulate small-bowel loops in what has been called an “abdominal cocoon” or sclerosing encapsulating peritonitis (SEP).[2829]
There is a strong association with patients receiving continuous ambulatory peritoneal dialysis (CAPD). Although rare among the entire population of patients on CAPD with an incidence of 0.7%, this rises to 19.4% in patients on CAPD for greater than 8 years.[30] Etiology, although unclear, is likely to be a result of chronic inflammation or infection. Other factors linked with SP include ventriculoperitoneal shunts, granulomatous disease, malignancy, familial Mediterranean fever, liver transplantation, abdominal surgery, protein S deficiency, luteinizing ovarian thecoma, and the beta-blocker practolol.[3031]
Imaging findings
Abdominal radiography may show focal or linear calcifications. In advanced stages, there may be pockets of small-bowel dilatation [Figure 17a].
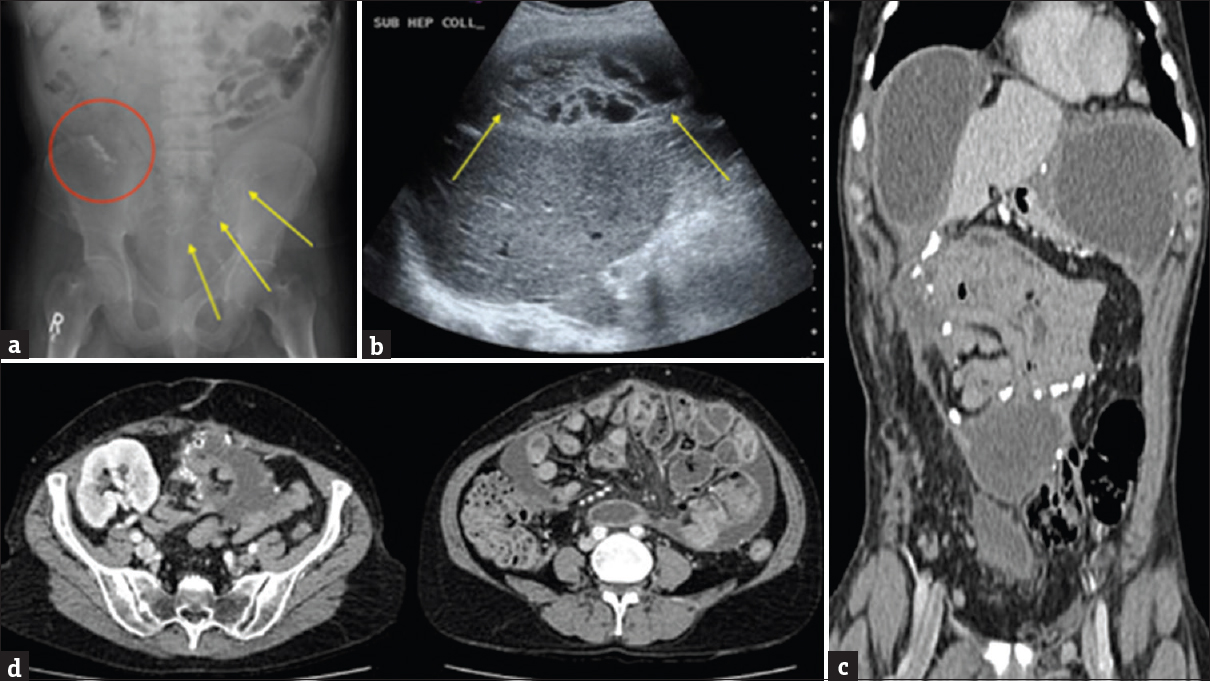
- Sclerosing peritonitis. (a) Abdominal radiograph in patient with known sclerosing peritonitis showing calcification in the right lower quadrant (red circle) with a paucity of bowel gas and gas filled bowel loops in the left upper quadrant. Note the JJ stent in the transplant ureter (yellow arrows). In advanced stages there may be pockets of small bowel dilatation. (b) Selected ultrasound image showing a complex loculated intra-abdominal collection in the subhepatic region with thick septations (yellow arrows) in a patient with encapsulated sclerosing peritonitis. (c) Coronal contrast-enhanced computed tomography selected image of the same patient in picture B, showing smooth, calcified thickening of the peritoneum. (d) Axial contrast-enhanced computed tomography images from a patient with a history of peritoneal dialysis showing smooth, calcified thickening of the peritoneum with encapsulation of the small bowel loops with multiple fluid collections. Note the transplant kidney in the right iliac fossa.
US scan findings are not specific but will show loculated fluid collections. It may also show trilaminar small-bowel wall thickening and foci of calcification[28] [Figure 17b].
CT shows smooth or nodular thickening of the peritoneum that can enhance postcontrast.[31] It eventually progresses to envelop the small-bowel loops with focal areas of upstream intestinal dilatation [Figures 17c and d]. The SEP form of the disease is classically widely and densely calcified. Patients on CAPD often have free intra-abdominal fluid, so the presence of ascites is nonpecific. However, loculated fluid collections trapped within the fibrotic, calcified peritoneum are often described in SEP [Figure 17c and d], and are seen to persist in patients who are no longer on CAPD.
Potential mimics of sclerosing peritonitis
Peritoneal calcification and loculated fluid collections can be seen in tuberculosis and peritoneal carcinomatosis, specifically pseudomyxoma. Spontaneous bacterial peritonitis can show smooth peritoneal thickening and bowel encapsulation mimicking SEP.[31]
CONCLUSION
Benign fibrosing diseases of the abdomen are a relatively rare, varied, and complex group of conditions that have both overlapping and more specific imaging features. It is important to recognize that there are a number of malignant and infective/inflammatory processes that can also mimic these benign conditions. To ensure a correct diagnosis, thorough clinical and histopathological assessment is required to support the imaging findings presented in this review.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Available FREE in open access from: http://www.clinicalimagingscience.org/text.asp?2018/8/1/21/233661.
REFERENCES
- A new clinicopathological entity of igG4-related autoimmune disease. J Gastroenterol. 2003;38:982-4.
- [Google Scholar]
- Systemic IgG4-related sclerosing disease: Spectrum of imaging findings and differential diagnosis. AJR Am J Roentgenol. 2012;199:W276-82.
- [Google Scholar]
- Chronic inflammatory sclerosis of the pancreas – An autonomous pancreatic disease? Am J Dig Dis. 1961;6:688-98.
- [Google Scholar]
- IgG4-related sclerosing disease: Autoimmune pancreatitis and extrapancreatic manifestations. Radiographics. 2011;31:1379-402.
- [Google Scholar]
- Autoimmune pancreatitis: An update on classification, diagnosis, natural history and management. Curr Gastroenterol Rep. 2012;14:95-105.
- [Google Scholar]
- Lymphoplasmacytic sclerosing pancreatitis (autoimmune pancreatitis): Evaluation with multidetector CT. Radiographics. 2008;28:157-70.
- [Google Scholar]
- Autoimmune pancreatitis: An illustrated guide to diagnosis. Clin Radiol. 2013;68:422-32.
- [Google Scholar]
- Diagnosis of autoimmune pancreatitis using its five cardinal features: Introducing the Mayo clinic's HISORt criteria. J Gastroenterol. 2007;42(Suppl 18):39-41.
- [Google Scholar]
- Retroperitoneal fibrosis: Role of imaging in diagnosis and follow-up. Radiographics. 2013;33:535-52.
- [Google Scholar]
- US case of the day. Retroperitoneal fibrosis with perirenal involvement. Radiographics. 1995;15:1024-6.
- [Google Scholar]
- Retroperitoneal fibrosis: A review of clinical features and imaging findings. AJR Am J Roentgenol. 2008;191:423-31.
- [Google Scholar]
- From the archives of the AFIP: Benign fibrous tumors and tumorlike lesions of the mesentery: Radiologic-pathologic correlation. Radiographics. 2008;28:583-607.
- [Google Scholar]
- Inflammatory pseudotumor: The great mimicker. AJR Am J Roentgenol. 2012;198:W217-27.
- [Google Scholar]
- Mesenteric panniculitis – A review and guide for the (confused) radiologist. GI106-ED-X poster RSNA; 29 November, 04 December 2015
- [Google Scholar]
- Is mesenteric panniculitis truely a paraneoplastic phenomenon. A matched pair analysis? Eur J Radiol. 2013;82:1853-9.
- [Google Scholar]
- Mesenteric panniculitis. Part 2: Prevalence and natural course: MDCT prospective study. JBR-BTR. 2011;94:241-6.
- [Google Scholar]
- CT findings in sclerosing mesenteritis (panniculitis): Spectrum of disease. Radiographics. 2003;23:1561-7.
- [Google Scholar]
- The CT appearances of sclerosing mesenteritis and associated diseases. Clin Radiol. 2006;61:652-8.
- [Google Scholar]
- Sclerosing mesenteritis: Imaging findings in 17 patients. AJR Am J Roentgenol. 1999;172:625-9.
- [Google Scholar]
- Gastrointestinal carcinoids: Imaging features with clinicopathologic comparison. Radiographics. 2007;27:237-57.
- [Google Scholar]
- Radiologic manifestations of sclerosing cholangitis with emphasis on MR cholangiopancreatography. Radiographics. 2000;20:959-75.
- [Google Scholar]
- From the archives of the AFIP: Musculoskeletal fibromatoses: Radiologic-pathologic correlation. Radiographics. 2009;29:2143-73.
- [Google Scholar]
- Mesenteric fibromatosis with involvement of the gastrointestinal tract. A GIST simulator: A study of 25 cases. Am J Clin Pathol. 2004;121:93-8.
- [Google Scholar]
- Imaging features of encapsulating peritoneal sclerosis in continuous ambulatory peritoneal dialysis patients. AJR Am J Roentgenol. 2010;195:W50-4.
- [Google Scholar]
- Abdominal cocoon: Preoperative diagnostic clues from radiologic imaging with pathologic correlation. AJR Am J Roentgenol. 2004;182:639-41.
- [Google Scholar]
- Sclerosing peritonitis: The experience in Australia. Nephrol Dial Transplant. 1998;13:154-9.
- [Google Scholar]
- Encapsulating peritoneal sclerosis – Correlation of radiological findings at CT with underlying pathogenesis. Clin Radiol. 2014;69:103-9.
- [Google Scholar]






